Abstract
Background
Antiarrhythmic and antiepileptic drugs share some mechanisms of actions. Therefore, possibility of interactions between these in epileptic patients with cardiac arrhythmias is quite considerable. Herein, we attempted to assess interactions between propafenone and four conventional antiepileptic drugs: carbamazepine, valproate, phenytoin and phenobarbital.
Methods
Effects of propafenone on seizures were determined in the electroconvulsive threshold test in mice. Interactions between propafenone and antiepileptic drugs were estimated in the model of maximal electroshock. Motor coordination was evaluated in the chimney test, while long-term memory in the passive-avoidance task. Brain concentrations of antiepileptics were determined by fluorescence polarization immunoassay.
Results
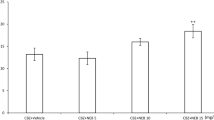
Propafenone up to 50 mg/kg did not affect the electroconvulsive threshold, significantly enhancing this parameter at doses of 60–90 mg/kg. Applied at its subthreshold doses, propafenone potentiated the antielectroshock action of all four tested classical antiepileptics: carbamazepine, valproate, phenytoin, and phenobarbital. Propafenone alone and in combinations with antiepileptics impaired neither motor performance nor long-term memory in mice. Propafenone did not change brain concentration of phenytoin and phenobarbital; however, it significantly decreased brain levels of carbamazepine and increased those of valproate.
Conclusions
Propafenone exhibits its own anticonvulsant effect and enhances the action of classical antiepileptic drugs against electrically induced convulsions in mice. Further investigations are required to determine the effect of propafenone on antiepileptic therapy in humans.
Similar content being viewed by others
References
Scholz H. Classification and mechanism of action of antiarrhythmic drugs. Fundam Clin Pharmacol 1994;8(5):385–90.
von Philipsborn G, Gries J, Hofmann HP, Kreiskott H, Kretzschmar R, Müller CD, et al. Pharmacological studies on propafenone and its main metabolite 5-hydroxypropafenone. Arzneimittelforschung 1984;34(11):1489–97.
D’Orazio JL, Curtis JA. Overdose of propafenone surreptitiously sold as Percocet. J Emerg Med 2011;41(2):172–5.
Borowicz KK, Banach M. Antiarrhythmic drugs and epilepsy. Pharmacol Rep 2014;66(4):545–51.
Benarroch EE. HCN channels: function and clinical implications. Neurology 2013;80(3):304–10.
Roubille F, Tardif JC. New therapeutic targets in cardiology, heart failure and arrhythmia: HCN channels. Circulation 2013;127(19):1620–9.
Lipkind GM, Fozzard HA. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol Pharmacol 2010;78(4):631–8.
Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol 2010;9(4):413–24.
Farber NB, Jiang XP, Heinkel C, Nemmers B. Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol Psychiatry 2002;7(7):726–33.
Jallon P. Epilepsy and the heart. Rev Neurol (Paris) 1997;153(3):173–84.
Castel-Branco MM, Alves GL, Figueiredo IV, Falcão AC, Caramona MM. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drug. Methods Find Exp Clin Pharmacol 2009; 31(2):101–6.
Borowicz KK, Banach M, Zarczuk R, íukasik D, íuszczki JJ, Czuczwar SJ. Acute and chronic treatment with mianserin differentially affects the anticonvulsant activity of conventional antiepileptic drugs in the mouse maximal electroshock model. Psychopharmacology 2007;195(2):167–74.
Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 1949;96(2):99–113.
Boissier JR, Tardy J, Diverres JC. Une nouvelle methode simple pour explorer l’action tranquilisante: le test de la cheminee. Med Exp (Basel) 1960;3:81–4.
Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2015;3:CD005049.
Chimienti M, Cullen Jr. MT, Casadei G. Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmias. Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. Eur Heart J 1995;16(12):1943–51.
Ito N, Tashiro T, Morishige N, Nishimi M, Hayashida Y, Takeuchi K, et al. Efficacy of propafenone hydrochloride in preventing postoperative atrial fibrillation after coronary artery bypass grafting. Heart Surg Forum 2010; 13(4):E223–7.
Kowey PR, Yannicelli D, Investigators Amsterdam ECOPPA-II. Effectiveness of oral propafenone for the prevention of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 2004;94(5):663–5.
Meinertz T, Lip GY, Lombardi F, Sadowski ZP, Kalsch B, Camez A, et al. ERAFT Investigators. Efficacy and safety of propafenone sustained release in the prophylaxis of symptomatic paroxysmal atrial fibrillation (The European Rythmol/Rytmonorm Atrial Fibrillation Trial [ERAFT] Study). Am J Cardiol 2002;90(12):1300–6.
Zehender M, Hohnloser S, Geibel A, Furtwangler A, Olschewski M, Meinertz T, et al. Short-term and long-term treatment with propafenone: determinants determinants of arrhythmia suppression, persistence of efficacy, arrhythmogenesis, and side effects in patients with symptoms. Br Heart J 1992;67(6): 491–7.
Gulizia M, Mangiameli S, Orazi S, Chiarandà G, Piccione G, Di Giovanni N, et al. PITAGORA Study Investigators. A randomized comparison of amiodarone and class IC antiarrhythmic drugs to treat atrial fibrillation in patients paced for sinus node disease: the Prevention Investigation and Treatment: A Group for Observation and Research on Atrial arrhythmias (PITAGORA) trial. Am Heart J 2008;155(1):100–7.
Fernández J, Lligoña L, Puigdemont A, Guitart R, Riu JL, Arboix M. Tissue distribution of propafenone in the rat after intravenous administration. Eur J Drug Metab Pharmacokinet 1991;16(1):23–7.
Latini R, Marchi S, Riva E, Cavalli A, Cazzaniga MG, Maggioni AP, et al. Distribution of propafenone and its active metabolite, 5-hydroxypropafenone, in human tissues. Am Heart J 1987;113(3):843–4.
Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia 1995;36(Suppl 2):S2–12.
Preissner S, Kroll K, Dunkel M, Senger C, Goldsobel G, Kuzman D, et al. SuperCYP: a comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res 2010;38:D237–43. Database issue.
Zhou SF, Zhou ZW, Yang LP, Cai JP. Substrates, inducers, inhibitors and structure- activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem 2009;16(27):3480–675.
Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2006;2(6):875–94.
Reagan-Shaw S, Nihal M, Ahmad N. Dos translation from animal to human studies revisited. FASEB J 2007;22(3):659–62.
Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011;20(5):359–68.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banach, M., Piskorska, B. & Borowicz-Reutt, K.K. Propafenone enhances the anticonvulsant action of classical antiepileptic drugs in the mouse maximal electroshock model. Pharmacol. Rep 68, 555–560 (2016). https://doi.org/10.1016/j.pharep.2016.01.002
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2016.01.002