Abstract
The renin–angiotensin system (RAS) is related to physiological adaptations induced by exercise. Angiotensin-converting enzyme (ACE) 2 is a major regulator of the RAS in tissues, as it metabolizes angiotensin (Ang) II to Ang-(1–7). The aim of this study was to determine the effects of ACE2 deficiency on physical performance and physiological adaptations induced by voluntary running. Physical performance, body composition and plasma angiotensin levels, as well as tissue morphology and gene expression of RAS components in the left ventricle (LV) and skeletal muscle (gastrocnemius), were evaluated in ACE2-deficient (ACE2−/y) and wild-type (ACE2+/y) mice after 6 weeks of voluntary wheel running. ACE2−/y mice run less than ACE2+/y mice (19±4.7 vs. 26±12.6 revolutions per day × 100, P<0.01). The ACE2+/y group presented a lower fat mass (15±1.1%) and higher muscle mass (76.6±1.6%) after 6 weeks of voluntary running compared with the sedentary control group (fat mass: 18.3±2.1%; muscle mass: 72.7±2.2). However, no change in body composition was observed in ACE2−/y mice after exercise. Heart and skeletal muscle hypertrophy was observed only in trained ACE2+/y mice. Besides a small decrease in Ang I in ACE2−/y mice, plasma levels of angiotensin peptides remained unchanged by exercise or ACE2 deficiency. In the LV of trained animals, AT2 gene expression was higher in ACE2+/y compared with ACE2−/y mice. ACE2 deficiency leads to an increase in AT1 gene expression in skeletal muscle. ACE expression in soleus was increased in all exercised groups. ACE2 deficiency affects physical performance and impairs cardiac and skeletal muscle adaptations to exercise.
Similar content being viewed by others
Introduction
Physical exercise training has been shown to be effective in the prevention and treatment of cardiovascular diseases.1, 2, 3, 4 There is considerable evidence that the renin–angiotensin system (RAS) is involved in physiological adaptations induced by exercise. Studies suggest that the cardioprotective and antihypertensive mechanisms of exercise are associated with reductions in angiotensin-converting enzyme (ACE) activity and angiotensin (Ang) II levels.5, 6 In rats, swimming training increases Ang-(1–7) and Mas expression in the heart.7 In addition, oral treatment with Ang-(1–7) and exercise training produce similar cardiovascular effects in spontaneously hypertensive rats.8
ACE2 is an enzyme homologous to ACE that cleaves the peptide Ang II to generate Ang-(1–7). The ACE2/Ang-(1–7)/Mas axis represents a significant protective system in pathological conditions as demonstrated in several studies.9, 10, 11, 12 Oral treatment with Ang-(1–7) reduces the risk and severity of cardiovascular diseases13, 14 and the absence of Mas, the Ang-(1–7) receptor, leads to cardiovascular and metabolic impairments in mice.15
Mice with disrupted ACE2 gene showed impaired cardiac contractility, increased Ang II levels and upregulated expression of hypoxia-induced genes in the heart.16 Furthermore, these mice presented adverse ventricular remodeling after myocardial infarction by potentiation of Ang II effects through the AT1 receptor.17 We hypothesized that lack of ACE2 may also impair physiological adaptations due to physical exercise. Thus, the aim of the present study was to determine the effect of voluntary wheel running on the heart and skeletal muscle in ACE2−/y mice.
Methods
Experimental animals
The animals used in the study consisted of C57BL/6 male mice (ACE2+/y and ACE2−/y) separated into sedentary or trained groups (n=6–8 per group). Animals were 8 to 12 weeks of age and weighed between 26 and 30 g. They received food and water ad libitum and were kept in light/dark cycle of 12 h each with the temperature maintained between 22 and 25 °C and relative humidity between 60 and 65%. All experimental protocols were approved by the local Ethics Committee of the State of Berlin (LAGESO).
Aerobic training protocol
Exercise training (Exe) was performed with a voluntary activity wheel (11.5 cm diameter) placed inside the cage for 6 weeks (42 days). The activity wheel was connected to a computer (Tiny Tag, Gemini Data Loggers, Chichester, UK) to measure the number of rotations performed by the animals. Groups of sedentary animals (Sed) were used as controls. In all groups, only one animal was kept per cage.
After 6 weeks, all groups underwent an analysis of their body composition by nuclear magnetic resonance (LF90II; Bruker Optics Inc., Billerica, MA, USA).
