Abstract
Background
Cholesterol-dependent and independent mechanisms were proposed to explain anti-atherosclerotic action of statins in humans. However, their effects in murine models of atherosclerosis have not been consistently demonstrated. Here, we studied the effects of pravastatin on atherosclerosis in ApoE/LDLR−/− mice fed a control and atherogenic diet.
Methods
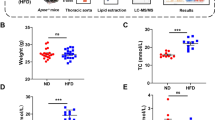
ApoE/LDLR−/− mice were fed a control (CHOW) or an atherogenic (Low Carbohydrate High Protein, LCHP) diet. Two doses of pravastatin (40 mg/kg and 100 mg/kg) were used. The anti-atherosclerotic effects of pravastatin in en face aorta, cross-sections of aortic roots and brachiocephalic artery (BCA) were analysed. The lipid profile was determined. Fourier Transform Infrared Spectroscopy followed by Fuzzy C-Means (FCM) clustering was used for the quantitative assessment of plaque composition.
Results
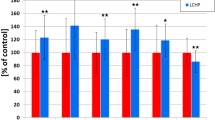
Treatment with pravastatin (100 mg/kg) decreased total and LDL cholesterol only in the LCHP group, but displayed a pronounced anti-atherosclerotic effect in BCA and abdominal aorta. The anti-atherosclerotic effect of pravastatin (100 mg/kg) in BCA was associated with significant alterations of the chemical plaque composition, including a fall in cholesterol and cholesterol esters contents independently on total cholesterol and LDL concentration in plasma.
Conclusions
Pravastatin at high (100 mg/kg), but not low dose displayed a pronounced anti-atherosclerotic effect in ApoE/LDLR−/− mice fed a CHOW or LCHP diet that was remarkable in BCA, visible in en face aorta, whereas it was not observed in aortic roots, suggesting that previous inconsistencies might have been due to the various sites of atherosclerotic plaque analysis.
Similar content being viewed by others
References
Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev 2004;25(1):81–93.
Takagi T, Matsuda M, Abe M, Kobayashi H, Fukuhara A, Komuro R, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis 2008;196(1):114–21.
Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 2007;27(8):1706–21.
Shaposhnik Z, Wang X, Trias J, Fraser H, Lusis AJ. The synergistic inhibition of atherogenesis in apoE2/2 mice between pravastatin and the sPLA2 inhibitor varespladib (A-002). J Lipid Res 2009;50:623–9.
Bea F, Blessing E, Bennett B, Levitz M, Wallace EP, Rosenfeld ME. Simvastatin promotes atherosclerotic plaque stability in apoE-deficient mice independently of lipid lowering. Arterioscler Thromb Vasc Biol 2002;22(11):1832–7.
Marzoll A, Melchior-Becker A, Cipollone F, Fischer JW. Small leucine-rich proteoglycans in atherosclerotic lesions: novel targets of chronic statin treatment? Cell Mol Med 2011;15(2):232–43.
van der Hoorn JWA, Kleemann R, Havekes LM, Kooistr T, Princen HMG, Jukem JW. Olmesartan and pravastatin additively reduce development of atherosclerosis in APOEM3Leiden transgenic mice. J Hypertens 2007;25:2454–62.
Byington RP, Davis BR, Plehn JF, White HD, Baker J, Cobbe SM, et al. Reduction of stroke events with pravastatin: the prospective pravastatin pooling (PPP) project. Circulation 2001;103:387–92.
Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol 2009;54(25):2358–62.
Kühnast S, van der Hoorn JWA, Pieterman EJ, van den Hoek AM, Sasiela WJ, Gusarova V, et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res 2014;55:2103–12.
Foo SY, Heller ER, Wykrzykowska J, Sullivan ChJ, Manning-Tobin JJ, Moore KJ, et al. Vascular effects of a low-carbohydrate high-protein diet. Proc Natl Acad Sci U S A 2009;106(36):15418–23.
Kostogrys RB, Franczyk-Żarów M, Maślak E, Gajda M, Mateuszuk Ł, Jackson CL, et al. Low carbohydrate, high protein diet promotes atherosclerosis in apolipoprotein E/low-density lipoprotein receptor double knockout mice (apoE/LDLR−/−). Atherosclerosis 2012;223:327–33.
