Abstract
Hydrogen sulfide (H2S) is increasingly recognized as a gasotransmitter with protective effects in the cardiovascular system. The aim of the study was to examine the effects of chronic NaHS treatment on blood pressure, vascular function and oxidative stress in an in vivo model of hypertension and oxidative stress. Male C57Bl6/J mice were rendered hypertensive with 0.7 mg kg−1 per day angiotensin II (AngII) for 14 days administered via implanted mini-pumps. The mice were treated with NaHS (10 μmol kg−1 per day) to deliver H2S or an inhibitor of cystathionine-γ-lyase, DL-propargylglycine (PPG 30 mg kg−1 per day) via intraperitoneal (i.p.) injection. Systolic blood pressure was measured and vascular function examined by myography. Vascular superoxide production was measured by lucigenin-enhanced chemiluminescence. AngII infusion significantly increased systolic blood pressure (P<0.001). This increase was significantly attenuated by treatment with NaHS (P<0.001). Both aortic endothelial function and NO bioavailability were significantly attenuated in the AngII group (P<0.01) but this attenuation was reversed by NaHS treatment. Similarly, aortic superoxide anion production was significantly enhanced by AngII (P<0.01), and this was reversed by NaHS treatment, and also exacerbated by PPG treatment (P<0.001). These data show that in a mouse model of hypertension and oxidative stress induced by AngII, exogenous H2S treatment in vivo reduces blood pressure, endothelial dysfunction and vascular oxidative stress, while inhibiting endogenous H2S production in vivo is deleterious. This furthers the evidence that H2S is a vasoprotective molecule that may be a useful treatment target in cardiovascular disease.
Similar content being viewed by others
Introduction
Hydrogen sulfide (H2S) is a gasotransmitter1 reported to have numerous physiological effects in diverse processes including metabolism, inflammation, the nervous system and the cardiovascular system. The cardiovascular effects of this molecule are currently of major interest and include vascular relaxation, cardioprotective and vasculoprotective effects.2, 3
In the cardiovascular system, H2S is produced primarily by the pyridoxyl'5'phosphate-dependent enzyme cystathionine-γ-lyase (CSE, EC 4.4.1.1), which is present in both endothelial cells4 and vascular smooth muscle cells.5, 6 Inhibition of CSE, with the irreversible inhibitor DL-propargylglycine (PPG), leads to an elevation of vascular tone in isolated aorta7 and an increase in blood pressure in vivo in rats.8 Most importantly, mice deficient in CSE display early endothelial dysfunction and hypertension.4 H2S is additionally reported to be produced by 3-mercaptopyruvate sulfurtransferase (EC 2.8.1.2) in concert with cysteine aminotransferase (EC 2.6.1.75) to metabolize cysteine.9
A key aspect of the biology of H2S is its anti-oxidant effects. H2S is a potent one-electron chemical reductant that is theoretically capable of scavenging free radicals by single electron or hydrogen atom transfer.10 Thus, H2S may participate in many reactions11 and is reported to scavenge reactive oxygen and nitrogen species.11, 12, 13, 14, 15, 16 However, the kinetics, reactivity and mechanism of H2S interactions with reactive oxygen species (ROS) are poorly understood under physiological conditions.10 Further cytoprotective effects of NaHS have been attributed to its ability to decrease lipid peroxidation,12 increase glutathione levels and boost endogenous anti-oxidant defences.17
Oxidative stress is an important feature in a number of cardiovascular disease states including hypertension, diabetes and atherosclerosis.18, 19 Blood vessels express NADPH oxidases; enzyme assemblies that contain catalytic ‘NOX’ subunits (or ‘NOXs’), of which at least three isoforms, NOX1, NOX2 and NOX4, are responsible for the production of ROS in the vasculature. Both NADPH oxidase activity and expression are upregulated in cardiovascular disease, with NOX2 playing a key role in the vascular dysfunction associated with these pathologies.20 Thus, strategies to limit oxidative stress in cardiovascular disease are sought and the antioxidant capacity of H2S makes it an attractive candidate. It is well recognized that angiotensin II (AngII) can increase ROS generation in the vasculature, predominantly via activation of NOXs such as NOX2. Indeed, AngII-mediated NADPH oxidase activation and ROS production has been implicated in atherosclerosis and hypertension.21 As such, the AngII-infusion model of hypertension exploits the ability of AngII to increase NADPH oxidase activity22 and superoxide generation, and is useful as a model of both increased oxidative stress and hypertension.23
While to date there have been many in vitro studies examining the anti-oxidant effects of H2S, in vivo studies focusing on vascular effects are lacking, so the physiological relevance of such findings is yet to be fully explored. The aim of this study was to investigate whether or not exogenous H2S can ameliorate vascular oxidative stress in vivo and thereby confer vasoprotection, using the AngII-induced oxidative stress and hypertension model in mice. These studies are an important next step from the in vitro evidence of H2S as an anti-oxidant16 to proving its capacity as a vasoprotectant in vivo.