Cardiac and skeletal muscle analysis
The running wheels were removed from the cages 48 h before the killing of the animals, to avoid acute effects of exercise. Blood was collected by cardiac puncture after anesthesia (ketamine 100 mg kg−1 and rompum 2 g per 10 g body weight). The heart was quickly removed, washed with saline (0.9% NaCl) and the cardiac structures were separated in the atrium, right ventricle and left ventricular+septum (LV). The effect of voluntary training on cardiac hypertrophy was assessed by the ratio cardiac mass/body weight.
The gastrocnemius and soleus muscles were dissected (right limb for gene expression analysis and left limb for morphological analysis). Skeletal muscles were fixed in paraformaldehyde and embedded in paraffin. Five-micrometer sections were produced, deparaffinized, rehydrated and stained with hematoxylin–eosin in saturated picric acid. Sections of skeletal muscle were photographed with a × 10 objective using a AVT-Horn Sony camera (Sony, Berlin, Germany) and Zeiss Axioplan-2 (Zeiss, Jena, Germany). 25 microscopic view fields were evaluated for each of the muscle types (gastrocnemius and soleus).
Gene expression
The left ventricle (LV) and gastrocnemius muscle were collected immediately on dry ice and stored at −80 °C to prevent degradation of RNA. Total RNA extraction was performed using Trizol (Invitrogen, Carlsbad, CA, USA) and purified using RNeasy columns (RNeasy Mini Kit, QIAGEN, Hilden, Germany) and DNAse I (Invitrogen) treatment to avoid genomic DNA contamination. RNA quantification and purity was assessed using a spectrophotometer (ND-1000 NanoDrop, Wilmington, DE, USA) and the 260/280 ratio was >1.8. The quantitative PCR analysis (AB7900, Applied Biosystems, Foster City, CA, USA and GoTaq, Promega, Madison, WI, USA) used 40 ng of reverse-transcribed RNA (cDNA) and SYBR green. Standard curves were performed with serial dilutions for the analysis of the efficiency of the primers (Biotez, Berlin, Germany) and only primers were used with an efficiency of at least 90%. Dissociation curves (‘Melting Curve’) were also carried out to check for the formation of primer dimers.
Angiotensin plasma levels
Blood samples were collected by cardiac puncture into Eppendorf tubes containing 50 μl of a protease inhibitor cocktail containing ethylenediaminetetraacetic acid (EDTA), pepstatin A, p-hydroxymercuribenzoic acid, phenanthroline and specific inhibitors for renin and aminopeptidases A and N to a final concentration of 5% v/v (Attoquant Diagnostics, Vienna, Austria) and centrifuged at 7000 r.p.m. for 8 min. The plasma samples were immediately frozen on dry ice and subsequently stored at −80 °C. The determination of plasma concentrations of angiotensins was performed by mass spectrometry using the RAS Fingerprint (Attoquant), which allows the simultaneous quantification of 10 angiotensin peptides in a sample of 1 ml plasma. Plasma of three animals was pooled for this measurement and four pools were analyzed per group.
Statistical analysis
Unpaired Student’s t-test was used for the comparison between the sedentary group and the trained group. Two-way analysis of variance was employed for repeated measures comparing the variables obtained before and after training. One-way analysis of variance was used for statistical analysis comparing training groups (ACE2+/y and ACE2−/y). In case of differences between groups, Tukey’s post-hoc test was used. The significance level for all experiments was set at P≤0.05 (GraphPad Software, Inc., San Diego, CA, USA).
Results
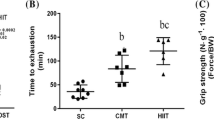
Physical performance
The ACE2−/y Exe (n=6) showed a lower performance in the voluntary exercise wheel when compared with the ACE2+/y Exe group (n=6). The reduction in the physical performance was observed during the whole 6 weeks of the training period (Figure 1). ACE2−/y performance was ~30% lower than the one of ACE2+/y mice.
Cardiac remodeling
The total mass of the heart and the weight of the LV were measured, to evaluate the effect of exercise on physiological cardiac hypertrophy. ACE2+/y Exe group presented a significantly higher heart mass (5.10±0.35 mg g−1) and LV weight (3.60±0.17 mg g−1) compared with the ACE2+/y Sed group (4.70±0.14 and 3.20±0.10 mg g−1, respectively). In contrast, voluntary exercise did not cause alterations in heart weight in ACE2−/y mice (Figure 2).
