Abstract
The aim of this paper was to review the up-to-date evidence base on pharmacology and clinical properties of vortioxetine.
Vortioxetine is a novel antidepressant, approved by the US Food and Drug Administration (FDA) for the treatment of major depressive disorder (MDD). Because vortioxetine exhibits both an antidepressant and anxiolytic effect, it may be effective in treating both depressive and anxiety disorders, such as generalized anxiety disorder (GAD). Based on its pharmacodynamics profile and preclinical studies, it is believe that the drug’s clinical action is mediated mainly by selective blockade of serotonin reuptake (by inhibiting the serotonin transporter [SERT]) and direct modulation of 5-HT receptors activity (such as 5-HT3, 5-HT7, 5-HT1D and 5-HT1B).
In patients with MDD the recommended doses range is 5–20 mg/day.
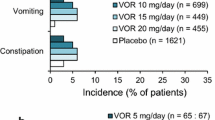
Vortioxetine was shown to be more effective than placebo both in MDD and GAD. In terms of side effects, nausea, vomiting, diarrhea, and dry mouth were most commonly observed in individuals receiving vortioxetine. In direct comparison to duloxetine, vortioxetine is found to have a smaller efficacy but had a lower risk of developing the common antidepressant-induced adverse effects.
Similar content being viewed by others
References
Lorenzo-Luaces L. Heterogeneity in the prognosis of major depression: from the common cold to a highly debilitating and recurrent illness. Epidemiol Psychiatr Sci 2015;24:466–72.
Kohler S, Cierpinsky K, Kronenberg G, Adli M. The serotonergic system in the neurobiology of depression: relevance for novel antidepressants. J Psychopharmacol 2016;30:13–22.
Lane RM. Antidepressant drug development: focus on triple monoamine reuptake inhibition. J Psychopharmacol 2015;29:526–44.
Guyatt G, Haynes B, Jaeschke R, Meade MO, Wilson M, Montori V, et al. The philosophy of evidence-based medicine. In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. McGraw-Hill; 2008. p. 9–16.
Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 2015;145:43–57.
D’Agostino A, English CD, Rey JA. Vortioxetine (brintellix): a new serotonergic antidepressant. P T 2015;40:36–40.
Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem 2011;54:3206–21.
Garnock-Jones KP. Vortioxetine a review of its use in major depressive disorder. CNS Drugs 2014;28:855–74.
Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry 2003;64(Suppl. (13)):5–12.
Mork A, Pehrson A, Brennum LT, Nielsen SM, Zhong H, Lassen AB, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 2012;340:666–75.
Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression. Biol Psychiatry 2003;53:193–203.
Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 2013;27:703–16.
Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther 2007;321:690–8.
Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord 2004;3:27–37.
Ramamoorthy R, Radhakrishnan M, Borah M. Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: an investigation in behaviour-based rodent models. Behav Pharm 2008;19:29–40.
European Medicines Agency: BRINTELLIX (vortioxetine)®. https://doi.org/www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002717/WC500159447.pdf. In: Agency EM, editor. 2013.
Brintellix (vortioxetine hydrobromide tablets): US prescribing information. https://doi.org/www.accessdata.fda.gov/drugsatfda_docs/label/2013/204447s000lbl.pdf. In: Administration FDA, editor. 2013.
Brintellix (TM). IDdb author IDDB MEETING REPORT 2009 Medicinal Chemistry — Fourth Anglo-Swedish Symposium (Part I). Lund, Sweden.
Areberg J, Sogaard B, Hojer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol 2012;111:198–205.
Gibb A, Deeks ED. Vortioxetine first global approval. Drugs 2014;74:135–45.
Areberg J, Luntang-Jensen M, Sogaard B, Nilausen DO. Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol 2012;110:401–4.
Lundbeck. Takeda and Lundbeck announce FDA approval of Brintellix™ (vortioxetine) for treatment of adults with major depressive disorder. Accessed from: https://doi.org/investor.lundbeck.com/releasedetail.cfm?releaseid=794050.2013.
Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant — what is the number needed to treat, number needed to harm and likelihood to be helped or harmed. Int J Clin Pract 2014;68:60–82.
Chen G, Lee R, Hojer AM, Buchbjerg JK, Serenko M, Zhao Z. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig 2013;33:727–36.
Uldam HK, Juhl M, Pedersen H, Dalgaard L. Biosynthesis and identification of an N-oxide/N-glucuronide metabolite and first synthesis of an N-O-glucuronide metabolite of Lu AA21004. Drug Metab Dispos 2011;39:2264–74.
Hvenegaard MG, Bang-Andersen B, Pedersen H, Jorgensen M, Puschl A, Dalgaard L. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos 2012;40:1357–65.
Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol 2016;30:242–52.
Katona CL, Katona CP. New generation multi-modal antidepressants: focus on vortioxetine for major depressive disorder. Neuropsychiatr Dis Treat 2014;10:349–54.
Spina E, Santoro V. Drug interactions with vortioxetine, a new multimodal antidepressant. Riv Psichiatr 2015;50:210–5.
Bortolato B, Miskowiak KW, Kohler CA, Maes M, Fernandes BS, Berk M, et al. Cognitive remission: a novel objective for the treatment of major depression? BMC Med 2016;14:9.
Pehrson AL, Cremers T, Betry C, van der Hart MG, Jorgensen L, Madsen M, et al. Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters-a rat microdialysis and electrophysiology study. Eur Neuropsychopharmacol 2013;23:133–45.
Betry C, Pehrson AL, Etievant A, Ebert B, Sanchez C, Haddjeri N. The rapid recovery of 5-HT cell firing induced by the antidepressant vortioxetine involves 5-HT(3) receptor antagonism. Int J Neuropsychopharmacol 2013;16:1115–27.
Dale E, Zhang H, Leiser SC, Xiao Y, Lu D, Yang CR, et al. Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus. J Psychopharmacol 2014;28:891–902.
El Mansari M, Lecours M, Blier P. Effects of acute and sustained administration of vortioxetine on the serotonin system in the hippocampus: electrophysiological studies in the rat brain. Psychopharmacology (Berl) 2015;232:2343–52.
Guilloux JP, Mendez-David I, Pehrson A, Guiard BP, Reperant C, Orvoen S, et al. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology 2013;73:147–59.
Li Y, Raaby KF, Sanchez C, Gulinello M. Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res 2013;256:520–8.
Overstreet DH, Wegener G. The flinders sensitive line rat model of depression-25 years and still producing. Pharmacol Rev 2013;65:143–55.
Betry C, Etievant A, Pehrson A, Sanchez C, Haddjeri N. Effect of the multimodal acting antidepressant vortioxetine on rat hippocampal plasticity and recognition memory. Prog Neuropsychopharmacol Biol Psychiatry 2015;58:38–46.
Westrich L, Haddjeri N, Dkhissi-Benyahya O, Sanchez C. Involvement of 5-HT (7) receptors in vortioxetine’s modulation of circadian rhythms and episodic memory in rodents. Neuropharmacology 2015;89:382–90.
du Jardin KG, Jensen JB, Sanchez C, Pehrson AL. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. Eur Neuropsychopharmacol 2014;24:160–71.
Jensen JB, du Jardin KG, Song D, Budac D, Smagin G, Sanchez C, et al. Vortioxetine, but not escitalopram or duloxetine, reverses memory impairment induced by central 5-HT depletion in rats: evidence for direct 5-HT receptor modulation. Eur Neuropsychopharmacol 2014;24:148–59.
Mork A, Montezinho LP, Miller S, Trippodi-Murphy C, Plath N, Li Y, et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav 2013;105:41–50.
Wallace A, Pehrson AL, Sanchez C, Morilak DA. Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol 2014;17:1695–706.
