Abstract
Background
We have shown previously that epicutaneous (EC) immunization with protein antigen induces T suppressor cells that alleviate inflammatory response in contact hypersensitivity reactions, in an animal model of multiple sclerosis, and in TNBS-induced colitis.
Methods
DBA/1 mice were EC immunized with type II collagen (COLL II) spread over a gauze patch on days 0 and 4. On day 7, patches were removed and mice were intradermally (id) immunized with COLL II in CFA to induce collagen-induced arthritis (CIA).
Results
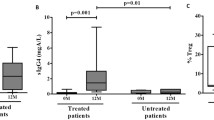
Our work shows that EC immunization with 100 μg of COLL II prior to CIA induction reduces disease severity as determined by macroscopic evaluation. Reduced disease severity after EC immunization with COLL II correlates with milder histological changes found in joint sections. Experiments with the three non-cross-reacting antigens COLL II, ovalbumin (OVA) and myelin basic protein (MBP) showed that skin-induced suppression is antigen non-specific. Transfer experiments show that EC immunization with COLL II induces suppressor cells that belong to the population of CD4+ CD8+ double positive lymphocytes. Flow cytometry experiments showed increased percentage of CD4+ CD8+ RORγt+ cells in axillary and inguinal lymph nodes isolated from mice patched with COLL II.
Conclusion
Maneuver of EC immunization with a protein antigen that induces suppressor cells to inhibit inflammatory responses may become an attractive, noninvasive, needle-free therapeutic method for different clinical situations.
Similar content being viewed by others
Abbreviations
- Ab:
-
antibody
- ALNC:
-
axillary and inguinal lymph node cells
- anti-CPP:
-
Anti-cyclic citrullinated peptide
- CFA:
-
complete Freund’s adjuvants
- CHS:
-
contact hypersensitivity reactions
- CIA:
-
collagen-induced arthritis
- COLL II:
-
type II collagen
- EAE:
-
experimental autoimmune encephalomyelitis
- EC:
-
epicutaneous
- FBS:
-
fetal bovine serum
- id :
-
intradermally
- IFA:
-
incomplete Freund’s adjuvants
- Ig:
-
immunoglobulin
- IL:
-
interleukin
- ip :
-
intraperitoneally
- iv :
-
intravenous
- LPS:
-
lipopolysaccharide
- MBP:
-
myelin basic protein
- MPO:
-
myeloperoxidase
- NK:
-
natural killer
- NKT:
-
natural killer T cell
- OVA:
-
ovalbumin
- PBS:
-
phosphate buffered saline
- RA:
-
rheumatoid arthritis
- RC:
-
rabbit complement
- TGF-β:
-
transforming growth factor beta
- Tc:
-
T cytotoxic cell
- Th:
-
T helper cell
- TNBS-induced colitis:
-
the 2,4,6-trinitrobenzene sulfonic acid-induced colitis
- TNP-Ig:
-
TNP conjugated mouse immunoglobulins
- Ts:
-
T suppressor cell
References
Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005;4:130–6.
Chapel H, Haeney M, Misbah S, Snowden N. Essential of clinical immunology, 10. Oxford, United Kingdom: Blackwell Publishing Ltd.; 2006. p. 178–200.
Rap P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 2008;11: 81–110.
Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol 2009;5:531–41.
Mackenzie NM. New therapeutics that treat rheumatoid arthritis by blocking T-cell activation. Drug Discov Today 2006;11:952–6.
Szczepanik M, Bryniarski K, Tutaj M, Ptak M, Skrzeczynska J, Askenase PW, et al. Epicutaneous immunization induces αβ T-cell receptor CD4 CD8 double-positive non-specific suppressor T cells that inhibit contact sensitivity via transforming growth factor-β. Immunology 2005;115:42–54.
Ptak W, Szczepanik M, Bryniarski K, Tutaj M, Ptak M. Epicutaneous application of protein antigens incorporated into cosmetic cream induces antigen-nonspecific unresponsiveness in mice and affects the cell-mediated immune response. Int Arch Allergy Immunol 2002;128:8–14.
Majewska-Szczepanik M, Zemelka-Wiacek M, Ptak W, Wen L, Szczepanik M. Epicutaneous immunization with DNP-BSA induces CD4+ CD25+ Treg cells that inhibit Tc1-mediated CS. Immunol Cell Biol 2012;90:784–95.
