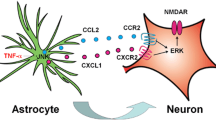
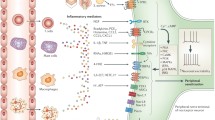
Abstract
The treatment of neuropathic pain resulting from nervous system malfunction remains a challenging problem for doctors and scientists. The lower effectiveness of conventionally used analgesics in neuropathic pain is associated with complex and not fully understood mechanisms of its development. Undoubtedly, interactions between immune and nervous system are crucial for maintenance of painful neuropathy. Nerve injury induces glial cell activation and thus enhances the production of numerous pronociceptive factors by these cells, including interleukins and chemokines. Increased release of those factors reduces the analgesic efficacy of opioids, which is significantly lower in neuropathic pain than in other painful conditions. This review discusses the role of chemokines from all four subfamilies as essential mediators of neuron-glia interactions occurring under neuropathic pain conditions. Based on available data, we analyse the influence of chemokines on opioid properties. Finally, we identify new direct and indirect pharmacological targets whose modulation may result in effective therapy of neuropathic pain, possibly in combination with opioids.
Similar content being viewed by others
References
Merskey H., Bogduk N. Classification of Chronic Pain., doi:https://doi.org/10.1002/ana.20394.
Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain 2015;156:1003–7, doi:https://doi.org/10.1097/j.pain.0000000000000160.
Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770, doi:https://doi.org/10.1186/1471-2458-11-770.
Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999;353:1959–64, doi:https://doi.org/10.1016/S0140-6736(99)01307-0.
McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev 2013, doi:https://doi.org/10.1002/14651858.CD006146pub2.
Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3–S14, doi:https://doi.org/10.4065/mcp.2009.0649.
Vorobeychik Y, Gordin V, Mao J, Chen L. Combination therapy for neuropathic pain. CNS Drugs 2011;25:1023–34, doi:https://doi.org/10.2165/11596280-000000000-00000.
Mao J, Gold MS, Backonja Misha M. Combination drug therapy for chronic pain: a call for more clinical studies. J Pain 2011;12:157–66, doi:https://doi.org/10.1016/j.jpain.2010.07.006.
Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol 2012;12:55–61, doi:https://doi.org/10.1016/j.coph.2011.10.007.
Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 2000;101:745–57, doi:https://doi.org/10.1016/S0306-4522(00)00396-1.
Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 2009;114:4613–23.
Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 2002;112:23–38, doi:https://doi.org/10.1016/S0306-4522(02)00065-9.
Taipa R, Ferreira V, Brochado P, Robinson A, Reis I, Marques F, et al. Inflammatory pathology markers (activated microglia and reactive astrocytes) in early and late onset Alzheimer disease: a post-mortem study. Neuropathol Appl Neurobiol n.d. n/a -n/a. 10.1111/nan.12445.
Mancera P, Wappenhans B, Cordobilla B, Virgili N, Pugliese M, Rueda F, et al. Natural docosahexaenoic acid in the triglyceride form attenuates in vitro microglial activation and ameliorates autoimmune encephalomyelitis in mice. Nutrients 2017;9:681, doi:https://doi.org/10.3390/nu9070681.
Kazakos EI, Kountouras J, Polyzos SA, Deretzi G. Novel aspects of defensins’ involvement in virus-induced autoimmunity in the central nervous system. Med Hypotheses 2017;102:33–6, doi:https://doi.org/10.1016/j.mehy.2017.02.020.
Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Mika J. Microglial inhibition influences XCL1/XCR1 expression and causes analgesic effects in a mouse model of diabetic neuropathy. Anesthesiology 2016;125:573–89.
Ji R-R, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013;154:S10–28, doi:https://doi.org/10.1016/j.pain.2013.06.022.
Popiolek-Barczyk K, Kolosowska N, Piotrowska A, Makuch W, Rojewska E, Jurga AM, et al. Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast 2015;2015:, doi:https://doi.org/10.1155/2015/676473.
Kwiatkowski K, Piotrowska A, Rojewska E, Makuch W, Jurga A, Slusarczyk J, et al. Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 2016;64:68–78, doi:https://doi.org/10.1016/j.pnpbp.2015.07.005.