Materials and methods
Animals
All experimental procedures involving the use of animals were approved by the RMIT Animal Ethics Committee before the commencement of this project. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23 revised 1996). All animals were housed in the RMIT Animal Facility, RMIT University, Bundoora West Campus on a 12-h day/night cycle at room temperature of 20±2 °C. Male C57BL/6J mice, 10 weeks of age were purchased from the Animal Resource Centre, Western Australia.
Angiotensin II-induced hypertension
Mice (C57Bl6/J, male 10 weeks) were anesthetized with 2% isoflurane in oxygen, and an osmotic mini-pump (Alzet micro-osmotic pump model 1004; Alzet DURECT Corporation, Cupertino, CA, USA) was implanted subcutaneously between the scapulae. Mice were infused for 2 weeks with angiotensin II (AngII) 0.7 mg kg−1 per day, prepared in AngII buffer (composition: 3 ml of 5 M NaCl (150 mM NaCl), 1 ml of 1 M acetic acid (10 mM) in 100 ml dH2O). The animals were divided into four groups; (1) no treatment, (2) AngII infusion, (3) AngII infusion and PPG 3 mg kg−1 per day i.p., (4) AngII infusion and NaHS 10 μmol kg−1 per day, i.p.
Blood pressure measurement
The systolic blood pressure of the animals was measured 1 day before the insertion of the mini-pumps and 7 and 14 days after the procedure, using the non-invasive tail cuff apparatus (ADInstruments, Sydney, NSW, Australia). Systolic blood pressure was averaged from four to six consecutive measurements taken at intervals of 1–2 min. After measurement of systolic blood pressure on day 14, mice were culled in a humane manner via CO2 asphyxiation (95% CO2, 5%O2), followed by cervical dislocation and decapitation. The aorta was dissected out and washed in ice-cold oxygenated Krebs’ solution (composition in mM: NaCl 119, KCl 4.7, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, CaCl2 2.5, glucose 5.5, EDTA 0.026, pH 7.4).
Myograph experiments
Abdominal aortic rings (∼2 mm length) were mounted in 7 ml myograph chambers, where they were maintained in Krebs’ solution at 37 °C, continuously supplied with carbogen (95% O2, 5%CO2). Changes in isometric force were recorded using Myograph Interface model 610M (ADInstruments) and the Myodac data acquisition system (Myodac 2.01, Myonic Software, Copenhagen, Denmark). The aortic rings were allowed to equilibrate for 20 min under zero force then a 5-mN resting tension was applied. Following a 15-min equilibration period at 5 mN, the aortic rings were maximally contracted using the thromboxane A2 mimetic, U46619 1 μM (U4max). The aortic rings were washed with Krebs’ solution and the tension allowed to return to baseline. Concentration response curves to the endothelium-dependent dilator acetylcholine (ACh 1 nM–10 μM), the NO donor sodium nitroprusside (SNP 0.1 nM–10 μM), the KATP channel opener levcromakalim (LKM 1 nM-10 μM), NaHS (1 μM–3 mM) or the CSE substrate L-cysteine (10 μM–3 mM) were constructed in vessels pre-constricted with the thromboxane analogue U46619. Pre-contraction to U46619 was submaximal (∼50% U4max) and not significantly different between groups. At the end of each concentration response curve, 10 μM nifedipine was added to test vascular smooth muscle cell function. In the NO bioavailability experiments, the vessels were contracted to 20–30% of U4max using titrated concentration of U46619, and after the contraction stabilized (∼10 min), L-NAME 100 μM was added. After the contraction to L-NAME stabilized for 30 min, the contraction value was taken and compared with U4max.
Superoxide production from vascular tissue
NADPH oxidase-driven superoxide production in abdominal aorta was measured using lucigenin-enhanced chemiluminescence. Sections of abdominal aorta (3 mm long) were pre-incubated for 45 min at 37 °C in Krebs-HEPES buffer (composition (mM): NaCl 99.9, KCl 4.7, KH2PO4 1.0, MgSO4·7 H2O 1.2, D-glucose 11.0, NaHCO3 25.0, CaCl2·2 H2O 2.5, Na HEPES 20.0, pH 7.4) containing diethylthiocarbamic acid (1 mM) to inactivate superoxide dismutase, and NADPH (100 μM) as a substrate for NADPH oxidase. Diphenylene iodonium (1 μM) was used to inhibit NADPH oxidase in some wells. In all, 300 μl of Krebs-HEPES buffer containing lucigenin (5 μM) was placed in separate wells of a white 96-well Opti-plate which was loaded into a Polarstar Optima photon counter (BMG Labtech, Melbourne, VIC, Australia) to measure background photon emission at 37 °C over a 30-min period. After background counting was completed, a single ring of aorta was added to each well, in semi-darkness, and photon emission was measured for 30 min. Superoxide production for each ring segment was calculated by subtracting the background chemiluminescence signal from the signal in the presence of the artery (103 counts per second) and then normalized to dry tissue weight (in mg).
