Abstract
Background
Prolonged use of aspirin, a commonly prescribed non steroidal anti-inflammatory drug, is well known to produce gastrointestinal toxicity which could be minimized by various anti-secretory agents. The present study was carried out to evaluate the protective effect of artesunate against aspirin induced gastric injury in rats.
Methods
Gastric injury was induced in fasted Wistar rats by oral administration of aspirin. The effect of 50 and 150 mg/kg of artesunate was studied on macroscopic changes, gastric secretions, histology, oxidative stress and inflammatory markers in the stomach tissue after 5 h of induction of gastric injury. Immunohistochemical analysis for the expression of IL-1β, IL-6, NF-κB(p65) and COX-2 was also carried out. The effect of artesunate was compared with that of standard anti-ulcer drug famotidine (20 mg/kg).
Results
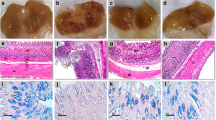
Artesunate pretreatment produced a dose-dependent reduction in aspirin induced gastric injury and restored the gastric juice parameters. It normalized the tissue levels of oxidative stress markers (glutathione, malondialdehyde and superoxide dismutase activity) and mediators of inflammation (myeloperoxidase and TNF-α). The protection afforded by artesunate was evident from the histoarchitecture of stomach tissue and marked reduction in tissue expression of IL-1β, IL-6, NF-κB(p65) and COX-2. The effect of artesunate was found to be comparable to that of standard drug famotidine.
Conclusion
Artesunate markedly ameliorated aspirin induced gastric injury in rats by targeting oxidative stress and COX-2 dependent as well as COX-2 independent proinflammatory signaling pathways and could have a therapeutic potential in gastric ulcer disease.
Similar content being viewed by others
References
Paez Espinosa EV, Murad JP, Khasawneh FT. Aspirin: pharmacology and clinical applications. Thrombosis 2012;2012:173124.
Hennekens CH, Sechenova O, Hollar D, Serebruany VL. Dose of aspirin in the treatment and prevention of cardiovascular disease: current and future directions. J Cardiovasc Pharmacol Ther 2006;11(3):170–6.
Valkhoff VE, Sturkenboom MC, Kuipers EJ. Risk factors for gastrointestinal bleeding associated with low-dose aspirin. Best Pract Res Clin Gastroenterol 2012;26(2):125–40.
Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Fuberg CD, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008;118(18):1894–909.
Tamura A, Murakami K, Kadota J. Prevalence and independent factors for gastroduodenal ulcers/erosions in asymptomatic patients taking low-dose aspirin and gastroprotective agents: the OITA-GF study. QJM 2011;104(2):133–9.
Yeomans ND, Lanas AI, Talley NJ, Thomson ABR, Daneshjoo R, Eriksson B, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther 2005;22:795–801.
Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 2011;48(2):107–11.
Laine L. Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am 1996;6(3):489–504.
Keiser J, Veneziano V, Rinaldi L, Mezzino L, Duthaler U, Cringoli G. Anthelmintic activity of artesunate against Fasciola hepatica in naturally infected sheep. Res Vet Sci 2010;88(1):107–10.
Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis 2008;47(6):804–11.
Algamal MA, Marei GIK, Saad MMG, Abdelgaleil SAM. Antimicrobial and phytotoxic properties of artemisinin and related derivatives. World Appl Sci J 2013;28(10):1382–8.
Kumar VL, Guruprasad B, Chaudhary P. Antimalarial drug artesunate affords protection against carrageenan induced acute inflammation in rat. Biocell 2014;38(1–3):1–5.
Guruprasad B, Chaudhary P, Choedon T, Kumar VL. Artesunate ameliorates functional limitations in freund’s complete adjuvant-induced monoarthritis in rat by maintaining oxidative homeostasis and inhibiting COX-2 expression. Inflammation 2015;38(3):1028–35.
Wang Q, Wu LM, Zhao Y, Zhang XL, Wang NP. The anticancer effect of artesunate and its mechanism. Yao Xue Xue Bao 2002;37(6):477–8.
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol 2001;18(4):767–73.
Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 2003;64(2):382–94.
Jiao Y, Ge CM, Meng QH, Cao JP, Fan SJ. Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin 2007;28(7):1045–56.
Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, et al. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappa-B and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology 2007;46(6):920–6.
Yang Z, Ding J, Yang C, Gao Y, Li X, Chen X, et al. Immunomodulatory and anti-inflammatory properties of artesunate in experimental colitis. Curr Med Chem 2012;19(26):4541–51.
Verma S, Kumar VL. Attenuation of gastric mucosal damage by artesunate in rat: modulation of oxidative stress and NFkB mediated signaling. Chem Biol Interact 2016;257:46–53.
Musumba C, Pritchard DM, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther 2009;30(6):517–31.
