Abstract
Background
Uterotonic mediators: endothelin-1 (ET-1), arginine vasopressin (AVP), and nitric oxide (NO) play important roles in the regulation of uterine contractility. We hypothesize that NO affects both ET-1 or AVP. Therefore, this study investigated the involvement of extended exogenous NO release in the regulation of responses of the human non-pregnant myometrium to ET-1 and AVP.
Methods
Specimens were obtained from 10 premenopausal women, undergoing hysterectomy for benign gynecological disorders. Responses of the myometrial strips to ET-1 or AVP in the absence and presence of an exogenous NO donor (diethylenetriamine; DETA/NO; 10−4 mol/L) were recorded under isometric conditions. To inhibit endogenous NO, a competitive inhibitor of NO synthase, L-NG-nitroarginine (L-NNA) was added to the organ bath.
Results
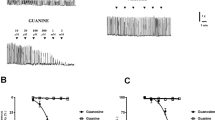
ET-1 enhanced the spontaneous contractile activity of the myometrium more powerfully (p s< 0.01) than AVP. Preincubation with exogenous NO weakened ET-1- or AVP-induced increases in this contractile activity (p s< 0.05). However, unexpected results were obtained after preincubation with L-NNA and with DETA/NO then added. Both ET-1 and AVP induced augmented contractile effects in almost all concentrations compared with the responses to these peptides alone or after NOS synthase inhibition (both p s< 0.01).
Conclusions
This study demonstrated for the first time that extended incubation with a NO donor influences the uterine muscle response evoked by ET-1 and AVP. Both endogenous and exogenous NO is involved in the control of the uterine responses to ET-1 or AVP of non-pregnant myometrium. Furthermore, both peptides stimulate increased uterine contractility when the local imbalance between the constrictive and relaxing mediators takes place.
Similar content being viewed by others
Abbreviations
- AUC:
-
area-under-the-curve
- AVP:
-
arginine vasopressin
- cGMP:
-
cyclic guanosine monophosphate
- DETA/NO:
-
diethylenetriamine
- ET-1:
-
endothelin-1
- KCa2+:
-
calcium-dependent potassium
- L-NMA:
-
N-nitro-L-arginine-methyl-ester
- L-NNA:
-
L-NG-nitroarginine
- NO:
-
nitric oxide
- NOS:
-
NO synthase
- cNOS:
-
constitutive NOS
- eNOS NOS3:
-
endothelial NOS
- sGC:
-
guanylyl cyclase
- SR:
-
sarcoplasmic reticulum
References
Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update 2010;16:725–44, doi: https://doi.org/10.1093/humupd/dmq016.
Fanchin R, Ayoubi JM. Uterine dynamics: impact on the human reproduction process. Reprod Biomed Online 2009;18(Suppl 2):57–62.
Dallot E, Pouchelet M, Gouhier N, Cabrol D, Ferré F, Breuiller-Fouché M. Contraction of cultured human uterine smooth muscle cells after stimulation with endothelin-1. Biol Reprod 2003;68:937–42, doi:https://doi.org/10.1095/biolreprod.102.00836.
Tanfin Z, Leiber D, Robin P, Oyeniran C, Breuiller-Fouché M. Endothelin-1: physiological and pathological roles in myometrium. Int J Biochem Cell Biol 2011;43:299–302, doi:https://doi.org/10.1016/j.biocel.2010.10.009.
Akerlund M. Vasopressin and oxytocin in normal reproduction and in the pathophysiology of preterm labour and primary dysmenorrhoea. Development of receptor antagonists for therapeutic use in these conditions. Rocz Akad Med w Bialymstoku 2004;49:18–21.
Khalil RA. Modulators of the vascular endothelin receptor in blood pressure regulation and hypertension. Curr Mol Pharmacol 2011;4:176–86.
Hyndman KA, Pollock JS. Nitric oxide and the A and B of endothelin of sodium homeostasis. Curr Opin Nephrol Hypertens 2013;22:26–31, doi:https://doi.org/10.1097/MNH.0b013e32835b4edc.
Redmond EM, Cahill PA, Hodges R, Zhang S, Sitzmann JV. Regulation of endothelin receptors by nitric oxide in cultured rat vascular smooth muscle cells. J Cell Physiol 1996;166:469–79, doi:https://doi.org/10.1002/(SICI)1097-4652(199603)166:3<469::AID-JCP1>3.0.œ;2-N.
Gong L, Gao F, Li J, Li J, Yu X, Ma X, et al. Oxytocin-induced membrane hyperpolarization in pain-sensitive dorsal root ganglia neurons mediated by Ca2+/nNOS/NO/KATP pathway. Neuroscience 2015;289:417–28, doi:https://doi.org/10.1016/j.neuroscience.2014.12.058.