CSE activity assay
The H2S production rate in various tissues was measured as described previously24 with modifications. In brief, tissues were collected from mice, weighed and homogenized in ice-cold potassium phosphate buffer, pH 7.4. The reaction mixture contained 100 mM potassium phosphate buffer, pH 7.4, 10 mM L-cysteine, 2 mM pyridoxyl'5'phosphate and 10% (w/v) tissue homogenates. Cryovial test tubes (2 ml) were used to trap H2S, each containing 0.5 ml 1% zinc acetate and a filter paper of 4 cm2 to increase the air/fluid surface area. The reaction was performed in 50 ml falcon tubes, each falcon tube contained a trapping solution and a reaction mixture tube, and it was sealed by a double layer of parafilm. The reaction was initiated by transferring the tubes into a 37 °C shaking water bath. After incubation for 90 min, the tubes were put on ice for another 30 min to stop the reaction and to ensure complete trapping of H2S. The contents of the trapping tube were transferred into test tubes each containing 3.5 ml water. Subsequently, 0.5 ml of 20 mM N,N-dimethyl-p-phenylenediamine sulfate prepared in 7.2 M HCl was added followed by 0.4 ml of 30 mM FeCl3 prepared in 1.2 M HCl. The absorbance of the resulting solution was measured at 670 nm after 20 min with a spectrophotometer. The H2S produced from the reaction was calculated from a calibration curve of standard H2S solution prepared by dissolving Na2S in deoxygenated water under anoxic conditions. A single calibration curve was used to calculate H2S concentration from each experiment. H2S production was normalized to the wet tissue weight and expressed as μmol g−1 per minute.
Data analysis and statistics
Results are expressed as mean±standard error of the mean (s.e.m.) with the number of experiments denoted by n. Concentration response curves to ACh were expressed as a percentage reversal of the U46619 pre-contraction. These data were computer fitted to a sigmoidal curve using non-linear regression (Graphpad Prism 5, Graphpad Software Inc, La Jolla, CA, USA) to provide an estimate of the concentration of agonist causing a 50% relaxation (pEC50 value; in –log mol l−1). Statistical analysis was performed using either unpaired t-tests or by one-way analysis of variance (ANOVA) with post hoc tests applied as appropriate and as stated in the text (GraphPad Prism, Version 5). P<0.05 was considered as statistically significant.
Drugs and reagents
All drugs and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA). All drugs were dissolved in dH2O, with the exception of LKM, which was dissolved in methanol.
Results
Effect of AngII infusion on systolic blood pressure
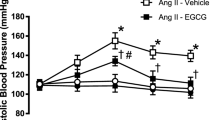
There was no difference in the systolic blood pressure between the experimental groups at the commencement of the study (data not shown). AngII infusion (0.7 mg kg−1 per day) for 14 days caused a significant increase in systolic blood pressure (Control 120±2 vs. AngII 171±3 mm Hg, P<0.001). Concomitant treatment with NaHS (10 μmol kg−1 per day, i.p.) significantly attenuated this rise in systolic blood pressure (P<0.001); however, treatment with the CSE inhibitor PPG (3 mg kg−1 per day, i.p.) had no effect on the AngII-induced increase in systolic blood pressure (Figure 1).
Effect of NaHS (10 μmol kg−1 per day) and the CSE inhibitor PPG (3 mg kg−1 per day) on the AngII (0.7 mg kg−1 per day)-induced increase in systolic blood pressure in C57Bl6/J mice. Systolic blood pressure was measured on day 14 after commencing the AngII infusion. *P<0.001 vs. Control, #P<0.001 vs. AngII, one-way ANOVA, post hoc Dunnett’s test, n=10–12.
Vascular responses in aorta from AngII-infused mice
Effect of AngII infusion on endothelial function
In aortic rings the vasorelaxant response to the endothelium-dependent vasodilator ACh was significantly reduced in the AngII and the AngII+PPG-treated groups compared with the control group (P<0.001). Combining AngII+NaHS reversed this endothelial dysfunction (P<0.001, Figure 2a, Table 1).
(a) Concentration response curves to the endothelium-dependent vasodilator acetylcholine (ACh), in aorta from mice chronically infused with AngII for 14 days and treated with either NaHS or the CSE inhibitor PPG. ○ Control, • AngII, ▴ AngII+NaHS and ▪ AngII+PPG. Response is expressed as % reversal of the pre-contraction to U46619. ***P<0.001 max relaxation vs. control, ###P<0.001 max relaxation vs. AngII, one-way ANOVA, post hoc Dunnett’s test, n=8–10. (b) Endogenous vascular nitric oxide bioavailability determined by the contraction response to L-NAME (100 μM) in aortic rings from mice chronically infused with AngII for 14 days and treated with either the CSE inhibitor PPG or NaHS. The contraction response to L-NAME is presented as percentage of the maximum contraction to U46619 1 μM. ***P<0.001, vs. control, ###P<0.001 vs. AngII, one-way ANOVA, post hoc Dunnett’s test, n=4.