Piotrowska A, Kwiatkowski K, Rojewska E, Slusarczyk J, Makuch W, Basta-Kaim A, et al. Direct and indirect pharmacological modulation of CCL2/CCR2 pathway results in attenuation of neuropathic pain—in vivo and in vitro evidence. J Neuroimmunol 2016;297:9–19, doi:https://doi.org/10.1016/j.jneuroim.2016.04.017.
Rojewska E, Popiolek-Barczyk K, Jurga AM, Makuch W, Przewlocka B, Mika J. Involvement of pro- and antinociceptive factors in minocycline analgesia in rat neuropathic pain model. J Neuroimmunol 2014;277(1–2):57–66, doi:https://doi.org/10.1016/j.jneuroim.2014.09.020.
Ochi-ishi R, Nagata K, Inoue T, Tozaki-Saitoh H, Tsuda M, Inoue K. Involvement of the chemokine CCL3 and the purinoceptor P2X7 in the spinal cord in paclitaxel-induced mechanical allodynia. Mol Pain 2014;10:53, doi:https://doi.org/10.1186/1744-8069-10-53.
Marinelli C, Di Liddo R, Facci L, Bertalot T, Conconi MT, Zusso M, et al. Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J Neuroinflammation 2015;12:244, doi:https://doi.org/10.1186/s12974-015-0458-6.
Pilat D, Rojewska E, Jurga AM, Piotrowska A, Makuch W, Przewlocka B, et al. IL-1 receptor antagonist improves morphine and buprenorphine efficacy in a rat neuropathic pain model. Eur J Pharmacol 2015;764:240–8, doi:https://doi.org/10.1016/j.ejphar.2015.05.058.
Pilat D, Piotrowska A, Rojewska E, Jurga A, Slusarczyk J, Makuch W, et al. Blockade of IL-18 signaling diminished neuropathic pain and enhanced the efficacy of morphine and buprenorphine. Mol Cell Neurosci 2016;71:114–24, doi:https://doi.org/10.1016/j.mcn.2015.12.013.
Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia — evidence from in vivo and in vitro studies. Neuropharmacology 2016;108:207–19, doi:https://doi.org/10.1016/j.neuropharm.2016.04.024.
Kwiatkowski K, Piotrowska A, Rojewska E, Makuch W, Mika J. The RS504393 influences the level of nociceptive factors and enhances opioid analgesic potency in neuropathic rats. J Neuroimmune Pharmacol 2017;12:402–19, doi:https://doi.org/10.1007/s11481-017-9729-6.
Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem 2001;130:169–75.
Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep 2008;60(3):297–307, doi:https://doi.org/10.1080/15360280902901404.
Cheon SY, Kim EJ, Kim JM, Kam EH, Ko BW, Koo B-N. Regulation of microglia and macrophage polarization via apoptosis signal-regulating kinase 1 silencing after ischemic/hypoxic injury. Front Mol Neurosci 2017;10:261, doi:https://doi.org/10.3389/fnmol.2017.00261.
Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw 2012;12:41–7, doi:https://doi.org/10.4110/in.2012.12.2.41.
Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005;115:71–83.
Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci 2005;28:661–9, doi:https://doi.org/10.1016/j.tins.2005.10.001.
Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol 2007;560:142–9, doi:https://doi.org/10.1016/j.ejphar.2007.01.013.
Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 2007;21:686–98, doi:https://doi.org/10.1016/j.bbi.2006.10.012.
Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol 2004;25:75–84, doi:https://doi.org/10.1016/j.it.2003.12.005.
Cartier L, Hartley O, Dubois-Dauphin M, Krause K-H. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Rev 2005;48:16–42, doi:https://doi.org/10.1016/j.brainresrev.2004.07.021.
Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol 2004;28:443–60, doi:https://doi.org/10.1016/j.dci.2003.09.006.
Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 2009;31:711–21, doi:https://doi.org/10.1016/j.immuni.2009.09.010.
Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000;12:121–7.
Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 2007;4:590–601, doi:https://doi.org/10.1016/j.nurt.2007.07.004.
Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995;270:1811–5.
Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev 2002;13:455–81, doi:https://doi.org/10.1016/S1359-6101(02)00045-X.
Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol 2004;25:201–9, doi:https://doi.org/10.1016/j.it.2004.02.006.
Lei Y, Takahama Y. XCL1 and XCR1 in the immune system. Microbes Infect 2012;14:262–7, doi:https://doi.org/10.1016/j.micinf.2011.10.003.