Li Y, Abdourahman A, Tamm JA, Pehrson AL, Sanchez C, Gulinello M. Reversal of age-associated cognitive deficits is accompanied by increased plasticity-related gene expression after chronic antidepressant administration in middle-aged mice. Pharmacol Biochem Behav 2015;135:70–82.
Goetghebeur P, Dias R. Comparison of haloperidol, risperidone, sertindole, and modafinil to reverse an attentional set-shifting impairment following subchronic PCP administration in the rat-a back translational study. Psychopharmacology (Berl) 2009;202:287–93.
Theunissen EL, Street D, Hojer AM, Vermeeren A, van Oers A, Ramaekers JG. A randomized trial on the acute and steady-state effects of a new antidepressant, vortioxetine (Lu AA21004), on actual driving and cognition. Clin Pharmacol Ther 2013;93:493–501.
Berhan A, Barker A. Vortioxetine in the treatment of adult patients with major depressive disorder: a meta-analysis of randomized double-blind controlled trials. BMC Psychiatry 2014; 14:276.
Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, et al. Vortioxetine: a meta-analysis of 12 short-term, randomized, placebo-controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci 2015;40:174–86.
Meeker AS, Herink MC, Haxby DG, Hartung DM. The safety and efficacy of vortioxetine for acute treatment of major depressive disorder: a systematic review and meta-analysis. Syst Rev 2015;4:21.
Fu J, Chen Y. The efficacy and safety of 5 mg/d Vortioxetine compared to placebo for major depressive disorder: a meta-analysis. Psychopharmacology (Berl) 2015;232:7–16.
Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol 2016;26:979–93.
Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol 2015;2015.
Llorca PM, Lancon C, Brignone M, Rive B, Salah S, Ereshefsky L, et al. Relative efficacy and tolerability of vortioxetine versus selected antidepressants by indirect comparisons of similar clinical studies. Curr Med Res Opin 2014;30:2589–606.
Li G, Wang X, Ma D. Vortioxetine versus duloxetine in the treatment of patients with major depressive disorder: a meta-analysis of randomized controlled trials. Clin Drug Investig 2016;36:509–17.
Wang G, Gislum M, Filippov G, Montgomery S. Comparison of vortioxetine versus venlafaxine XR in adults in Asia with major depressive disorder: a randomized, double-blind study. Curr Med Res Opin 2015;31:785–94.
Montgomery SA, Nielsen RZ, Poulsen LH, Haggstrom L. A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol 2014;29:470–82.
Rothschild AJ, Mahableshwarkar AR, Jacobsen P, Yan M, Sheehan DV. Vortioxetine (Lu AA21004) 5 mg in generalized anxiety disorder: results of an 8-week randomized, double-blind, placebo-controlled clinical trial in the United States. Eur Neuropsychopharmacol 2012;22:858–66.
Pae CU, Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, et al. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatr Res 2015;64:88–98.
Citrome L. Vortioxetine for major depressive disorder: an indirect comparison with duloxetine, escitalopram, levomilnacipran, sertraline, venlafaxine, and vilazodone, using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord 2016; 196:225–33.
NICE. Technology appraisal guidance. Vortioxetine for treating major depressive episodes (TA367). National Institute for Health and Care Excellence; 2015.
Fu J, Peng L, Li X. The efficacy and safety of multiple doses of vortioxetine for generalized anxiety disorder: a meta-analysis. Neuropsychiatr Dis Treat 2016;12:951–9.
FDA Drug Safety Communication: FDA approves brand name change for antidepressant drug Brintellix (vortioxetine) to avoid confusion with antiplatelet drug Brilinta (ticagrelor) https://doi.org/www.fda.gov/Drugs/DrugSafety/ucm497942.htm.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sowa-Kućma, M., Pańczyszyn-Trzewik, P., Misztak, P. et al. Vortioxetine: A review of the pharmacology and clinical profile of the novel antidepressant. Pharmacol. Rep 69, 595–601 (2017). https://doi.org/10.1016/j.pharep.2017.01.030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2017.01.030