Abstract
Background
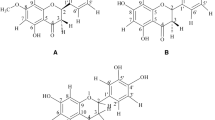
Considering the therapeutic potential of phenolic compounds, the purpose of the present study was to investigate the mechanisms involved in the relaxation induced by cryptostrobin and catechin, isolated from Eugenia mattosii D. Legrand leaves, in the aorta of spontaneously hypertensive rats (SHR).
Methods
The thoracic aorta was isolated from SHR and kept in the organ bath system by recording contractile or relaxant responses.
Results
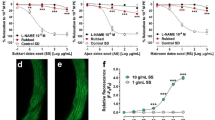
The addition of cumulative concentrations of cryptostrobin and catechin induced endothelium-dependent and-independent relaxation in aorta rings from SHR, as well as both compounds were effective in reducing phenylephrine-induced contraction. Pretreatment of aortic rings with Nm-nitro-L-arginine methylester (L-NAME, an inhibitor of nitric oxide synthase) or 1H-[1,2,4] oxadiazolo[4,3-a] quinoxalin-1-one (ODQ, an inhibitor of soluble guanylate cyclase), resulted in a significant change of relaxant effect induced by catechin, and a slight influence on cryptostrobin-induced relaxation. Muscarinic receptor and potassium channels are involved in catechin-induced relaxation as assessed using atropine (a muscarinic receptor antagonist), tetraethylammonium (a non-selective K+ channel blocker) and glibenclamide (an ATP-sensitive K+ channel blocker). Conversely, cryptostrobin, but not catechin, blunted the contraction induced by the addition of phenylephrine in a calcium-free solution. Besides that, cryptostrobin attenuated the contraction of rat aorta rings induced by internal Ca2+ release and external Ca2+ influx.
Conclusions
These findings indicated that cryptostrobin and catechin alter vascular smooth muscle reactivity, and this effect may be involved, at least in part, by enhancing the endothelium NO/cGMP pathway and potassium channels activation. In addition, cryptostrobin reduced the phenylephrine, KC1 and CaCl2-induced contractions in a calcium-free solution.
Similar content being viewed by others
References
Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 2015; 14:111–29, doi: https://doi.org/10.1038/nrd4510
Fong HHS. Integration of herbal medicine into modern medical practices: issues and prospects. Integr Cancer Ther 2002;1:287–93, doi:https://doi.org/10.1177/153473540200100313 discussion 293.
Cragg CM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta Gen Subj 2013;1830:3670–95, doi:https://doi.org/10.1016/j.bbagen.2013.02.008.
Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep 2008;25:475, doi:https://doi.org/10.1039/b514294f.
Braicu C, Ladomery MR, Chedea VS, Irimie A, Berindan- Neagoe I. The relationship between the structure and biological actions of green tea catechins. Food Chem 2013;141:3282–9, doi:https://doi.org/10.1016/j.foodchem.2013.05.122.
Franco V, Oparil S, Carretero OA. Hypertensive therapy: part I. Circulation 2004;109:2953–8, doi:https://doi.org/10.1161/01.CIR.0000132614.41493.B5.
Egan BM. Prediction of incident hypertension. Health implications of data mining in the “Big Data” era. J Hypertens 2013;31:2123–4, doi:http://dx.doi.org/10.1097/HJH.0b013e328365b932.
Ignarro LJ, Napoli C, Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res 2002;90:21–8.
Harnett KM, Biancani P. Calcium-dependent and calcium-independent contractions in smooth muscles. Am J Med 2003;115(Suppl 3A):24S–30S.
Li A-N, Li S, Zhang Y-J, Xu X-R, Chen Y-M, Li H-B. Resources and biological activities of natural polyphenols. Nutrients 2014;6:6020–47, doi:https://doi.org/10.3390/nu6126020.
Khan N, Khymenets O, Urpi-Sarda M, Tulipani S, Garcia-Aloy M, Monagas M, et al. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014;6:844–80, doi:http://dx.doi.org/10.3390/nu6020844.
Mattera R, Benvenuto M, Giganti M, Tresoldi I, Pluchinotta F, Bergante S, et al. Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients 2017;9:523, doi:http://dx.doi.org/10.3390/nu9050523.
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270–8, doi:http://dx.doi.org/10.4161/oxim.2.5.9498.
Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000;101:1899–906.