Fox JC, Nakayama T, Tyler RC, Sander TL, Yoshie O, Volkman BF. Structural and agonist properties of XCL2, the other member of the C-chemokine subfamily. Cytokine 2015;71:302–11, doi:https://doi.org/10.1016/j.cyto.2014.11.010.
Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther 2010;126:56–68, doi:https://doi.org/10.1016/j.pharmthera.2010.01.002.
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610–21, doi:https://doi.org/10.1056/NEJMra052723.
Mines M, Ding Y, Fan G-H. The many roles of chemokine receptors in neurodegenerative disorders: emerging new therapeutical strategies. Curr Med Chem 2007;14:2456–70.
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 2001;21:5027–35.
Liou J-T, Mao C-C, Ching-Wah Sum D, Liu F-C, Lai Y-S, Li J-C, et al. Peritoneal administration of Met-RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J Pain 2013;14:24–35, doi:https://doi.org/10.1016/j.jpain.2012.09.015.
Pevida M, Lastra A, Meana A, Hidalgo A, Baamonde A, Menendez L. The chemokine CCL5 induces CCR1-mediated hyperalgesia in mice inoculated with NCTC 2472 tumoral cells. Neuroscience 2014;259:113–25, doi:https://doi.org/10.1016/j.neuroscience.2013.11.055.
Benamar K, Geller EB, Adler MW. Elevated level of the proinflammatory chemokine, RANTES/CCL5, in the periaqueductal grey causes hyperalgesia in rats. Eur J Pharmacol 2008;592:93–5, doi:https://doi.org/10.1016/j.ejphar.2008.07.009.
Perrin FE, Lacroix S, Aviles-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1 alpha and interleukin-1beta in Wallerian degeneration. Brain 2005;128:854–66, doi:https://doi.org/10.1093/brain/awh407.
Dawes JM, Kiesewetter H, Perkins JR, Bennett DLH, McMahon SB. Chemokine expression in peripheral tissues from the Monosodium Iodoacetate model of chronic joint pain. Mol Pain 2013;9:57, doi:https://doi.org/10.1186/1744-8069-9-57.
Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 2007;27:8893–902, doi:https://doi.org/10.1523/JNEUROSCI.2209-07.2007.
Zychowska M, Rojewska E, Pilat D, Mika J. The role of some chemokines from the CXC subfamily in a mouse model of diabetic neuropathy. J Diabetes Res 2015;201:5, doi:https://doi.org/10.1155/2015/750182.
Xu W, Zhu M, Yuan S, Yu W. Spinal CXCL5 contributes to nerve injury-induced neuropathic pain via modulating GSK-3beta phosphorylation and activity in rats. Neurosci Lett 2016;634:52–9, doi:https://doi.org/10.1016/j.neulet.2016.10.004.
Zhu M, Yuan ST, Yu WL Jia LL, Sun Y. CXCL13 regulates the trafficking of GluN2B-containing NMDA receptor via IL-17 in the development of remifentanil-induced hyperalgesia in rats. Neurosci Lett 2017;648:26–33, doi:https://doi.org/10.1016/j.neulet.2017.03.044.
Cao D-L, Zhang Z-J, Xie R-G, Jiang B-C, Ji R-R, Gao Y-J. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol 2014;261:328–36, doi:https://doi.org/10.1016/j.expneurol.2014.05.014.
Wang Z, Du T, Zhang R. JNK in spinal cord facilitates bone cancer pain in rats through modulation of CXCL1. J Huazhong Univ Sci Technol Med Sci = Hua Zhong Ke Ji Da Xue Bao Yi Xue Ying Wen Ban = Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban 2016;36:88–94, doi:https://doi.org/10.1007/s11596-016-1547-1.
Omari KM, John G, Lango R, Raine CS. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia 2006;53:24–31, doi:https://doi.org/10.1002/glia.20246.
Nunemaker CS, Chung HG, Verrilli GM, Corbin KL, Upadhye A, Sharma PR. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J Endocrinol 2014;222:267–76, doi:https://doi.org/10.1530/JOE-14-0126.
Dawes JM, Calvo M, Perkins JR, Paterson KJ, Kiesewetter H, Hobbs C, et al. CXCL5 mediates UVB irradiation-induced pain. Sci Transl Med 2011;3:90ra60, doi:https://doi.org/10.1126/scitranslmed.3002193.