Body composition
Body composition (% fat, muscle and water) was analyzed before and after 6 weeks of training by magnetic resonance. No difference was observed in the body weight between groups, before or after the voluntary exercise training (Table 1). As expected, the ACE2+/y Exe group presented a lower percentage of fat and increased lean mass compared with the ACE2+/y Sed group (Table 1). This improvement in body composition was not observed in the ACE2−/y Exe group. Interestingly, the percentage of fat in the ACE2−/y Sed group was significantly lower (P<0.05) compared with the sedentary ACE2+/y group (Table 1).
Morphological changes in skeletal muscle
The morphological analysis of skeletal muscle in the ACE2+/y group indicated that voluntary exercise increased soleus diameter (Figure 3b), and the number of the nuclei in the gastrocnemius (Figure 3c) and the soleus muscle (Figure 3d). No morphological changes were observed in the skeletal muscles of ACE2−/y mice after exercise training.
Gene expression of RAS components
Components of the RAS (ACE, ACE2, Mas, AT1 and AT2 receptors) were analyzed by quantitative PCR in the LV and gastrocnemius muscle. Mas expression increased only in the LV of the ACE2+/y Exe group (Figure 4a). AT2 gene expression was significantly lower in ACE2−/y subjected to voluntary exercise, compared with sedentary controls, and to the ACE2+/y Exe group (Figure 4c). No differences in the AT1 and ACE gene expression in the LV were observed between all groups (Figure 4b and d), whereas ACE2 was downregulated after exercise in the ACE2+/y mice (Figure 4e). However, analysis of gene expression in skeletal muscle showed an increase in Mas expression only in ACE2−/y Exe mice compared with sedentary controls (Figure 5a). Voluntary exercise did not alter the muscular expression of AT1 (Figure 5b) and AT2 receptors (Figure 5c), and ACE2 (Figure 5e). An increase in muscular ACE gene expression was observed in both trained groups (Figure 5d).
Angiotensin levels
We measured the plasma concentrations of Ang-(1–8), Ang-(3–8), Ang-(1–10), Ang-(2–7), Ang-(1–7), Ang-(3–7), Ang-(1–5), Ang-(1–9), Ang-(2–8) and Ang-(2–10). Ang-(2–7), Ang-(3–7) and Ang-(2–10) were not detectable. No significant differences were observed in Ang-(3–8), Ang-(2–8) (data not shown), Ang II (Figure 6b), Ang-(1–7) (Figure 6d), Ang-(1–5) (Figure 6e) and Ang-(1–8)/Ang-(1–7) levels (Figure 6f) between groups. The levels of Ang I (Figure 6a) were higher in the ACE2+/y Exe group and the ratio Ang II/Ang I (Figure 6c) was lower in the ACE2+/y Exe group compared with the ACE2+/y Sed group.
Discussion
The present study showed for the first time that ACE2 deficiency affects physical performance and leads to impaired physiological adaptations to exercise. At the end of the sixth week of training, ACE2−/y mice showed less rotations per day in comparison with ACE2+/y. The physiological remodeling of the heart and muscle, and the improvement of body composition induced by exercise were absent in ACE2 knockout mice
There are no studies in the literature that evaluate the effect of ACE2 deficiency on physical performance. However, the effect of bleomycin treatment on the performance was studied in ACE2-deficient and control mice.18 Bleomycin treatment is a model used in mice to induce acute respiratory distress syndrome. The deletion of the ACE2 gene worsened lung injury induced by bleomycin. A treadmill test showed that both groups (ACE2+/y and ACE2−/y) treated with bleomycin presented reduced exercise capacity; however, the reduction was greater in the ACE2−/y group. This result was interpreted as evidence that the deletion of the ACE2 gene worsens lung injury in this model. The physical performance of the ACE2−/y mice without bleomycin treatment was not evaluated in this study. Thus, it may well be that the reduced physical performance already at baseline in ACE2−/y mice, which we reveal in our study, confounded the results shown by Rey-Parra et al.18 Several physiological adaptations induced by physical training have been demonstrated in the heart and skeletal muscle.19, 20, 21 Cardiac hypertrophy can occur in different ways, with physiological remodeling resulting from physical training22 and pathological remodeling associated with contractile dysfunction and heart failure.23 In the present study, we only observed an increase of cardiac ventricular mass in trained ACE2+/y mice. The reduced volume of training (30% less than ACE+/y) in ACE2−/y mice may only partially explain the total absence of physiological cardiac hypertrophy in these animals. Guimarães et al.24 evaluated the effect of swimming training in Mas-knockout mice. These mice presented cardiac hypertrophy after training; however, this was associated with deleterious cardiac effects such as an increased deposition of extracellular matrix proteins. Voluntary running increased Mas expression in the LV of ACE2+/y (Figure 4a). This confirms data of Filho et al.,7 who first described that aerobic swimming training induces an increase in Ang-(1–7) associated with an increase in Mas (mRNA and protein) in the LV of trained spontaneously hypertensive rats. Subsequent studies confirmed the activation of the ACE2/Ang-(1–7)/Mas axis induced by exercise in the heart and vessels.25, 26 Taken together, these data provide evidence that physiological cardiac hypertrophy is depending on an intact ACE2/Ang-(1–7)/Mas axis.