Bharti S, Wahane VD, Kumar VL. Protective effect of Calotropis procera latex extracts on experimentally induced gastric ulcers in rat. J Ethanopharmacol 2010;127(2):440–4.
Adachi K, Komazawa Y, Mihara T, Azumi T, Fujisawa T, Katsube T. Comparative study of the speed of acid-suppressing effects of oral administration of cimetidine and famotidine. J Gastroenterol Hepatol 2005;20(7):1012–5.
Hamiza OO, Rehman MU, Tahir M, Khan R, Khan AQ, Lateef A, et al. Amelioration of 1,2 dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in wistar rats. Asian Pac J Cancer Prev 2012;13(9):4393–402.
Chakraborty SP, Karmahapatra S, Sahu SK, Pramanik P, Roy S. Amelioratory effect of nanoconjugated vancomycin on spleen during VRSA-induced oxidative stress. Patholog Res Int 2011;2011:420198.
Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 1982;60(3):618–22.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70–7.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351–8.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47(3):469–74.
Onishi M, Odajima T. Degradation of apotransferrin by the myeloperoxidase system. Inflamm Regen 2010;30(1):63–7.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374(9699):1449–61.
Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012;107(3):345–60.
Alberts MJ, Bergman DL, Molner E, Jovanovic BD, Ushiwata I, Teruya J. Antiplatelet effect of aspirin in patients with cerebrovascular disease. Stroke 2004;35(1):175–8.
Iwamoto J, Saito Y, Honda A, Matsuzaki Y. Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy. World J Gastroenterol 2013;19(11):1673–82.
Halter F, Tarnawski AS, Schmassmann A. Peskar BM: Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and Perspectives. Gut 2001;49(3):443–53.
Kauffman G. Aspirin-induced gastric mucosal injury: lessons learned from animal models. Gastroenterology 1989;96(Suppl. 2 Pt 2):606–14.
Wang GZ, Huang GP, Yin GL, Zhou G, Guo CJ, Xie CG, et al. Aspirin can elicit the recurrence of gastric ulcer induced with acetic acid in rats. Cell Physiol Biochem 2007;20(1–4):205–12.
Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomized, double-blind, placebo-controlled trial. Lancet 2009;374(9684):119–25.
Wang Z, Hasegawa J, Wang X, Matsuda A, Tokuda T, Miura N, et al. Protective effects of ginger against aspirin-induced gastric ulcers in rats. Yonago Acta Med 2011;54(1):11–9.
Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A 2005;102(2):431–6.
Olza J, Aguilera CM, Gil-Campos M, Leis R, Bueno G, Martinez-Jimenez MD, et al. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care 2012;35(11):2373–6.
Zhang JY, Wu QF, Wan Y, Song SD, Xu J, Xu XS, et al. Protective role of hydrogen-rich water on aspirin-induced gastric mucosal damage in rats. World J Gastroenterol 2014;20(6):1614–22.
Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 2012;188(12):6287–99.
Kamisah Y, Qodriyah HMS, Chua KH, Nur Azlina MF. Vitamin E: A potential therapy for gastric mucosal injury. Pharm Biol 2014;52(12):1591–7.
Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol 2006;149(6):611–23.
Stark LA, Reid K, Sansom OJ, Din FV, Guichard S, Mayer I, et al. Aspirin activates the NF-kappa B signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis 2007;28(5):968–76.
Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9(5):361–71.
Baeuerle PA. Pro-inflammatory signaling: last pieces in the NF-κB puzzle? Curr Biol 1998;8(1):R19–22.
Magierowskia M, Magierowskaa K, Hubalewska-Mazgajb M, Adamskic J, Bakalarzc D, Sliwowskia Z, et al. Interaction between endogenous carbon monoxide and hydrogensulfide in the mechanism of gastroprotection against acute aspirin-induced gastric damage. Pharmacol Res 2016;114:235–50.
Takeuchi K, Takayama S, Izuhara C. Comparative effects of the anti-platelet drugs, clopidogrel, ticlopidine, and cilostazol on aspirin-induced gastric bleeding and damage in rats. Life Sci 2014;110(2):77–85.
Sakai T, Ishihara K, Saigenji K, Hoti’a K. Recovery of mucin content in surface layer of rat gastric mucosa after HCl-aspirin-induced mucosal damage. J Gastroenterol 1997;32(2):157–63.
Melcarne L, Garcia Iglesias P, Calvet X. Management of NSAID-associated peptic ulcer disease. Expert Rev Gastroenterol Hepatol 2016;10(6):723–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, S., Kumar, V.L. Artesunate affords protection against aspirin-induced gastric injury by targeting oxidative stress and proinflammatory signaling. Pharmacol. Rep 70, 390–397 (2018). https://doi.org/10.1016/j.pharep.2017.06.003
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2017.06.003