Wang J, Geng Y-J, Guo B, Klima T, Lal BN, Willerson JT, et al. Near-infrared spectroscopic characterization of human advanced atherosclerotic plaques. J Am Coll Cardiol 2002;39(8):1305–13.
Caplan JD, Waxman S, Nesto RW, Muller JE. Near-infrared spectrscopy for the detection of vulnerable coronary artery plaques. J Am Coll Cardiol 2006;47(8): C92–6.
Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging 2008;1(5): 638–48.
Wrobel TP, Majzner K, Baranska M. Protein profile in vascular wall of atherosclerotic mice analyzed ex vivo using FT-IR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 2012;96:940–5.
Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in knockout mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci U S A 1994;91:4431–5.
Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51.
Lloyd DJ, Helmering J, Kaufman SA, Turk J, Silva M, Vasquez S, et al. A volumetric method for quantifying atherosclerosis in mice by using microCT: comparison to en face. PLoS One 2011;6(April (4)):18800, doi:https://doi.org/10.1371/journal.pone.0018800.
Rosenfeld ME, Carson KG, Johnson JL, Williams H, Jackson CL, Schwartz SM. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep 2002;4(3):238–42.
Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler Thromb Vasc Biol 2003;23(8): 1449–54.
Schroeter MR, Humboldt T, Schäfer K, Konstantinides S. Rosuvastatin reduces atherosclerotic lesions and promotes progenitor cell mobilisation and recruitment in apolipoprotein E knockout mice. Atherosclerosis 2009;205: 63–73.
Nachtigal P, Pospisilova N, Jamborova G, Pospechova K, Solichova D, Andrys C, et al. Atorvastatin has hypolipidemic and anti-inflammatory effects in apoE/ LDL receptor-double-knockout mice. Life Sci 2008;82(13–14):708–17.
Kuhnast S, Louwe MC, Heemskerk MM, Pieterman EJ, van Klinken JB, van den Berg SAA, et al. Niacin reduces atherosclerosis development in APOE*3Leiden. CETP mice mainly by reducing NonHDL cholesterol. PLoS One 2013;8(June (6)):e66467, doi:https://doi.org/10.1371/journal.pone.0066467.
Kwak BR, Veillard N, Pelli G, Mulhaupt F, James RW, Chanson M, et al. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation 2003;107(7):1033–9.
Dunoyer-Geindre S, Kwak BR, Pelli G, Roth I, Satta N, Fish RJ, et al. Immunization of LDL receptor-deficient mice with beta2-glycoprotein 1 or human serum albumin induces a more inflammatory phenotype in atherosclerotic plaques. Thromb Haemost 2007;97(1):129–38.
Zhou X, Li D, Yan W, Li W. Pravastatin prevents aortic atherosclerosis via modulation of signal transduction and activation of transcription 3 (STAT3) to attenuate interleukin-6 (IL-6) action in ApoE knockout mice. Int J Mol Sci 2008;9(11):2253–64.
Paumelle R, Staels B. Peroxisome proliferator-activated receptors mediate pleiotropic actions of statins. Circ Res 2007;100(10):1394–405.
Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator- activated receptors and cancers: complex stories. Nat Rev Cancer 2004;4(1):61–70.
Prinz V, Endres M. Statins and stroke: prevention and beyond. Curr Opin Neurol 2011;24(1):75–80.
Bruder-Nascimento T, Callera GE, Montezano AC, He Y, Antunes TT, Cat AN, et al. Vascular injury in diabetic db/db mice is ameliorated by atorvastatin: role of Rac1/2-sensitive nox-dependent pathways. Clin Sci (Lond) 2015;128(7):411–23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kostogrys, R.B., Franczyk-Zarow, M., Gasior-Glogowska, M. et al. Anti-atherosclerotic effects of pravastatin in brachiocephalic artery in comparison with en face aorta and aortic roots in ApoE/LDLR−/− mice. Pharmacol. Rep 69, 112–118 (2017). https://doi.org/10.1016/j.pharep.2016.09.014
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2016.09.014