Abstract
Background
To assess the therapeutic efficacy, safety and tolerability of Guluronic acid (G2013) in patients with ankylosing spondylitis (AS) patients.
Methods
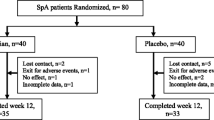
This investigation was a 12-week randomized, placebo-controlled, phase I/II clinical trial involving 75 AS patients that were randomly divided into 3 groups: 25 as placebo, 25 Guluronic acid and 25 naproxen groups. Patients who had AS with active disease at baseline according to the modified New York criteria were considered for this trial. The primary consequence measure was the Appraisement of Spondyloarthritis International Society (ASAS) 20 response-rate at week 12.
Results
There were no statistically significant differences between groups at the entry. ASAS20 response at week 12 was achieved (60.8%) in patients receiving Guluronic acid compared with — (68.4% of) — patients in the naproxen group (p > 0.05) and (21.0%) of patients in the placebo group. In comparison with the placebo group from the baseline to week 12, patients who received Guluronic acid and naproxen showed significantly greater improvement in all secondary endpoints. Moreover, Guluronic acid decreased some inflammatory parameters more dramatically than naproxen and placebo group. Patients in the naproxen group had more incidence of gastrointestinal and others adverse events in comparison with Guluronic acid and placebo groups.
Conclusion
The present research indicated that Guluronic acid and naproxen are similar in terms of efficacy. However, Guluronic acid had more notable safety characteristics identifying information than naproxen. Accordingly, it is proposed that Guluronic acid could be appropriate for management of AS.
Clinical trial identifier; IRCT2016091813739N4.
Similar content being viewed by others
References
Tsui FW, Tsui HW, Akram A, Haroon N, Inman RD. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl Clin Genet 2014;7:105–15.
Sun L, Wu R, Xue Q, Wang F, Lu P. Risk factors of uveitis in ankylosing spondylitis: An observational study. Medicine (Baltimore) 2016;95:e4233.
Huang F, Gu J, Liu Y, Zhu P, Zheng Y, Fu J, et al. Efficacy and safety of celecoxib in chinese patients with ankylosing spondylitis: a 6-week randomized, double-blinded study with 6-week open-label extension treatment. Curr Ther Res Clin Exp 2014;76:126–33.
Raychaudhuri SP, Deodhar A. The classification and diagnostic criteria of ankylosing spondylitis. J Autoimmun 2014;48:128–33.
Kenna TJ, Robinson PC, Haroon N. Endoplasmic reticulum aminopeptidases in the pathogenesis of ankylosing spondylitis. Rheumatology (Oxford) 2015;54:1549–56.
Balazcs E, Sieper J, Bickham K, Mehta A, Frontera N, Stryszak P, et al. A randomized, clinical trial to assess the relative efficacy and tolerability of two doses of etoricoxib versus naproxen in patients with ankylosing spondylitis. BMC Musculoskelet Disord 2016;17:426.
Wang R, Dasgupta A, Ward MM. Comparative efficacy of non-steroidal anti-inflammatory drugs in ankylosing spondylitis: a Bayesian network meta-analysis of clinical trials. Ann Rheum Dis 2016;75:1152–60.
Haibel H, Brandt H, Song I, Brandt A, Listing J, Rudwaleit M, et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis 2007;66:419–21.
Mansour M, Cheema GS, Naguwa SM, Greenspan A, Borchers AT, Keen CL, et al. Ankylosing spondylitis: a contemporary perspective on diagnosis and treatment. Semin Arthritis Rheum 2007;36:210–23.
Sieper J, Listing J, Poddubnyy D, Song I-H, Hermann K-G, Callhoff J, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43.
Afraei S, Azizi G, Zargar SJ, Sedaghat R, Mirshafiey A. New therapeutic approach by G2013 in experimental model of multiple sclerosis. Acta Neurol Belg 2015;115:259–66.
Mortazavi-Jahromi SS, Farazmand A, Motamed N, Navabi SS, Mirshafiey A. Effects of guluronic acid (G2013) on SHIP1, SOCS1 induction and related molecules in TLR4 signaling pathway. Int Immunopharmacol 2018;55:323–9.
Hajivalili M, Pourgholi F, Majidi J, Aghebati-Maleki L, Movassaghpour A, Samadi KH, et al. G2013 modulates TLR4 signaling pathway in IRAK-1 and TARF-6 dependent and miR-146a independent manner. Cell Mol Biol (Noisyle-Grand) 2016;62:1–5.
Fard NA, Tabrizian N, Mirzaei R, Hadjati J, Zavareh FT, Nodeh ARS, et al. Efficacy and safety of G2013 as a novel immunosuppressive agent on differentiation, maturation and function of human dendritic cells. Iran J Public Health 2017;46:216–21.
Kuznetsova O, Tymofyeyev Y. Expansion of the modified Zelen’s approach randomization and dynamic randomization with partial block supplies at the centers to unequal allocation. Contemp Clin Trials 2011;32:962–72.
Wanders A, Dvd Heijde, Landewé R, Béhier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005;52:1756–65.
Zochling J, Bohl-Bühler MH, Baraliakos X, Feldtkeller E, Braun J. Nonsteroidal anti-inflammatory drug use in ankylosing spondylitis—a population-based survey. Clin Rheumatol 2006;25:794–800.
van der Heijde D, Baraf HS, Ramos-Remus C, Calin A, Weaver AL, et al. Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a fifty-two-week, randomized, controlled study. Arthritis Rheum 2005;52:1205–15.
Dougados M, Béhier JM, Jolchine I, Calin A, van der Heijde D, et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum 2001;44:180–5.
Nazeri S, Khadem Azarian S, Fattahi MJ, Sedaghat R, et al. Preclinical and pharmacotoxicology evaluation of α-1-guluronic acid (G2013) as a nonsteroidal anti-inflammatory drug with immunomodulatory property. Immunopharmacol Immunotoxicol 2017;39:59–65.
Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker-Hermann E, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 2012;71:1616–22.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nazeri, S., Jamshidi, A.R., Mahmoudi, M. et al. The safety and efficacy of Guluronic acid (G2013) in ankylosing spondylitis: A randomized controlled parallel clinical trial. Pharmacol. Rep 71, 393–398 (2019). https://doi.org/10.1016/j.pharep.2019.02.002
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2019.02.002