Zemelka-Wiącek M, Majewska-Szczepanik M, Ptak W, Szczepanik M. Epicutaneous immunization with protein antigen induces antigen-non-specific suppression of CD8T cell mediated contact sensitivity. Pharmacol Rep 2012;64:1485–96.
Majewska-Szczepanik M, Strzępa A, Dorożyńska I, Motyl S, Banach T, Szczepanik M. Epicutaneous immunization with hapten-conjugated protein antigen alleviates contact sensitivity mediated by three different types of effector cells. Pharmacol Rep 2012;64:919–26.
Szczepanik M, Tutaj M, Bryniarski K, Dittel BN. Epicutaneously induced TGF-beta-dependent tolerance inhibits experimental autoimmune encephalomyelitis. J Neuroimmunol 2005;164:105–14.
Tutaj M, Szczepanik M. Epicutaneous (EC) immunization with myelin basic protein (MBP) induces TCRαβ+ CD4+ CD8+ double positive suppressor cells that protect from experimental autoimmune encephalomyelitis (EAE). J Autoimmun 2007;28:208–15.
Majewska M, Zając K, Kubera M, Bryniarski K, Ptak M, Basta-Kaim A, et al. Effect of ovoalbumin on the survival of an H-Y incompatible skin graft in C57BL/6 mice. Pharmacol Rep 2006;58:439–42.
Majewska-Szczepanik M, Góralska M, Marcińska K, Zemelka-Wiącek M, Strzępa A, Dorożyńska I, et al. Epicutaneous immunization with protein antygen TNP-lg alleviates TNBS-induced colitis in mice. Pharmacol Rep 2012;64:1497–504.
Marcińska K, Majewska-Szczepanik M, Maresz K, Szczepanik M. Epicutaneous immunization with collagen induces TCRαβ T suppressor cells that inhibit collagen-induced arthritis. Int Arch Allergy Immunol 2015;166:121–34.
Szczepanik M, Lewis J, Geba GP, Ptak W, Askenase PW. Positive regulatory gamma delta T cells in contact sensitivity: augmented responses by in vivo treatment with anti-gamma delta monoclonal antibody, or anti-V gamma 5 or V delta 4. Immunol Invest 1998;27:1–15.
Juryńczyk M, Walczak A, Jurewicz A, Jesionek-Kupnicka D, Szczepanik M, Selmaj K. lmmune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol 2010;68:593–601.
Walczak A, Siger M, Ciach A, Szczepanik M, Selmaj K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol 2013;70: 1105–9.
Hoyne GF, Lamb JR. Regulation of T cell function in mucosal tolerance. Immunol Cell Biol 1997;75:197–201.
Stingl G, Koning F, Hamada H, Yokoyama WM, Tschachler E, Bluestone JA, et al. Thy-1+ dendritic epidermal cells express T3 antigen and the T-cell receptor gamma chain. Proc Natl Acad Sci USA 1987;84:4586–90.
Chen Y, Inobe JI, Kuchroo VK, Baron JL, Janeway CA, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA 1996;93:388–91.
Szczepanik M. Skin-induced tolerance as a new needle free therapeutic strategy. Pharmacol Rep 2014;66:192–7.
Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanism and therapeutic potential. Clin Sci (Lond) 2012;122:487–511.
Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17 deficient mice. J Immunol 2003;171: 6173–7.
Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for spontaneous development of destructive arthritis in mice deficient in IL-1 receptor agonist. Proc Natl Acad Sci USA 2003;100:5986–90.
He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA 2007;104:15817–22.
Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggrevates dextran sulfate sodium-induced colitis in mice. Clin Immunol 2004;110:55–62.
Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med 2006;203:2715–25.
Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 2013;121:2647–58.
Afzali B, Mitchell PJ, Edozie FC, Povoleri GAM, Dowson SE, Demandt L, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT-3- dependent manner. Eur J Immunol 2013;43:2043–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marcińska, K., Majewska-Szczepanik, M., Lazar, A. et al. Epicutaneous (EC) immunization with type II collagen (COLL II) induces CD4+ CD8+ T suppressor cells that protect from collagen-induced arthritis (CIA). Pharmacol. Rep 68, 483–489 (2016). https://doi.org/10.1016/j.pharep.2015.11.004
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2015.11.004