Abstract
Background
From a theoretical point of view, cholecalciferol (vitamin D3) as a precursor of calcitriol, a representative of secosteroids, may have neuroprotective properties and affect seizure phenomena.
Methods
In the present study, interactions between cholecalciferol and three second generation antiepileptic drugs (oxcarbazepine, lamotrigine, and topiramate) were studied in the maximal electroshock test in mice. Effects of drugs on motor coordination, long-term memory and explorative behavior of animals were evaluated in the chimney test, passive-avoidance task and plus-maze test, respectively.
Results
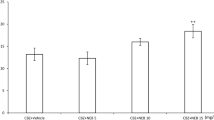
Cholecalciferol applied ip at doses of 37.5–75 μg/kg significantly raised the electroconvulsive threshold. Cholecalciferol, administered at the subthreshold dose of 18.75 μg, potentiated the anticonvulsant activity of oxcarbazepine and lamotrigine, but did not change their brain concentrations, therefore the revealed interactions seem to be pharmacodynamic. Furthermore, the action of cholecalciferol was not dependent on its conversion to calcitriol. The anticonvulsant effect of topiramate was enhanced by cholecalciferol applied at the higher dose of 37.5 μg/kg, at which it also increased the brain level of topiramate. As regards adverse effects, cholecalciferol, antiepileptic drugs, and their combinations did not significantly impair motor coordination or long-term memory in mice. Moreover, cholecalciferol did not show either anxiolytic or anxiogenic properties.
Conclusion
Our findings show that cholecalciferol has not only its own anticonvulsant action but also enhances efficacy of certain antiepileptic drugs, at least in experimental conditions.
Similar content being viewed by others
References
Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D3. Biochemistry (Moscow) 2004;69(7):738–41.
Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13(3):100–5.
Teagarden DL, Meador KJ, Loring DW. Low vitamin D levels are common in patients with epilepsy. Epilepsy Res 2014;108(8):1352–6.
Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology 1998;135(4):319–23.
Gupta MM, Grover DN. Hypocalcemia and convulsions. Postgrad Med J 1977;53(620):330–3.
Holló A, Clemens Z, Lakatos P. Epilepsy vitamin D. Int J Neurosci 2014;124(6): 387–93.
McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res 1999;40(3):173–7.
McGrath J. Does ‘imprinting’ with low prenatal vitamin D contributes to the risk of various adult disorders? Med Hypotheses 2001;56(3):367–71.
Janjoppi L, Katayama MH, Scorza FA, Folgueira MA, Brentani M, Pansani AP, et al. Expression of vitamin D receptor mRNA in the hippocampal formation of rats submitted to a model of temporal lobe epilepsy induced by pilocarpine. Brain Res Bull 2008;76(5):480–4.
Kalueff AV, Minasyan A, Keisala T, Kuuslahti M, Miettinen S, Tuohimaa P. Increased severity of chemically induced seizures in mice with partially deleted vitamin D receptor gene. Neurosci Lett 2006;394(1):69–73.
Siegel A, Malkowitz L, Moscowitz MJ, Christakos S. Administration of 1,25-hydroxyvitamin D3 results in the elevation of hippocampal seizure threshold levels in rats. Brain Res 1984;298(1):125–9.
Kalueff AV, Minasyan A, Tuohimaa P. Anticonvulsant effects of 1,25-dihydroxyvitamin D in chemically induced seizures in mice. Brain Res Bull 2005;67(1– 2):156–60.
Holló A, Clemens Z, Kamondi A, Lakatos P, Szűcs A. Correction of vitamin D deficiency improves seizure control in epilepsy: a pilot study. Epilepsy Behav 2012;24(1):131–3.
Borowicz KK, Morawska M, Furmanek-Karwowska K, Luszczki JJ, Czuczwar SJ. Cholecalciferol enhances the anticonvulsant effect of conventional antiepileptic drugs in the mouse model of maximal electroshock. Eur. J. Pharmacol. 2007;573(1–3):111–5.
Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007;4(1):18–61.
Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 1949;96(2):99–113.
Boissier JR, Tardy J, Diverres JC. Une nouvelle methode simple pour explorer l’action tranquilisante: le test de la cheminee. Med Exp (Basel) 1960;3:81–4.
Venault P, Chapouthier G, De Carvalho LP, Simiand J, Morre M, Dodd RH, et al. Benzodiazepines impair and beta-carbolines enhance performance in learning and memory tasks. Nature 1986;321(6073):864–6.
Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. JoVE 2008;22(22):1088–91.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2007;22(3):659–61.
Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev 2008;13(1):6–20.
Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM, et al. hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001;21(1): 98–108.
Nguyen TM, Lieberherr M, Fritsch J, Guillozo H, Alvarez ML, Fitouri Z, et al. The rapid effects of 1,25-dihydroxyvitamin D3 require the vitamin D receptor and influence 24-hydroxylase activity: studies in human skin fibroblasts bearing vitamin D receptor mutations. J Biol Chem 2004;279(9):7591–7.
Norman AW, Henry HL, Bishop JE, Song X, Bula C, Okamura AW. Different shapes of the steroid hormone 1α,25(OH)2-vitamin D3 act as agonists for two different receptors in the vitamin D endocrine system to mediate genomic and rapid responses. Steroids 2001;66(3–5):147–58.
Zanatta L, Goulart PB, Gonçalves R, Pierozan P, Winkelmann-Duarte EC, Woehl VM, et al. 1α,25-dihydroxyvitamin D(3) mechanism of action: modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta 2012;1823(10):1708–19.
Boland R, De Boland AR, Buitrago C, Morelli S, Santillán G, Vazquez G, et al. Non-genomic stimulation of tyrosine phosphorylation cascades by 1,25(OH)(2)D(3) by VDR-dependent and -independent mechanisms in muscle cells. Steroids 2002;67(6):477–82.
Doroudi M, Olivares-Navarrete R, Hyzy SL, Boyan BD, Schwartz Z. Signaling components of the 1α,25(OH)2D3-dependent Pdia3 receptor complex are required for Wnt5a calcium-dependent signaling. Biochim Biophys Acta 2014;1843(11):2365–75.
Holick MF, Trvela TE, Holick SA, Schnoes HK, DeLuca HF, Gallagher BM. Synthesis of 1α-hydroxy[6-3H]vitamin D3 and its metabolism to 1α,25-dihydroxy[6-3H]vitamin D3 in the rat. J Biol Chem 1976;251(4):1020–1.
Kułak W, Sobaniec W, Wojtal K, Czuczwar SJ. Calcium modulation in epilepsy. Pol J Pharmacol 2004;56(1):29–41.
Mintzer S, Boppana P, Toguri J, DeSantis A. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia 2006;47(3):510–5.
Burne THJ, McGratf JJ, Eyles DW, Mackay-Sim A. Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res 2005;157(2): 299–308.
Foreman MM, Hanania T,Eller M. Anxiolytic effect sof lamotrigine and JZP-4 in the elevated plus maze and in the four plate conflict test. Eur J Pharmacol 2009;602(2–3):316–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borowicz, K.K., Morawska, D. & Morawska, M. Effect of cholecalciferol on the anticonvulsant action of some second generation antiepileptic drugs in the mouse model of maximal electroshock. Pharmacol. Rep 67, 875–880 (2015). https://doi.org/10.1016/j.pharep.2015.01.012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2015.01.012