The ACE2/Ang-(1–7)/Mas axis may also be involved in the adaptation of skeletal muscle to exercise. The increase of Mas expression by exercise in the gastrocnemius muscle of ACE2−/y but not of ACE2+/y mice (Figure 5a) can be explained by the fact that Ang II has been shown to increase Mas expression in muscles.27 We did not measure the muscular levels of Ang II but Ang II plasma level were unchanged in ACE2−/y compared with ACE2+/y mice corroborating earlier data.28 Recent studies showed that Ang II induces skeletal muscle atrophy by activating the ubiquitin-proteasome system.29, 30 In addition, some pathological models of muscular atrophy (that is, Ang II and lipopolysaccharide treatment and muscular immobilization) induced an increased expression of Mas.27 Increased Mas expression is probably a compensatory effect, as we and others have shown that the ACE2/Ang-(1–7)/Mas axis is protective in muscular atrophy models.28, 29, 30 The genetic deletion of Mas induced a highly deteriorated muscular architecture, increased fibrosis and diminished muscle strength in a mouse model of muscular dystrophy.30 In the present study, we have not investigated whether ACE2 deficiency leads to muscular fibrosis; however, Riquelme et al.31 showed that ACE2 overexpression in mdx mice reduces fibrosis and decreases infiltration of inflammatory cells. Moreover, muscular dystrophy models presented improvements in locomotor activity and muscle strength when treated with Ang-(1–7).32, 33, 34
There are no data in the literature regarding the effect of Ang-(1–7) administration on physical performance. However, an ongoing study in our laboratory shows that oral treatment with Ang-(1–7) decrease muscle damage and maintains the muscle strength after an eccentric exercise session. These data strongly indicate a relationship between Ang-(1–7) and physical performance.
The ACE2/Ang-(1–7)/Mas axis seems to be also pivotal for muscular hypertrophy induced by physical exercise, as ACE2−/y mice did not show an increase in soleus diameter and in the number of nuclei in soleus and gastrocnemius muscles after running, in contrast to control mice (Figure 3). Furthermore, the increase in lean (muscle) mass and the reduction in fat mass observed in the body composition analysis of wild-type mice after running was absent in ACE2−/y animals (Table 1). These observations suggest that the absence of ACE2 affects skeletal muscle adaptations to physiological and pathological challenges, and these effects are mediated by the change in the relative concentrations of its substrate Ang II, which is deleterious, and its metabolite Ang-(1–7), which is protective for the muscle. We did not observe strong alterations in the plasma concentrations of angiotensin peptides in ACE2−/y mice. However, the most important effects of ACE2 on the peptide levels are expected to happen locally in the tissue, in close proximity to the relevant receptors, AT1 and Mas. One limitation of our study is that we did not measure the local angiotensin concentrations in the heart and skeletal muscle. However we would expect that local muscular Ang II levels are elevated, as it has been shown for the kidney and placenta of ACE2-deficient animals in the absence of changes in plasma levels.35
However, it cannot be excluded that the recently discovered effect of ACE2 on tryptophan uptake in the gut and the concentrations of this amino acid in the blood36 may also contribute to decreased muscle strength in ACE2−/y mice. Accordingly, tryptophan was recently shown to stimulate the expression of myogenic genes.37
The evidence presented in this study shows that absence of ACE2 impairs physiological adaptations to exercise in skeletal and cardiac muscle, probably due to the effects of ACE2 deficiency on the local RAS in muscular tissue.
Future studies including ACE2−/y mice performing exercise at the same level as the controls will be necessary to assess physiological adaptations in these conditions. Furthermore, due to the multiple substrates of ACE2 and various functions of this enzyme several other hormones, neuropeptides and neurotransmitters may be involved in the exercise phenotype of ACE2-/y mice. Moreover, the control of physical activity is very complex and not only related to various biochemical and physiological systems but also to brain reward pathways.38 Since ACE2 is expressed in the brain, a central contribution to the low running performance in ACE2-/y mice cannot be excluded.