Effect of AngII infusion on endogenous nitric oxide bioavailability
The contraction response to L-NAME (100 μM) was significantly reduced in the AngII and the AngII+PPG-treated groups compared with that of the control group (P<0.001), indicative of decreased endogenous NO bioavailability. Combining AngII+NaHS restored the response to L-NAME to a level comparable to that of the control (P<0.001, Figure 2b, Table 1).
Effects of AngII infusion on vascular smooth muscle function
The sensitivity to the vasorelaxant response to the NO donor SNP was significantly reduced in the AngII and the AngII+PPG-treated groups compared with that of the control (P<0.05). However, the maximal relaxation response was unaffected. Combining AngII+NaHS restored the sensitivity to SNP back to the level of the control (Figure 3a). The vasorelaxant response to the KATP channel opener LKM was not different across the treatment groups (Figure 3b, Table 1).
Concentration response curves to the (a) NO donor SNP and (b) KATP channel opener LKM in aorta from mice chronically infused with AngII for 14 days and treated with either the CSE inhibitor PPG or NaHS. ○ Control, • AngII, ▴ AngII+NaHS and ▪ AngII+PPG. Responses are expressed as % reversal of the pre-contraction to U46619. Φ=P<0.05 EC50, vs. control, ANOVA, post hoc Dunnett’s test, n=5–9.
Effect of AngII infusion treatment on NADPH-dependent vascular superoxide production
Chronic angiotensin infusion (14 days) in mice lead to a significant increase in NADPH-dependent superoxide generation in isolated aortae (P<0.01), an effect that was further augmented by combining AngII+PPG (P<0.001). Combining AngII+NaHS significantly reduced superoxide production in the aorta of these mice, back to the levels seen in the control group (P<0.01, Figure 4).
NADPH (100 μmol l−1)-stimulated superoxide levels in mouse aortic rings, after 14 days infusion with AngII (0.7 mg kg−1 per day) with additional treatment with either the CSE inhibitor PPG (3 mg kg−1 per day) or NaHS (10 μmol/kg−1 per day). Superoxide was measured by lucigenin (5 μmol l−1)-enhanced chemiluminescence in the absence (open bars) and presence (closed bars) of the NADPH oxidase inhibitor diphenylene iodonium (DPI) (1 μmol l−1). Values are expressed as 103 counts per second per milligram of dry tissue. **P<0.01, ***P<0.001, vs. control, ##P<0.01 vs. AngII; one-way ANOVA, post hoc Dunnett’s test, n=9–13.
Effect of AngII infusion on vascular CSE activity
In aorta the vasorelaxation response to NaHS was unaffected by AngII infusion, or any of the treatments (PPG or NaHS) indicating no change in the ability of the vessels to respond to exogenous H2S (Figure 5a). Maximum vasorelaxation induced by the CSE substrate, L-cysteine was significantly inhibited in the PPG-treated group (P<0.01) and slightly inhibited by AngII infusion alone, indicative of a reduction in endogenous H2S generation. Treatment with NaHS reversed the inhibitory effect of Ang II infusion on L-cysteine mediated vasorelaxation (Figure 5b). In addition, H2S production in aorta from AngII-infused mice was virtually abolished by PPG treatment (P<0.001) and significantly attenuated in the AngII-treated group compared with the control (P<0.05). Treatment with NaHS restored aortic H2S production back to the levels of the control (P<0.05, Figure 5c).
Concentration response curves in aorta from mice chronically infused with AngII for 14 days and treated with either the CSE inhibitor PPG or NaHS, n=4–6. ○ Control, • AngII, ▴ AngII+NaHS and ▪ AngII+PPG. (a) The vasorelaxation response to exogenous H2S via NaHS, (b) the vasorelaxation response to the CSE substrate L-cysteine, ##P<0.01 maximum relaxation vs. control, one-way ANOVA, n=5. (c) Aortic H2S production assessed by the methylene blue assay *P<0.05, ***P<0.001, vs. control, #P<0.05 vs. AngII, one-way ANOVA, post hoc Dunnett’s test, n=4.
Discussion
This study presents data that highlight the potential importance of H2S as a vasoprotectant molecule. Chronic AngII infusion in mice for 14 days caused hypertension, increased vascular superoxide production, endothelial dysfunction, reduction in endogenous NO bioavailability, and a decrease in NO signalling and endogenous H2S production via CSE. All these effects were reversed by supplying exogenous H2S. The increase in vascular superoxide production and a decrease in endogenous H2S production were exacerbated by inhibition of the endogenous H2S producing enzyme CSE. This study supports previous reports of the anti-oxidant,16, 25 cardioprotective,26 and cytoprotective27, 28 effects of H2S and extends the repertoire of H2S to specifically include vasoprotectant actions in vivo.
