Abstract
Study Design
Cadaveric study.
Objective
To establish the safety and efficacy of magnetically controlled growing rods (MCGRs) after magnetic resonance imaging (MRI) exposure.
Summary of Background Data
MCGRs are new and promising devices for the treatment of early-onset scoliosis (EOS). A significant percentage of EOS patients have concurrent spinal abnormalities that need to be monitored with MRI. There are major concerns of the MRI compatibility of MCGRs because of the reliance of the lengthening mechanism on strongly ferromagnetic actuators.
Methods
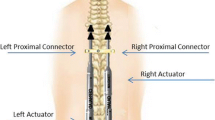
Six fresh-frozen adult cadaveric torsos were used. After thawing, MRI was performed four times each: baseline, after implantation of T2–T3 thoracic rib hooks and L5–S1 pedicle screws, and twice after MCGR implantation. Dual MCGRs were implanted in varying configurations and connected at each end with cross connectors, creating a closed circuit to maximize MRI-induced heating. Temperature measurements and tissue biopsies were obtained to evaluate thermal injury. MCGRs were tested for changes to structural integrity and functionality. MRI images obtained before and after MCGR implantation were evaluated.
Results
Average temperatures increased incrementally by 1.1°C, 1.3°C, and 0.5°C after each subsequent scan, consistent with control site temperature increases of 1.1°C, 0.8°C, and 0.4°C. Greatest cumulative temperature change of +3.6°C was observed adjacent to the right-sided actuator, which is below the 6°C threshold cited in literature for clinically detectable thermal injury. Histologic analysis revealed no signs of heat-induced injury. All MCGR actuators continued to function properly according to the manufacturer’s specifications and maintained structural integrity. Significant imaging artifacts were observed, with the greatest amount when dual MCGRs were implanted in standard/offset configuration.
Conclusions
We demonstrate minimal MRI-induced temperature change, no observable thermal tissue injury, preservation of MCGR-lengthening functionality, and no structural damage to MCGRs after multiple MRI scans. Expectedly, the ferromagnetic actuators produced substantial MR imaging artifacts.
Level of Evidence
Level V.
Similar content being viewed by others
References
Yang S, Andras LM, Redding GJ, Skaggs DL. Early-onset scoliosis: a review of history, current treatment, and future directions. Pediatrics 2016:137.
Cunin V. Early-onset scoliosis: current treatment. Orthop Traumatol Surg Res 2015;101:S109–18.
Robinson CM, McMaster MJ. Juvenile idiopathic scoliosis. Curve patterns and prognosis in one hundred and nine patients. J Bone Joint Surg Am 1996;78:1140–8.
Jenks M, Craig J, Higgins J, et al. The MAGEC system for spinal lengthening in children with scoliosis: a NICE Medical Technology Guidance. Appl Health Econ Health Policy 2014;12:587–99.
Hickey BA, Towriss C, Baxter G, et al. Early experience of MAGEC magnetic growing rods in the treatment of early onset scoliosis. Eur Spine J 2014;23(Suppl 1):S61–5.
La Rosa G, Oggiano L, Ruzzini L. Magnetically controlled growing rods for the management of early-onset scoliosis: a preliminary report. J Pediatr Orthop 2017;37:79–85.
Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am 2010;92:2533–43.
Akbarnia BA, Cheung K, Noordeen H, et al. Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine (Phila Pa 1976) 2013;38:665–70.
Wang S, Zhang J, Qiu G, et al. Dual growing rods technique for congenital scoliosis: more than 2 years outcomes: preliminary results of a single center. Spine (Phila Pa 1976) 2012;37:E1639–44.
Takaso M, Moriya H, Kitahara H, et al. New remote-controlled growing-rod spinal instrumentation possibly applicable for scoliosis in young children. J Orthop Sci 1998;3:336–40.
Budd HR, Stokes OM, Meakin J, et al. Safety and compatibility of magnetic-controlled growing rods and magnetic resonance imaging. Eur Spine J 2016;25:578–82.
Eroglu M, Demirkiran G, Kocyigit IA, et al. Magnetic Resonance imaging safety of magnetically controlled growing rods in an in vivo animal model. Spine 2017;42:E504–8.
Gupta P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Is a magnetic resonance image screening necessary? Spine (Phila Pa 1976) 1998;23:206–10.
Dempsey MF, Condon B, Hadley DM. Investigation of the factors responsible for burns during MRI. J Magn Reson Imaging 2001;13: 627–31.
Marshman LA, Strong G, Trewhella M, et al. Minimizing ferromagnetic artefact with metallic lumbar total disc arthroplasty devices at adjacent segments: technical note. Spine (Phila Pa 1976) 2010;35:252–6.
Talbot BS, Weinberg EP. MR imaging with metal-suppression sequences for evaluation of total joint arthroplasty. Radiographics 2016;36:209–25.
Suh JS, Jeong EK, Shin KH, et al. Minimizing artifacts caused by metallic implants at MR imaging: experimental and clinical studies. AJR Am J Roentgenol 1998;171:1207–13.
Dannawi Z, Altaf F, Harshavardhana NS, et al. Early results of a remotely-operated magnetic growth rod in early-onset scoliosis. Bone Joint J 2013;95-B:75–80.
Yoon WW, Sedra F, Shah S, et al. Improvement of pulmonary function in children with early-onset scoliosis using magnetic growth rods. Spine (Phila Pa 1976) 2014;39:1196–202.
Cheung KM, Cheung JP, Samartzis D, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet 2012;379:1967–74.
Goldstein LS, Dewhirst MW, Repacholi M, Kheifets L. Summary, conclusions and recommendations: adverse temperature levels in the human body. Int J Hyperthermia 2003;19:373–84.
Gupta A, Subhas N, Primak AN, et al. Metal artifact reduction. Radiol Clin 2015;53:531–47.
Yamazaki M, Ideta T, Kudo S, Nakazawa M. Evaluation of artificial hip joint with radiofrequency heating issues during MRI examination: a comparison between 1.5 T and 3 T. Nihon Hoshasen Gijutsu Gakkai zasshi 2016;72:480–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author disclosures: SP (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study; other from NuVasive, outside the submitted work); YHC (grants from Ellipse Technologies, NuVasive, and Scoliosis Research Society, during the conduct of the study); SFW (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study); AG (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study); RN (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study); TA (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study); Jon-PD (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study); DMW (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study); RCG (grants from Ellipse Technologies, grants from NuVasive, grants from Scoliosis Research Society, during the conduct of the study); DAG (grants from Ellipse Technologies, NuVasive, and the Scoliosis Research Society, during the conduct of the study).
This research was supported by Ellipse Technologies, NuVasive, and Scoliosis Research Society Grants. We gratefully acknowledge the contributions of the Northwell Health Bioskills Education Center for their facilities and support staff, and Meredith Akerman for her statistical support.
Rights and permissions
About this article
Cite this article
Poon, S., Chen, Y.H., Wendolowski, S.F. et al. Cadaveric Study of the Safety and Device Functionality of Magnetically Controlled Growing Rods After Exposure to Magnetic Resonance Imaging. Spine Deform 6, 290–298 (2018). https://doi.org/10.1016/j.jspd.2017.11.003
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jspd.2017.11.003