References
Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK American Heart Association Council on Clinical Cardiology Subcommittee on Exercise Rh and Prevention American Heart Association Council on Nutrition PyA Metabolism Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003; 107: 3109–3116.
Pescatello L, Franklin B, Fagard R, Farquhar W, Kelley G, Ray C . American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 2004; 36: 533–553.
Lee SE, Youn JC, Lee HS, Park S, Lee SH, Cho IJ, Shim CY, Hong GR, Choi D, Kang SM . Left atrial volume index is an independent predictor of hypertensive response to exercise in patients with hypertension. Hypertens Res 2015; 38: 137–142.
Leggio M, Mazza A, Cruciani G, Sgorbini L, Pugliese M, Bendini MG, Severi P, Jesi AP . Effects of exercise training on systo-diastolic ventricular dysfunction in patients with hypertension: an echocardiographic study with tissue velocity and strain imaging evaluation. Hypertens Res 2014; 37: 649–654.
Negrão CE, Middlekauff HR . Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J Appl Physiol 2008; 104: 577–578.
Kar S, Gao L, Zucker IH . Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol (1985) 2010; 108: 923–932.
Filho AG, Ferreira AJ, Santos SH, Neves SR, Silva Camargos ER, Becker LK, Belchior HA, Dias-Peixoto MF, Pinheiro SV, Santos RA . Selective increase of angiotensin(1-7) and its receptor in hearts of spontaneously hypertensive rats subjected to physical training. Exp Physiol 2008; 93: 589–598.
Bertagnolli M, Casali KR, De Sousa FB, Rigatto K, Becker L, Santos SH, Dias LD, Pinto G, Dartora DR, Schaan BD, Milan RD, Irigoyen MC, Santos RA . An orally active angiotensin-(1-7) inclusion compound and exercise training produce similar cardiovascular effects in spontaneously hypertensive rats. Peptides 2014; 51: 65–73.
Valdes G, Neves L, Anton L, Corthorn J, Chacon C, Germain AM, Merril DC, Sarao R, Penninger J, Ferrario CM, Brosnihan KB . Distribution of angiotensin-(1-7) and ACE2 in human placenta of early and term normotensive pregnancy and preeclampsia. J Hypertens 2004; 22: S65–S65.
Santos RA, Frézard F, Ferreira AJ . Angiotensin-(1-7): blood, heart, and blood vessels. Curr Med Chem Cardiovasc Hematol Agents 2005; 3: 383–391.
Iwata M, Cowling RT, Yeo SJ, Greenberg B . Targeting the ACE2-Ang-(1-7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol 2011; 51: 542–547.
Iwanami J, Mogi M, Tsukuda K, Wang XL, Nakaoka H, Ohshima K, Chisaka T, Bai HY, Kanno H, Min LJ, Horiuchi M . Role of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis in the hypotensive effect of azilsartan. Hypertens Res 2014; 37: 616–620.
Marques FD, Melo MB, Souza LE, Irigoyen MC, Sinisterra RD, de Sousa FB, Savergnini SQ, Braga VB, Ferreira AJ, Santos RA . Beneficial effects of long-term administration of an oral formulation of Angiotensin-(1-7) in infarcted rats. Int J Hypertens 2012; 2012: 795452.
Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA . An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo) 2011; 66: 837–841.
Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M . Genetically altered animal models for Mas and angiotensin-(1-7). Exp Physiol 2008; 93: 528–537.
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM . Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002; 417: 822–828.
Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY . Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2009; 2: 446–455.
Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, Thébaud B . Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med (Berl) 2012; 90: 637–647.
Malek MH, Hüttemann M, Lee I, Coburn JW . Similar skeletal muscle angiogenic and mitochondrial signalling following 8 weeks of endurance exercise in mice: discontinuous versus continuous training. Exp Physiol 2013; 98: 807–818.
Budiono BP, See Hoe LE, Peart JN, Sabapathy S, Ashton KJ, Haseler LJ, Headrick JP . Voluntary running in mice beneficially modulates myocardial ischemic tolerance, signaling kinases, and gene expression patterns. Am J Physiol Regul Integr Comp Physiol 2012; 302: R1091–R1100.
Fujimaki S, Hidaka R, Asashima M, Takemasa T, Kuwabara T . Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running. J Biol Chem 2014; 289: 7399–7412.
Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA . Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 2001; 90: 1900–1908.
van Berlo JH, Maillet M, Molkentin JD . Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 2013; 123: 37–45.
Guimarães GG, Santos SH, Oliveira ML, Pimenta-Velloso EP, Motta DF, Martins AS, Alenina N, Bader M, Santos RA, Campagnole-Santos MJ . Exercise induces renin-angiotensin system unbalance and high collagen expression in the heart of Mas-deficient mice. Peptides 2012; 38: 54–61.
Silva SD, Zampieri TT, Ruggeri A, Ceroni A, Aragão DS, Fernandes FB, Casarini DE, Michelini LC . Downregulation of the vascular Renin-Angiotensin system by aerobic training- focus on the balance between vasoconstrictor and vasodilator axes. Circ J 2015; 79: 1372–1380.
Guimaraes GG, Santos SH, Oliveira ML, Pimenta-Velloso EP, Motta DF, Martins AS, Alenina N, Bader M, Santos RA, Campagnole-Santos MJ . Exercise induces renin-angiotensin system unbalance and high collagen expression in the heart of Mas-deficient mice. Peptides 2012; 38: 54–61.
Morales MG, Abrigo J, Meneses C, Cisternas F, Simon F, Cabello-Verrugio C . Expression of the Mas receptor is upregulated in skeletal muscle wasting. Histochem Cell Biol 2015; 143: 131–141.
Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM . Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 2006; 116: 2218–2225.
Kadoguchi T, Kinugawa S, Takada S, Fukushima A, Furihata T, Homma T, Masaki Y, Mizushima W, Nishikawa M, Takahashi M, Yokota T, Matsushima S, Okita K, Tsutsui H . Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp Physiol 2015; 100: 312–322.
Du Bois P, Pablo Tortola C, Lodka D, Kny M, Schmidt F, Song K, Schmidt S, Bassel-Duby R, Olson EN, Fielitz J . Angiotensin II induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ Res 2015; 117: 424–436.
Riquelme C, Acuña MJ, Torrejón J, Rebolledo D, Cabrera D, Santos RA, Brandan E . ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS ONE 2014; 9: e93449.
Sabharwal R, Chapleau MW . Autonomic, locomotor and cardiac abnormalities in a mouse model of muscular dystrophy: targeting the renin-angiotensin system. Exp Physiol 2014; 99: 627–631.
Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E . Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-beta signalling. Hum Mol Genet 2014; 23: 1237–1249.
Morales MG, Abrigo J, Acuña MJ, Santos RA, Bader M, Brandan E, Simon F, Olguin H, Cabrera D, Cabello-Verrugio C . Angiotensin-(1-7) attenuates disuse skeletal muscle atrophy via the Mas receptor. Dis Model Mech 2016 pii: dmm.023390.
Bharadwaj MS, Strawn WB, Groban L, Yamaleyeva LM, Chappell MC, Horta C, Atkins K, Firmes L, Gurley SB, Brosnihan KB . Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension 2011; 58: 852–858.
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM . ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012; 487: 477–481.
Dukes A, Davis C, El Refaey M, Upadhyay S, Mork S, Arounleut P, Johnson MH, Hill WD, Isales CM, Hamrick MW . The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition 2015; 31: 1018–1024.
Garland T, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC . The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 2011; 214 (Pt 2): 206–229.
Acknowledgements
We thank FAPEMIG, CAPES, PROBRAL and CNPq. The Brazilian fellowship BJT 407352/2013-9 to NA and the DAAD/CNPq program PROBRAL to NA and RAS supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Motta-Santos, D., dos Santos, R., Oliveira, M. et al. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 39, 506–512 (2016). https://doi.org/10.1038/hr.2016.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.28
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
COVID-19 and aerobic exercise: possible role of angiotensin converting enzyme 2
Archives of Public Health (2022)
-
Lower limb strength training (LLST) modulates serum and urinary levels of renin angiotensin system molecules in healthy young males
Sport Sciences for Health (2022)
-
Angiotensin-(1–7) oral formulation improves physical performance in mountain bike athletes: a double‐blinded crossover study
BMC Sports Science, Medicine and Rehabilitation (2021)
-
ACE2 in Brain Physiology and Pathophysiology: Evidence from Transgenic Animal Models
Neurochemical Research (2019)
-
Correlation between the 24-h urinary angiotensinogen or aldosterone level and muscle mass: Japan shimanami health promoting program study
Hypertension Research (2018)