AngII infusion caused a marked increase in systolic blood pressure that was reduced by NaHS. NaHS is a known vasorelaxant with effects in both conduit arteries7, 29 and resistance-like vessels.30, 31, 32 The molecular mechanism of vasorelaxation induced by H2S is controversial, with roles for K+ channels, Ca2+ channels and Cl− channels all being implicated. More recently, it has been reported that H2S is a phosphodiesterase inhibitor and cGMP-dependent protein kinase (PKG-I) activator33, 34 and that vasorelaxation is reliant on underlying cGMP production. While the mechanism(s) via which H2S donors elicit vasorelaxation remain to be fully elucidated it is well recognized that H2S donors are effective vasodilators both in vitro and also in vivo. Indeed, intravenous administration of NaHS8 or the H2S donor compound GYY413735 have been shown to reduce blood pressure in vivo. It is most likely that the vasorelaxant actions of H2S contribute to the NaHS-induced antihypertensive effect observed in the current study, but this is possibly not the only reason NaHS reduces blood pressure in the AngII-infusion model of hypertension. AngII activates AT1 receptors (AT1Rs) to cause vasoconstriction and signals by markedly increasing vascular NADPH oxidase activity.36 The increased superoxide production will lead to a reduction in NO bioavailability, which in turn leads to increased vascular tone,21, 36 thus a reduction in superoxide levels by H2S could also impede the development of increased vascular tone to AngII.
Another possible mechanism of the protective effects seen by NaHS in this study is an interaction with the AT1R itself. It is well known that the AT1R has two extracellular disulfide bonds that are important for AngII binding. When these disulfide bonds are reduced by thiol-reducing agents (for example, dithiothreitol37 or N-acetylcysteine38 in cellular binding assays, AngII binding to the AT1R is inhibited. Similarly, dithiothreitol reduces the vasocontraction response to AngII in in vitro vascular assays39 thus it is established that reducing agents can decrease AngII binding to the AT1R and block downstream signalling events. Interestingly, AT2 receptor-mediated effects are enhanced by dithiothreitol.40 H2S is a known thiol reducing agent and there is evidence that it too can inhibit AngII-mediated signalling.41 Thus, a possible mechanism in this study is that NaHS is causing an inhibition of AngII binding to AT1R, ameliorating AngII-mediated signalling and inhibiting the progression of the pathology of the model and furthermore NaHS may be enhancing AT2 receptor-mediated effects (vasodilatation and anti-proliferative effects) that counteract the effects of the AT1R.42
NaHS treatment inhibited NADPH-dependent superoxide production in aorta ex vivo as assessed by lucigenin-enhanced chemiluminescence. It has previously been shown that H2S can inhibit NADPH oxidase in cell-based studies, where it inhibits NOX1 expression and Rac1 activity in vascular smooth muscle cells43 and gp91phox expression in endothelial cells.44 Additionally, recent in vitro studies have shown an anti-oxidant and vasoprotective effect of NaHS.16 This is the first report to show that chronic treatment with an H2S donor can affect superoxide production in vivo in a hypertension model. Previous work has shown a similar effect in an atherosclerosis model45 suggesting a role for H2S as a vascular anti-oxidant. In addition, H2S has been reported to act as a scavenger for a variety of ROS, including superoxide,16, 46, 47 hydrogen peroxide,43 peroxynitrite12, 13 and hypochloride.15 Thus, H2S may both inhibit vascular ROS production and act as a scavenger of ROS, increasing its anti-oxidant potential.
Endothelium-derived NO is destroyed by oxidative stress as NO and superoxide rapidly react to form peroxynitrite.48, 49 The detrimental outcomes of this reaction are twofold. First, the beneficial vascular actions of NO are impaired and second peroxynitrite is a highly reactive molecule which can cause protein nitration and lipid peroxidation.50 Further, peroxynitrite causes single-strand DNA breakage and it oxidizes tetrahydrobiopterin, an important endogenous nitric oxide (eNOS) co-factor. Depletion of tetrahydrobiopterin is one trigger for the uncoupling of eNOS which then produces superoxide instead of NO51 adding to the oxidant load. This increased oxidative stress is an important factor in the development of hypertension.52 An important finding of this study is that NaHS treatment prevented endothelial dysfunction caused by AngII infusion. The endothelial dysfunction in this model is known to be due to increased superoxide production since it is reversed by scavengers of ROS and exacerbated by the deficiency of key endogenous anti-oxidants.53, 54 The endothelial impairment shown in this study is likely to be due to a reduction in NO bioavailability. NO bioavailability was assessed in this study by examining the contractile response to L-NAME, an inhibitor of eNOS. These experiments reflect the availability of endogenous NO release which is controlling basal tone.55 The contractile response to L-NAME was significantly impaired in the AngII-treated group whereas the response in the NaHS-treated group was comparable to that of the control. These data confirm that NO bioavailability is impaired under oxidative stress, and that NaHS treatment can reverse this impairment. Thus, the ability of H2S to inhibit NADPH oxidase-derived superoxide would protect the vasculature from peroxynitrite-mediated damage. Additionally, H2S donors have been shown to elevate glutathione levels,17 increase the activity of glutathione peroxidase and glutathione reductase,56 increase the activity and expression of superoxide dismutase,57, 58 and increase eNOS phosphorylation59 and activity60 and expression61 implying H2S has the capacity for a multi-faceted enhancement of anti-oxidant mechanisms and the protection of endogenous NO.