Yoshida S, Arakawa F, Higuchi F, Ishibashi Y, Goto M, Sugita Y, et al. Gene expression analysis of rheumatoid arthritis synovial lining regions by cDNA microarray combined with laser microdissection: up-regulation of inflammation-associated STAT1,IRF1, CXCL9, CXCL10, and CCL5. Scand J Rheumatol 2012;41:170–9, doi:https://doi.org/10.3109/03009742.2011.623137.
Guan X-H, Fu Q-C, Shi D, Bu H-L, Song Z-P, Xiong B-R, et al. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol 2015;263:39–49, doi:https://doi.org/10.1016/j.expneurol.2014.09.019.
McColl SR, Mahalingam S, Staykova M, Tylaska LA, Fisher KE, Strick CA, et al. Expression of rat I-TAC/CXCL11/SCYA11 during central nervous system inflammation: comparison with other CXCR3 ligands. Lab Invest 2004;84:1418–29, doi:https://doi.org/10.1038/labinvest.3700155.
Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun 2007;21:581–91, doi:https://doi.org/10.1016/j.bbi.2006.12.003.
Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol Pain 2009;5:48, doi:https://doi.org/10.1186/1744-8069-5-48.
Shen W, Hu X-M, Liu Y-N, Han Y, Chen L-P, Wang C-C, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014;11:75, doi:https://doi.org/10.1186/1742-2094-11-75.
Ikejima H, Imanishi T, Tsujioka H, Kashiwagi M, Kuroi A, Tanimoto T, et al. Upregulation of fractalkine and its receptor, CX3CR1, is associated with coronary plaque rupture in patients with unstable angina pectoris. Circ J 2010;74:337–45.
Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv 2010;10:263–70, doi:https://doi.org/10.1124/mi.10.5.3.
Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res 1998;53:260–7, doi:https://doi.org/10.1002/(SICI)1097-4547(19980715)53:2<260:AID-JNR15>3.0.CO;2-A.
Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 2002;22:6696–703 20026699.
Dansereau M-A, Gosselin R-D, Pohl M, Pommier B, Mechighel P, Mauborgne A, et al. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem 2008;106:757–69, doi:https://doi.org/10.1111/j.1471-4159.2008.05429.x.
White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv 2009;9:188–95, doi:https://doi.org/10.1124/mi.9.4.7.
Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci 2003;100:7947–52.
Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau M-A, Kitabgi P, et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci 2011;31:5865–75, doi:https://doi.org/10.1523/JNEUROSCI.5986-10.2011.
Gao Y-J, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu Z-Z, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 2009;29:4096–108, doi:https://doi.org/10.1523/JNEUROSCI.3623-08.2009.
Gao Y-J, Ji R-R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 2009;2:11–7, doi:https://doi.org/10.2174/1876386300902010011.
Hu J-H, Zheng X-Y, Yang J-P, Wang L-N, Ji F-H. Involvement of spinal monocyte chemoattractant protein-1 (MCP-1) in cancer-induced bone pain in rats. Neurosci Lett 2012;517:60–3, doi:https://doi.org/10.1016/j.neulet.2012.04.026.
Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, et al. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 2007;149:706–14, doi:https://doi.org/10.1016/j.neuroscience.2007.08.014.
Stammers AT, Liu J, Kwon BK. Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J Neurosci Res 2012;90:782–90, doi:https://doi.org/10.1002/jnr.22820.
Mummidi S, Ahuja SS, Gonzalez E, Anderson SA, Santiago EN, Stephan KT, et al. Genealogy of the CCR5 locus and chemokine system gene variants associated with altered rates of HIV-1 disease progression. Nat Med 1998;4:786–93.
Malon JT, Cao L. Calcitonin gene-related peptide contributes to peripheral nerve injury-induced mechanical hypersensitivity through CCL5 and p38 pathways. J Neuroimmunol 2016;297:68–75, doi:https://doi.org/10.1016/j.jneuroim.2016.05.003.
Yin Q, Fan Q, Zhao Y, Cheng M-Y, Liu H, Li J, et al. Spinal NF-kappaB and chemokine ligand 5 expression during spinal glial cell activation in a neuropathic pain model. PLoS One 2015;10:e0115120, doi:https://doi.org/10.1371/journal.pone.0115120.
