Abstract
Background
Furosemide is a loop diuretic drug frequently indicated in hypertension and fluid overload conditions such as congestive heart failure and hepatic cirrhosis.
Objective
The purpose of the study was to establish a population pharmacokinetic model for furosemide in Indian hypertensive and fluid overload patients, and to evaluate effects of covariates on the volume of distribution (V/F) and oral clearance (CL/F) of furosemide.
Methods
A total of 188 furosemide plasma sample concentrations from 63 patients with hypertension or fluid overload conditions were collected in this study. The population pharmacokinetic model for furosemide was built using Phoenix NLME 1.3 software. The covariates included age, sex, body surface area, bodyweight, height and creatinine clearance (CRCL).
Results
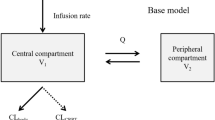
The pharmacokinetic data of furosemide was adequately explained by a two-compartment linear pharmacokinetic model with first-order absorption and an absorption lag-time. The mean values of CL/F and Vd/F of furosemide in the patients were 15.054 Lh−1 and 4.419 L, respectively. Analysis of covariates showed that CRCL was significantly influencing the clearance of furosemide.
Conclusion
The final population pharmacokinetic model was demonstrated to be appropriate and effective and it can be used to assess the pharmacokinetic parameters of furosemide in Indian patients with hypertension and fluid overload conditions.
Similar content being viewed by others
References
Kitsios GD, Mascari P, Ettunsi R, Gray AW. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: a meta-analysis. J Crit Care 2014;29:253–9.
Klausner EA, Lavy E, Stepensky D, Cserepes E, Barta M, Friedman M, et al. Furosemide pharmacokinetics and pharmacodynamics following gastroretentive dosage form administration to healthy volunteers. J Clin Pharmacol 2003;43:711–20.
Heidarimoghadam R, Farmany A. Rapid determination of furosemide in drug and blood plasma of wrestlers by a carboxy-MWCNT sensor. Mater Sci Eng C Mater Biol Appl 2016;58:1242–5.
Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension: part 2: loop diuretics and potassium-sparing agents. Expert Opin Pharmacother 2014;15:605–21.
Lamarche C, Pichette M, Ouimet D, Vallée M, Bell R, Ouellet G, et al. Pharmacokinetic and dynamic of furosemide in peritoneal dialysis patients. Perit Dial Int 2016;36:107–8.
Oh SW, Han SY. Loop diuretics in clinical practice. Electrolyte Blood Press 2015;13:17–21.
Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B. Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol 2016;310:958 9–71.
Zisaki A, Miskovic L, Hatzimanikatis V. Antihypertensive drugs metabolism: an update to pharmacokinetic profiles and computational approaches. Curr Pharm Des 2015;21:806–22.
Hu P, Chen J, Lui D, Zheng X, Zhao Q, Jiang J. Development of population pharmacokinetic model of icotinib with non-linear absorption characters in healthy Chinese volunteers to assess the CYP2C19 polymorphism and food-intake effect. Eur J Clin Pharmacol 2015;71:843–50.
Pene DT, Anic-Milic T, Oreskovic K, Padovan J, Brouwer KL, Zuo P, et al. Development of a population pharmacokinetic model to describe azithromycin wholeblood and plasma concentrations over time in healthy subjects. Antimicrob Agents Chemother 2013;57:3194–201.
Carlton LD, Patterson JH, Mattson CN, Schmith VD. The effects of epoprostenol on drug disposition. II: a pilot study of the pharmacokinetics of furosemide with and without epoprostenol in patients with congestive heart failure. J Clin Pharmacol 1996;36:257–64.
Wakelkamp M, Alván G, Gabrielsson J, Paintaud G. Pharmacodynamic modeling of furosemide tolerance after multiple intravenous administration. Clin Pharmacol Ther 1996;60:75–88.
Wakelkamp M, Alván G, Scheinin H, Gabrielsson J. The influence of drug input rate on the development of tolerance to frusemide. Br J Clin Pharmacol 1998;46:479–87.
Schoemaker RC, van dDer Vorst MM, van Heel IR, Cohen AF, Burggraaf J. Development of an optimal furosemide infusion strategy in infants with modeling and simulation. Clin Pharmacol Ther 2002;72:383–90.
Van Wart SA, Shoaf SE, Mallikaarjun S, Mager DE. Population-based meta-analysis of furosemide pharmacokinetics. Biopharm Drug Dispos 2014;35:119–33.
Feng S, Jiang J, Hu P, Zhang J, Liu T, Zhao Q, et al. A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy Chinese subjects. Acta Pharmacol Sin 2012;33:1353–8.
Huntjens DR, Liefaard LC, Nandy P, Drenth HK, Vermeulen A. Population pharmacokinetic modeling of tapentadol extended release (ER) in healthy subjects and patients with moderate or severe pain. Clin Drug Investig 2016;36:213–23.
Vestergaard B, Thygesen P, Kreilgaard M, Fels JJ, Lykkesfeldt J, Agersø H. The kidneys play a central role in the clearance of rhGH in rats. Eur J Pharm Sci 2016;86:29–33.
Munekage M, Ichikawa K, Kitagawa H, Ishihara K, Uehara H, Watanabe J, et al. Population pharmacokinetic analysis of daikenchuto, a traditional Japanese medicine (Kampo) in Japanese and US health volunteers. Drug Metab Dispos 2013;41:1256–63.
Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 2002;105:1348–53.
Silbert BI, Ho KM, Lipman J, Roberts JA, Corcoran TB, Morgan DJ, et al. Determinants of urinary output response to IV furosemide in acute kidney injury: a Pharmacokinetic/Pharmacodynamic study. Crit Care Med 2016, doi:https://doi.org/10.1097/ccm.0000000000001823.
Kerremans AL, Gribnau FW. Changes in pharmacokinetics and in effect of furosemide in the elderly. Clin Exp Hypertens A 1983;5:271–84.
Boles-Ponto LL, Schoenwald RD. Furosemide (frusemide): a pharmacokinetic/pharmacodynamic review (part I). Clin Pharmacokinet 1990;18:381–408.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodati, D., Yellu, N. Population pharmacokinetic modeling of furosemide in patients with hypertension and fluid overload conditions. Pharmacol. Rep 69, 492–496 (2017). https://doi.org/10.1016/j.pharep.2017.01.006
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2017.01.006