In addition to the impaired endogenous NO bioavailability in the AngII-infusion model, there was also impairment in the response to the NO donor SNP. The vasorelaxation response to the KATP channel opener LKM was the same in all groups, indicating that vascular smooth muscle cell vasorelaxant function per se was not affected; thus, the attenuation of SNP-induced relaxation indicates a problem in downstream NO signalling, rather than damage to the vascular smooth muscle. While maximal relaxation was preserved, the EC50 for SNP was significantly shifted to the right in the AngII-infused animals, compared with the control and the NaHS-treated group. This may suggest that the superoxide production of the aorta was sufficient to scavenge exogenously generated NO, or interfere with downstream NO signalling. Treatment with NaHS restored the sensitivity to the NO donor. There are a number of possible explanations for this effect. It may be that H2S is promoting cGMP-mediated signalling by inhibiting phosphodiesterase.33 Additionally, the activity and expression of the sGC can be directly impaired by oxidative stress due to oxidation of its ferrous heme which prevents NO-mediated activation of sGC.62 As a scavenger of ROS and a chemical reductant, NaHS may also be acting to protect the ferric form of sGC and therefore preserving the receptor for NO in the vascular smooth muscle cell, providing a further explanation for the improvement of both endothelial function and sensitivity to SNP in the NaHS-treated group.
PPG is a widely used inhibitor of CSE, which acts by irreversibly binding to the pyridoxyl-5-phosphate binding site.63 Despite quite poor cell permeability PPG does exhibit selectivity in inhibiting CSE64 and is a well-used pharmacological tool in this field. On the basis of using PPG as an inhibitor of CSE, at the same concentrations, previous studies have shown that endogenous H2S is involved in the regulation of basal blood vessel tone7 and indeed blood pressure.8 In this study, treatment with the CSE inhibitor PPG did not further increase blood pressure, or endothelial dysfunction, but did increase superoxide production and reduce vascular CSE activity. That inhibiting endogenous H2S production causes little further exacerbation of the deleterious effects of AngII infusion is not unexpected. A simple explanation for this is that endogenous H2S is not sufficient to ameliorate the hemodynamic and vascular changes induced by AngII. In addition, the H2S production data suggest that AngII infusion leads to a decrease in endogenous H2S anyway, so it follows that inhibiting CSE with PPG has little effect.
Interestingly, the NaHS treatment reversed the inhibitory effect of Ang II infusion on vascular H2S production, as L-cysteine-induced vasorelaxation was maintained in the Ang II infusion with the NaHS treatment. This suggests that the activity of CSE is sensitive to increased oxidative stress. To our knowledge, it has not been reported previously that NaHS can protect L-cysteine bioavailability, but this is a possibility. The data we present indeed indicate that NaHS treatment in vivo can protect CSE activity in the vasculature from Ang II infusion; however, we did not measure CSE protein levels or conduct other CSE activity assays to further investigate this. It is also possible that other sources of H2S are upregulated under conditions of CSE inhibition. It is reported that 3-mercaptopyruvate sulfurtransferase and cysteine aminotransferase can also produce H2S in vascular cells,9 although the regulation of this enzyme is not well understood, especially not in vivo. H2S can also be produced by CBS, although this enzyme is generally associated with the neural production.65 Further potential sources of H2S are from bound or acid-labile sulfur storage sites in mitochondria66 and other non-enzymatic sources, although the physiological relevance of these is yet to be determined. A more perplexing finding is that NaHS treatment restored CSE activity, as observed with both the vasorelaxation responses to L-cysteine and via aortic H2S production. The reason for this is unclear, but suggests that H2S may regulate CSE activity.
More work is still required though to examine the pharmacokinetics of NaHS. The t1/2 and the fate of the administered NaHS are not well understood, and it is noted that the present results are obtained from a single daily i.p. dose of NaHS for a relatively short period. The dose of NaHS used is appropriate as it has returned H2S production back to control levels, thus compensating for the decrease in endogenous H2S that is observed with chronic AngII infusion. H2S may react with methemoglobin to form sulfhemoglobin that acts as a sink for H2S, which subsequently releases H2S upon reduction. Another possibility for this effect is that H2S may signal through protein sulfhydration;67 thus, it is possible that NaHS treatment has a longer lasting effect. The field is plagued with a lack of tools, in particular selective and specific blockers of H2S producing enzymes and reliable scavengers although consistent and stable donors of H2S are now becoming available. Improvements in these will be most useful for advances in the study of H2S biology.