Duffy SJ, Keaney JF, Holbrook M, Gokce N, Swerdloff PL, Frei B, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001;104:151–6.
Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res 2009;58:537–52, doi:http://dx.doi.org/ 10.1007/S00011-009-0037-3.
Trippodo NC, Frohlich ED. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res 1981;48:309–19.
Doggrell SA, Brown L Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 1998;39:89–105.
Vechi Giovana, Tenfen Adrielli, Maina BoederAriela, Lorena Hernandez-Gomez, de Cordova Caio Mauricio Mendes, et al. Chemical composition and antimycoplasmic activity of Eugenia mattosii leaves, stems and isolated compounds. Nat Prod Commun 2019;14:37–40.
Meddings JB, Scott RB, Fick GH. Analysis and comparison of sigmoidal curves: application to dose-response data. Am J Physiol Liver Physiol 1989;257:G982–9, doi:https://doi.org/10.1152/ajpgi.l989.257.6.G982.
Da Silva R de CV de AF, Crestani S, de Souza P, Boligon AA, Athayde ML, Santos ARS, et al. Endothelium-dependent and independent vasorelaxation induced by an n-butanolic fraction of bark of Scutia buxifolia Reiss (Rhamanaceae). J Ethnopharmacol 2012;141:997–1004.
Gendron G, Gobeil F, Morin J, D’Orleans- Juste P, Regoli D. Contractile responses of aortae from WKY and SHR to vasoconstrictors. Clin Exp Hypertens 2004;26:511–23.
Vanhoutte PM. Endothelial dysfunction in hypertension. J Hypertens Suppl 1996;14:S83–93.
Liischer TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension 1979;1986(8):344–8.
Mateo AO, de Artinano MA. Effect of high extracellular Ca(2+) levels in spontaneously hypertensive rat aorta. Eur J Pharmacol 2001;432:177–85.
Levy BI, Duriez M, Phillipe M, Poitevin P, Michel JB. Effect of chronic dihydropyridine (isradipine) on the large arterial walls of spontaneously hypertensive rats. Circulation 1994;90:3024–33.
Vanhoutte PM, Shimokawa H, Feletou M, Tang EHC. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf) 2017;219:22–96, doi:http://dx.doi.org/10.1111/apha.l2646.
Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 1978;30:293–331.
Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–6, doi:https://doi.org/10.1038/288373a0.
Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol 1988;93:515–24.
Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem 1994;269:13725–8.
Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 2009;147:S193–201, doi:https://doi.org/10.1038/sj.bjp.0706458.
Francis SH, Busch JL, Corbin JD. Sibley D. cGMP-Dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 2010;62:525–63, doi:https://doi.org/10.1124/pr.ll0.002907.
Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987;327:524–6, doi:https://doi.org/10.1038/327524a0.
Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994;368:850–3, doi:https://doi.org/10.1038/368850a0.
Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995;268:C799–822.
Byun E-B, Korematsu S, Ishikawa T, Nishizuka T, Ohshima S, Kanda T, et al. Apple procyanidins induce hyperpolarization of rat aorta endothelial cells via activation of K+ channels. J Nutr Biochem 2012;23:278–86, doi:https://doi.org/10.1016/j.jnutbio.2010.12.005.
Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A 1994;91:7583–7.
Somlyo AP, Somlyo AV. Ca 2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by g proteins, kinases, and myosin phosphatase. Physiol Rev 2003;83:1325–58, doi:https://doi.org/10.1152/physrev.00023.2003.
Rembold CM. Regulation of contraction and relaxation in arterial smooth muscle. Hypertension 1979; 1992(20): 129–37.
Missiaen L, De Smedt H, Droogmans G, Himpens B, Casteels R. Calcium ion homeostasis in smooth muscle. Pharmacol Ther 1992;56:191–231.
Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 1984;308:693–8 n.d..
Berridge MJ. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J 1984;220:345–60.
Wong KK. Effect of endothelium on the aortic contraction induced by norepinephrine and KC1 in isolated rat aorta. Artery 1996;22:55–60.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vechi, G., da Silva, R.C.M.V.A.F.d., de Souza, P. et al. Cryptostrobin and catechin isolated from Eugenia mattosii D. Legrand leaves induce endothelium-dependent and independent relaxation in spontaneously hypertensive rat aorta. Pharmacol. Rep 71, 950–957 (2019). https://doi.org/10.1016/j.pharep.2019.05.006
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2019.05.006