Streefkerk JO, Pfaffendorf M, van Zwieten PA. Endothelium-dependent, vasopressin-induced contractions in rabbit renal arteries. J Cardiovasc Pharmacol 2003;42:703–9.
Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol 2012;24:609–28, doi:https://doi.org/10.1111/j.1365-2826.2012.02303.x.
Sharman A, Low J. Vasopressin and its role in critical care. Contin Educ Anaesth Crit Care Pain 2008;8:134–7, doi:https://doi.org/10.1093/bjaceaccp/mkn021.
Rosselli M, Imthurn B, Keller PJ, Jackson EK, Dubey RK. Circulatingnitric oxide (nitrite/nitrate) levels in postmenopausal women substituted with 17ß-estradiol and norethisterone acetate: a two-year follow-up study. Hypertension 1995;25:848–53.
Wimalawansa SJ. Nitric oxide: new evidence for novel therapeutic indications. Expert Opin Pharmacother 2008;9:1935–54, doi:https://doi.org/10.1517/14656566.9.11.1935.
Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res 2002;12:311–20.
Buxton ILO, Kaiser RA, Malmquist NA, Tichenor S. NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP. Br J Pharmacol 2001;134:206–14, doi:https://doi.org/10.1038/sj.bjp.0704226.
Orth TA, Shi S-Q, Williamson K, Shi L, Chambliss L, Coonrod DV, et al. Additive inhibitory effects of progesterone and sodium nitroprusside on uterine contractility during pregnancy. Reprod Sci 2011;18:868–75, doi:https://doi.org/10.1177/1933719111398141.
Norman J. Nitricoxide and the myometrium. PharmacolTher 1996;70:91–100.
Shimano M, Nakaya Y, Fukui R, Kamada M, Hamada Y, Maeda K, et al. Activation of Ca2+-Activated K+ channels in human myometrium by nitric oxide. Gynecol Obstet Invest 2000;49:249–54, doi:https://doi.org/10.1159/000010254.
Modzelewska B, Sipowicz MA, Saavedra JE, Keefer LK, Kostrzewska A. Involvement of K+ATP channels in nitric oxide-induced inhibition of spontaneous contractile activity of the nonpregnant human myometrium. Biochem Biophys Res Commun 1998;253:653–7, doi:https://doi.org/10.1006/bbrc.1998.9844.
Pearl JM, Nelson DP, Raake JL, Manning PB, Schwartz SM, Koons L, et al. Inhaled nitric oxide increases endothelin-1 levels: a potential cause of rebound pulmonaryhypertension. Crit Care Med 2002;30:89–93, doi:https://doi.org/10.1097/00003246-200201000-00014.
Boulanger C, Lüscher TF. Release of endothelin from the porcine aorta. Inhibition of endothelium-derived nitric oxide. J Clin Invest 1990;85:587–90, doi:https://doi.org/10.1172/JCI114477.
Johnson JA, Barman SA. Protein kinase C modulation of cyclic GMP in rat neonatal pulmonary vascular smooth muscle. Lung 2004;182:79–89, doi: https://doi.org/10.1007/s00408-003-1046-6.
Baksu B, Davas I, Baksu A, Akyol A, Gulbaba G. Plasma nitric oxide, endothelin-1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int J Gynecol Obstet 2005;90:112–7, doi: https://doi.org/10.1016/j.ijgo.2005.04.018.
Rossi NF. Regulation of vasopressin secretion by ETA and ETB receptors in compartmentalized rat hypothalamo-neurohypophysial explants. Am J Physiol Endocrinol Metab 2004;286:E535–41, doi:https://doi.org/10.1152/ajpendo.00344.2003.
Kostrzewska A, Modzelewska B, Kleszczewski T, Batra S. Effect of nitric oxide on responses of the human uterine arteries to vasopressin. Vascul Pharmacol 2008;48:9–13, doi:https://doi.org/10.1016/j.vph.2007.09.003.
Modzelewska B, Jóźwik MM, Jóźwik MM, Sulkowski S, Pędzińska-Betiuk A, Kleszczewski T, et al. Altered uterine contractility in response to ß-adrenoceptor agonists in ovarian cancer. J Physiol Sci 2017;67:711–22, doi: https://doi.org/10.1007/s12576-016-0500.
Thomas DD, Miranda KM, Espey MG, Citrin D, Jourd’heuil D, Paolocci N, et al. Guide for the use of nitric oxide (NO) donors as probes of the chemistry ofNO and related redox species in biological systems. Methods Enzymol 2002;359:84–105, doi:https://doi.org/10.1016/S0076-6879(02)59174-6.
Izumi H, Garfield RE. Relaxant effects of nitric oxide and cyclic GMP on pregnant rat uterine longitudinal smooth muscle. Eur J Obstet Gynecol Reprod Biol 1995;60:171–80, doi:https://doi.org/10.1016/0028-2243(95)02096-B.