Conclusions
These data show that in a mouse model of oxidative stress induced by AngII, exogenous H2S treatment in vivo reduces blood pressure, vascular oxidative stress, endothelial damage and protects NO bioavailability. Inhibiting endogenous H2S production in vivo exacerbated vascular superoxide formation. These data provide evidence that H2S is a vasoprotective molecule that may be a useful treatment target in cardiovascular disease.
References
Wang R . Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002; 16: 1792–1798.
Hart JL . Role of sulfur-containing gaseous substances in the cardiovascular system. Front Biosci (Elite Ed) 2011; 3: 736–749.
Streeter E, Ng HH, Hart JL . Hydrogen sulfide as a vasculoprotective factor. Med Gas Res 2013; 3: 9.
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R . H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008; 322: 587–590.
Zhao W, Zhang J, Lu Y, Wang R . The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 2001; 20: 6008–6016.
Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ES, Taylor EA, Moore PK . Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther 2008; 324: 876–882.
Al-Magableh MR, Hart JL . Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn Schmiedebergs Arch Pharmacol 2011; 383: 403–413.
Yan H, Du J, Tang C . The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 2004; 313: 22–27.
Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H . Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 2009; 146: 623–626.
Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Moller MN, Folkes LK, Garcia-Bereguiain MA, Gutierrez-Merino C, Wardman P, Denicola A, Radi R, Alvarez B . Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic Biol Med 2010; 50: 196–205.
Stasko A, Brezova V, Zalibera M, Biskupic S, Ondrias K . Electron transfer: a primary step in the reactions of sodium hydrosulphide, an H2S/HS− donor. Free Radic Res 2009; 43: 581–593.
Whiteman M, Armstong J, Chu S, Jia-Ling S, Wong B, Cheung N, Halliwell B, Moore P . The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite 'scavenger'? J Neurochem 2004; 90: 765–768.
Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I . Biochemical insight into physiological effects of H2S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem J 2012; 441: 609–621.
Lu M, Hu LF, Hu G, Bian JS . Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic Biol Med 2008; 45: 1705–1713.
Whiteman M, Cheung N, Zhu Y, Chu S, Siau J, Wong B, Armstrong J, Moore P . Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 2005; 343: 303–310.
Al-Magableh MR, Kemp-Harper BK, Ng HH, Miller AA, Hart JL . Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn Schmiedebergs Arch Pharmacol 2014; 387: 67–74.
Kimura Y, Goto Y, Kimura H . Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 2010; 12: 1–13.
Schulz E, Gori T, Munzel T . Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011; 34: 665–673.
Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, Laher I . Free radical biology of the cardiovascular system. Clin Sci (Lond) 2012; 123: 73–91.
Drummond GR, Selemidis S, Griendling KK, Sobey CG . Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 2011; 10: 453–471.
Cai H, Griendling KK, Harrison DG . The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 2003; 24: 471–478.
Cai H . NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 2005; 96: 818–822.
Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J . Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension 2009; 54: 338–344.
Stipanuk MH, Beck PW . Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 1982; 206: 267–277.
Predmore BL, Lefer DJ, Gojon G . Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 2012; 17: 119–140.
Lefer DJ . A new gaseous signalling molecule emerges: cardioprotective role of hydrogen sulphide. PNAS 2007; 104: 2.
Kimura Y, Kimura H . Hydrogen sulfide protects neurons from oxidative stress. FASEB J 2004; 18: 1165–1167.
Calvert JW, Coetzee WA, Lefer DJ . Novel insights into hydrogen sulfide–mediated cytoprotection. Antioxid Redox Signal 12: 1203–1217.
Zhao W, Wang R . H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 2002; 283: H474–H480.
Cheng Y, Ndisang J, Tang G, Cao K, Wang R . Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 2004; 287: 2316–2323.
Sun Y, Tang CS, Du JB, Jin HF . Hydrogen sulfide and vascular relaxation. Chin Med J (Engl) 2011; 124: 3816–3819.
Streeter E, Hart J, Badoer E . An investigation of the mechanisms of hydrogen sulfide-induced vasorelaxation in rat middle cerebral arteries. Naunyn Schmiedebergs Arch Pharmacol 2012; 385: 991–1002.
Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G . Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 2010; 30: 1998–2004.
Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G . cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS ONE 2012; 7: e53319.
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK . Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008; 117: 2351–2360.
Chrissobolis S, Faraci FM . The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med 2008; 14: 495–502.
Chang RS, Lotti VJ, Keegan ME . Inactivation of angiotensin II receptors in bovine adrenal cortex by dithiothreitol: further evidence for the essential nature of disulfide bonds. Biochem Pharmacol 1982; 31: 1903–1906.
Ullian ME, Gelasco AK, Fitzgibbon WR, Beck CN, Morinelli TA . N-acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells. J Am Soc Nephrol 2005; 16: 2346–2353.
Zhang JS, van Meel JC, Pfaffendorf M, van Zwieten PA . Inhibitory effect of dithiothreitol on angiotensin II-induced contractions mediated by AT1-receptors in rat portal vein and rabbit aorta. Naunyn Schmiedebergs Arch Pharmacol 1994; 349: 538–542.