Hang L-H, Shao D-H, Chen Z, Chen Y-F, Shu W-W, Zhao Z-G. Involvement of spinal CC chemokine ligand 5 in the development of bone cancer pain in rats. Basic Clin Pharmacol Toxicol 2013;113:325–8, doi:https://doi.org/10.1111/bcpt.12099.
Padi SSV, Shi XQ, Zhao YQ, Ruff MR, Baichoo N, Pert CB, et al. Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain 2012;153:95–106, doi:https://doi.org/10.1016/j.pain.2011.09.022.
Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Nalepa I, Mika J. Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target — evidence from a mouse diabetic neuropathy model. Int Immunopharmacol 2017;52:261–71, doi:https://doi.org/10.1016/j.intimp.2017.09.021.
Akimoto N, Honda K, Uta D, Beppu K, Ushijima Y, Matsuzaki Y, et al. CCL-1 in the spinal cord contributes to neuropathic pain induced by nerve injury. Cell Death Dis 2013;4:e679, doi:https://doi.org/10.1038/cddis.2013.198.
Saika F, Kiguchi N, Kobayashi Y, Fukazawa Y, Kishioka S. CC-chemokine ligand 4/macrophage inflammatory protein-1beta participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 2012;16:1271–80, doi:https://doi.org/10.1002/j.1532-2149.2012.00146.x.
Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain 2010;149:305–15, doi:https://doi.org/10.1016/j.pain.2010.02.025.
Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 2011;30:1864–73, doi:https://doi.org/10.1038/emboj.2011.89.
de Jong EK, Vinet J, Stanulovic VS, Meijer M, Wesseling E, Sjollema K, et al. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. FASEB J Off Publ Fed Am Soc Exp Biol 2008;22:4136–45, doi:https://doi.org/10.1096/fj.07-101907.
Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, et al. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglia. J Immunol 2002;168:3221–6.
Schmitz K, Pickert G, Wijnvoord N, Haussler A, Tegeder I. Dichotomy of CCL21 and CXCR3 in nerve injury-evoked and autoimmunity-evoked hyperalgesia. Brain Behav Immun 2013;32:186–200, doi:https://doi.org/10.1016/j.bbi.2013.04.011.
Strong JA, Xie W, Coyle DE, Zhang J-M. Microarray analysis of rat sensory ganglia after local inflammation implicates novel cytokines in pain. PLoS One 2012;7:e40779, doi:https://doi.org/10.1371/journal.pone.0040779.
Zhang Z-J, Cao D-L, Zhang X, Ji R-R, Gao Y-J. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013;154:2185–97, doi:https://doi.org/10.1016/j.pain.2013.07.002.
Kiguchi N, Kobayashi Y, Maeda T, Fukazawa Y, Tohya K, Kimura M, et al. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J Pharmacol Exp Ther 2012;340:577–87, doi:https://doi.org/10.1124/jpet.111.187724.
Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Spatiotemporal CCR1, CCL3(MIP-1 alpha), CXCR4, CXCL12(SDF-1 alpha) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J Neurosurg Spine 2011;14:583–97, doi:https://doi.org/10.3171/2010.12.SPINE10480.
Dubovy P, Klusakova I, Svizenska I, Brazda V. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol 2010;133:323–37, doi:https://doi.org/10.1007/s00418-010-0675-0.
Reaux-Le Goazigo A, Rivat C, Kitabgi P, Pohl M, Melik Parsadaniantz S. Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci 2012;36:2619–31, doi:https://doi.org/10.1111/j.1460-9568.2012.08179.x.
Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian J-L, et al. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 2016;32:27–40, doi:https://doi.org/10.1007/s12264-015-0007-4.
Hu X-M, Liu Y-N, Zhang H-L, Cao S-B, Zhang T, Chen L-P, et al. CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 2015;132:452–63, doi:https://doi.org/10.1111/jnc.12985.
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997;385:640–4, doi:https://doi.org/10.1038/385640a0.
Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain 2005;6:434–8, doi:https://doi.org/10.1016/j.jpain.2005.02.001.
Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci 2004;20:2294–302, doi:https://doi.org/10.1111/j.1460-9568.2004.03709.x.
Zhuang Z-Y, Kawasaki Y, Tan P-H, Wen Y-R, Huang J, Ji R-R. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun 2007;21:642–51, doi:https://doi.org/10.1016/j.bbi.2006.11.003.
Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the bad guys: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun 2007;21:131–46, doi:https://doi.org/10.1016/j.bbi.2006.10.011.
Zou W, Guo Q, Wang E, Cai J. Effect of intrathecal pumping morphine on immunological function in rats with formalin pain. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2005;30:157–61.
Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun 2009;23:75–84, doi:https://doi.org/10.1016/j.bbi.2008.07.005.
Franchi S, Panerai AE, Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 2007;21:767–74, doi:https://doi.org/10.1016/j.bbi.2007.01.001.
Zhang X, Wang J, Yu T, Du D, Jiang W. Minocycline can delay the development of morphine tolerance, but cannot reverse existing tolerance in the maintenance period of neuropathic pain in rats. Clin Exp Pharmacol Physiol 2015;42:94–101, doi:https://doi.org/10.1111/1440-1681.12316.
Rojewska E, Makuch W, Przewlocka B, Mika J. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology 2014;86:301–10, doi:https://doi.org/10.1016/j.neuropharm.2014.08.001.
Dorazil-Dudzik M, Mika J, MK-H Schafer, Li Y, Obara I, Wordliczek J, et al. The effects of local pentoxifylline and propentofylline treatment on formalin-induced pain and tumor necrosis factor-alpha messenger RNA levels in the inflamed tissue of the rat paw. Anesth Analg 2004;98:1566–73 table of contents.
Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, et al. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol 2006;2:279–91, doi:https://doi.org/10.1017/S1740925X0700035X.
Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology 2004;29:327–34, doi:https://doi.org/10.1038/sj.npp.1300315.
Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res 2001;39:281–6.
Wordliczek J, Szczepanik AM, Banach M, Turchan J, Zembala M, Siedlar M, et al. The effect of pentoxifiline on post-injury hyperalgesia in rats and postoperative pain in patients. Life Sci 2000;66:1155–64.
Zhao C, Guo R, Hu F, Chen P, Cui Y, Feng J, et al. Spinal MCP-1 contributes to the development of morphine antinociceptive tolerance in rats. Am J Med Sci 2012;344:473–9, doi:https://doi.org/10.1097/MAJ.0b013e31826a82ce.
Rivat C, Sebaihi S, Van Steenwinckel J, Fouquet S, Kitabgi P, Pohl M, et al. Src family kinases involved in CXCL12-induced loss of acute morphine analgesia. Brain Behav Immun 2014;38:38–52, doi:https://doi.org/10.1016/j.bbi.2013.11.010.
Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPSJ 2006;7:E865–70, doi:https://doi.org/10.1208/aapsj070484.
Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend 2007;88:36–41, doi:https://doi.org/10.1016/j.drugalcdep.2006.09.010.
Ye D, Bu H, Guo G, Shu B, Wang W, Guan X, et al. Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: involvement of Gi protein. J Mol Neurosci 2014;53:571–9, doi:https://doi.org/10.1007/s12031-013-0223-1.
Wang S-F, Dong C-G, Yang X, Yin J-J. Upregulation of (C-X-C motif) ligand 13 (CXCL13) attenuates morphine analgesia in rats with cancer-induced bone pain. Med Sci Monit 2016;22:4612–22.
Guo G, Peng Y, Xiong B, Liu D, Bu H, Tian X, et al. Involvement of chemokine CXCL11 in the development of morphine tolerance in rats with cancer-induced bone pain. J Neurochem 2017;141:553–64, doi:https://doi.org/10.1111/jnc.13919.
Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids 1999;64:64–75.
Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J Immunol 2004;173:594–9.
Szabo I, Chen X-H, Xin L, Adler MW, Howard OMZ, Oppenheim JJ, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A 2002;99:10276–81, doi:https://doi.org/10.1073/pnas.102327699.
Rojewska E, Zychowska M, Piotrowska A, Kreiner G, Nalepa I, Mika J. Involvement of macrophage inflammatory protein-1 family members in the development of diabetic neuropathy and their contribution to effectiveness of morphine. Front. Immunol. 2018;9:494, doi:https://doi.org/10.3389/fimmu.2018.00494.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwiatkowski, K., Mika, J. The importance of chemokines in neuropathic pain development and opioid analgesic potency. Pharmacol. Rep 70, 821–830 (2018). https://doi.org/10.1016/j.pharep.2018.01.006
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2018.01.006