Gagnon RC, Peterson JJ. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J Pharmacokinet Biopharm 1998;26:87–102, doi:https://doi.org/10.1023/A:1023228925137.
Modzelewska B, Sipowicz MAA, Saavedra JEE, Keefer LKK, Kostrzewska A. Involvement of K+ ATP channels in nitric oxide-induced inhibition of spontaneous contractile activity of the nonpregnant human myometrium. Biochem Biophys Res Commun 1998;253:653–7, doi:https://doi.org/10.1006/bbrc.1998.9844.
Momohara Y, Sakamoto S, Obayashi S, Aso T, Goto M, Azuma H. Roles of endogenous nitric oxide synthase inhibitors and endothelin-1 for regulating myometrial contractions during gestation in the rat. Mol Hum Reprod 2004;10:505–12, doi:https://doi.org/10.1093/molehr/gah072.
McGovern PG, Goldsmith LT, Schmidt CL, Von Hagen S, Linden M, Weiss G, et al. Effects of endothelin and relaxin on rat uterine segment contractility. Biol Reprod 1992;46:680–5.
Pierzynski P, Lemancewicz A, Reinheimer T, Akerlund M, Laudanski T. Inhibitory effect of barusiban and atosiban on oxytocin-induced contractions of myometrium from preterm and term pregnant women. J Soc Gynecol Investig 2004;11:384–7, doi:https://doi.org/10.1016/j.jsgi.2004.02.008.
Sanfilippo J, Erb T. Evaluation and management of dysmenorrhea in adolescents. Clin Obstet Gynecol 2008;51:257–67, doi:https://doi.org/10.1097/GRF.0b013e31816d2307.
Yuan W, Lopez Bernal A. Cyclic AMP signalling pathways in the regulation of uterine relaxation. BMC Pregnancy Childbirth 2007;7(Suppl 1), doi:https://doi.org/10.1186/1471-2393-7-S1-S10S10.
Tomczyk K, Rzymski P, Wilczak M. Have we achieved progress in tocolytic treatment? -results of a retrospective cohort study in a tertiary university hospital. Ginekol Pol 2015;86:504–8, doi:https://doi.org/10.17772/gp/57835.
Russell R, Doyle R, Turner J, Attkins N, Ramsey S, Weibley L, et al. In vitro and in vivo pharmacological characterisation of the potent and selective vasopressin V(1A) receptor antagonist 4-[4-(4-Chloro-phenyl)-5-[1,2,3]triazol-2-ylmethyl-4H-[1,2,4]triazol-3-yl]-piperidin-1-yl-(3,5-difluoro-phenyl) methanone (PF-00738245). Eur J Pharmacol 2011;670:347–55, doi:https://doi.org/10.1016/j.ejphar.2011.09.034.
Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008;371:1624–32, doi:https://doi.org/10.1016/S0140-6736(08)60695-9.
Héluy V, Germain G, Fournier T, Ferré F, Breuiller-Fouché M. Endothelin ETA receptors mediate human uterine smooth muscle contraction. Eur J Pharmacol 1995;285:89–94, doi:https://doi.org/10.1016/0014-2999(95)00388-2.
Bradley KK, Buxton ILO, Barber JE, McGaw T, Bradley ME. Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am J Physiol Physiol 1998;275:C1668–73, doi:https://doi.org/10.1152/ajpcell.1998.275.6.C1668.
Buxton IL. What is it about the uterus anyway? Proc West Pharmacol Soc 2003;46:1–8.
Raheja R, Gupta H, Pandey U, Deshpande SB. Lignocaine augments the in-vitro uterine contractions involving NO-guanylyl cyclase dependent mechanisms. Life Sci 2017;190:52–7, doi:https://doi.org/10.1016/j.lfs.2017.09.042.
Iyer AKV, Rojanasakul Y, Azad N. Nitrosothiol signaling and protein nitrosation in cell death. Nitric Oxide 2014;42:9–18, doi:https://doi.org/10.1016/j.niox.2014.07.002.
Ali AA, Coulter JA, Ogle CH, Migaud MM, Hirst DG, Robson T, et al. The contribution of N2O3 to the cytotoxicity of the nitric oxide donor DETA/NO: an emerging role for S-nitrosylation. Biosci Rep 2013;33, doi:https://doi.org/10.1042/BSR20120120.
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization ofa novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008;117:2351–60, doi:https://doi.org/10.1161/CIRCULATIONAHA.107.75346.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modzelewska, B., Jóźwik, M., Jóźwik, M. et al. The effects of extended nitric oxide release on responses of the human non-pregnant myometrium to endothelin-1 or vasopressin. Pharmacol. Rep 71, 892–898 (2019). https://doi.org/10.1016/j.pharep.2019.05.003
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2019.05.003