Heerding JN, Hines J, Fluharty SJ, Yee DK . Identification and function of disulfide bridges in the extracellular domains of the angiotensin II type 2 receptor. Biochemistry 2001; 40: 8369–8377.
Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, Du JB . Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res 2008; 31: 1619–1630.
Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE . AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther 2008; 120: 292–316.
Muzaffar S, Shukla N, Bond M, Newby AC, Angelini GD, Sparatore A, Del Soldato P, Jeremy JY . Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res 2008; 45: 521–528.
Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, Shukla N . H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol 2008; 155: 984–994.
Ford A, Al-Magableh M, Gaspari TA, Hart JL . Chronic NaHS treatment is vasoprotective in high fat fed ApoE−/− mice. Int J Vasc Med 2013; 2013: 915983.
Mitsuhashi H, Yamashita S, Ikeuchi H, Kuroiwa T, Kaneko Y, Hiromura K, Ueki K, Nojima Y . Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock 2005; 24: 529–534.
Streeter EY, Badoer E, Woodman OL, Hart JL . Effect of type 1 diabetes on the production and vasoactivity of hydrogen sulfide in rat middle cerebral arteries. Physiol Rep 2013; 1: e00111.
Gryglewski RJ, Palmer RM, Moncada S . Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986; 320: 454–456.
MacKenzie A, Martin W . Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics. Br J Pharmacol 1998; 124: 719–728.
Pacher P, Beckman JS, Liaudet L . Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87: 315–424.
Vasquez-Vivar J, Kalyanaraman B, Martasek P . The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res 2003; 37: 121–127.
Rodrigo R, Gonzalez J, Paoletto F . The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 2011; 34: 431–440.
Didion SP, Kinzenbaw DA, Faraci FM . Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension 2005; 46: 1147–1153.
Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM . Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension 2008; 51: 872–877.
Leo CH, Hart JL, Woodman OL . 3',4'-Dihydroxyflavonol reduces superoxide and improves nitric oxide function in diabetic rat mesenteric arteries. PLoS ONE 2011; 6: e20813.
Benetti LR, Campos D, Gurgueira SA, Vercesi AE, Guedes CE, Santos KL, Wallace JL, Teixeira SA, Florenzano J, Costa SK, Muscara MN, Ferreira HH . Hydrogen sulfide inhibits oxidative stress in lungs from allergic mice in vivo. Eur J Pharmacol 2012; 698: 463–469.
Sun WH, Liu F, Chen Y, Zhu YC . Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res Commun 2012; 421: 164–169.
Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH . Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun 2006; 351: 485–491.
Altaany Z, Yang G, Wang R . Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med 2013; 17: 879–888.
Kida M, Sugiyama T, Yoshimoto T, Ogawa Y . Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci 2013; 48: 211–215.
Meng J, Ganesan Adaikan P, Srilatha B . Hydrogen sulfide promotes nitric oxide production in corpus cavernosum by enhancing expression of endothelial nitric oxide synthase. Int J Impot Res 2013; 23: 86–90.
Liu YH, Yan CD, Bian JS . Hydrogen sulfide: a novel signaling molecule in the vascular system. J Cardiovasc Pharmacol 2011; 58: 560–569.
Johnston M, Jankowski D, Marcotte P, Tanaka H, Esaki N, Soda K, Walsh C . Suicide inactivation of bacterial cystathionine gamma-synthase and methionine gamma-lyase during processing of L-propargylglycine. Biochemistry 1979; 18: 4690–4701.
Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A . Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol 2013; 169: 922–932.
Kimura H . Hydrogen sulfide: its production, release and functions. Amino Acids 2011; 41: 113–121.
Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H . A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 2009; 11: 205–214.
Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH . H2S signals through protein S-sulfhydration. Sci Signal 2009; 2: ra72.
Acknowledgements
We wish to thank Jeffrey Moore (Monash University) for assistance with surgical procedures, They Ng (RMIT University) for assistance with biochemical assays and the RMIT animal facility staff. Grants from Ramaciotti Foundation, William Buckland Foundation and the School of Medical Sciences, RMIT University helped fund this work. MA was the recipient of a scholarship from the Hashemite University, Jordan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Magableh, M., Kemp-Harper, B. & Hart, J. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens Res 38, 13–20 (2015). https://doi.org/10.1038/hr.2014.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.125
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Vasorelaxation elicited by endogenous and exogenous hydrogen sulfide in mouse mesenteric arteries
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
Diallyl Trisulfide Suppresses Angiotensin II–Induced Vascular Remodeling Via Inhibition of Mitochondrial Fission
Cardiovascular Drugs and Therapy (2020)
-
Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice
Hypertension Research (2017)
-
The NOX2-derived reactive oxygen species damaged endothelial nitric oxide system via suppressed BKCa/SKCa in preeclampsia
Hypertension Research (2017)
-
How does Eucommia leaf extract prevent smooth muscle cell proliferation induced by high-fat diets at the aortic tunica media?
Hypertension Research (2017)