Abstract
Cupric oxide (CuO) has been comprehensively studied in the field of electrochemistry due to its high Tc-Superconducting property. The present work focus on two different CuO materials i.e. CuO-1 and CuO-2 nanocrystallites which are successfully synthesized from their oxalate and adipate precursors respectively. The calcination temperature for the synthesis of CuO from their precursors is ascertained by TGA analysis of the dicarboxylates. Both the CuO materials are thoroughly characterized by SEM–EDS, XRD, IR and XPS spectroscopic techniques. As a candidate for supercapacitor electrode material, CuO-1/C and CuO-2/C showed a specific capacitance of 4.15 F/g and 22.24 F/g using cyclic voltammetry, 10.4 F/g and 46.6 F/g using GCD curves respectively at a current density of 1 A/g. Also, the CuO-1/C and CuO-2/C showed a specific energy density (Es) 1.59 Wh kg−1 and 0.36 Wh kg−1 at a specific power density (Ps) of 0.02 kW kg−1 and 0.025 kW kg−1 respectively. Moreover, the CuO-2/C exhibits ≈ 96.1% coulombic efficiency following 1000 cycles, whereas, CuO-1/C lags in coulombic efficiency with only 51.8%. As a better candidate, CuO-2/C exhibited excellent rate capability with an outstanding cycling stability of 93.7% retention after 1,000 cycles. The factors contributing to the significant specific capacitance of CuO-2/C along with better stability and reproducibility are its low electrolyte resistance Rs (2.47Ω) and charge transfer resistance Rct (1.01 Ω).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Unsolicited and undesirable ecological impacts imposed by vast utilization of non-renewable fossil fuels have prompted severe concerns among scientific community to widen the renewable and sustainable energy production options along with efficient storage and conversion phenomena [1, 2]. The energy crisis has led to rapid technological developments in the electrochemical energy storage devices like batteries, supercapacitors, fuel cells etc [3, 4].
Though batteries are considered as major electrochemical energy storage system because of their high energy density and specific energy [5, 6] their poor life duration, low specific power and low power density, eco-toxicity, safety and environmental issues limit their practical use. Another low CO2 emission clean energy option i.e. fuel cell can provide able energy conversion rate. However, it has limitations like bulky size, higher installation cost and insufficient fuel storage capability [7].
Nowadays electrochemical energy storage systems like supercapacitors (SCs) have been receiving an immense importance in the energy sector as an environmentally friendly option. In comparison to a battery, SC has a longer life cycle, high power density storage, rapid charge–discharge mechanism and excellent rate capability. Generally, the performance of a SC is dependent on their electrode materials. Carbon-based materials are the most important SC electrode materials owing to their relatively low cost and systematic production process, as well as excellent electrical properties [8]. In present era of technological advancements, the use of SCs for the ‘Internet of Things (IoT) and Artificial Intelligence (AI)’ applications are attracting the researchers all over the world [9].
By considering superior electronic, mechanical and structural properties, the transition metal oxides are extensively studied as SC electrode material affords to add on benefits like large pseudo capacitance, low fabrication cost and high environmental stability [10]. In this framework, a variety of transition metal oxides such as MnO2, NiO, Co3O4, Fe3O4 etc. have been comprehensively studied [11]. Apart from these metal oxides, the cobalt Co(OH)2 based SC electrode materials are noteworthy due to their a high specific capacitance, good electrical conductivity, stability over a wide range of pH and robust cyclability [12,13,14]. In recent years, copper oxides have emerged as a highly promising candidate as a positive electrode material in batteries. The further modifications and hybridization of copper oxide with suitable pseudo-capacitive material to fabricate advanced high energy supercapacitor electrode has become a key research area [15].
Copper oxides can be a promising supercapacitor candidate owing to their low cost, high abundance, non-toxicity and easy shape tailoring at nano-scale. The p-type semiconductors tenorite (CuO) and cuprites (Cu2O) are the two popular forms of copper oxides with tunable band gap energy of 1.22–1.55 eV and 2.0–2.40 eV respectively [16,17,18].
Usually, the electrochemical performance of the nanocomposite electrodes in their supercapacitor cells is measured by using cyclic voltammetry (CV), galvanostatic current charging-discharging (GCD) cycling exercises and electrochemical impedance spectroscopy (EIS) [19, 20].
In scientific literature there are numerous reports on synthesis of CuO for the successful energy storage applications by adopting different synthetic methods like chemical bath deposition (CBD)[21], sol–gel[22], micro-emulsion technique [23], gamma (γ)-irradiation technique [24], microwave irradiation synthesis [25], sonochemical synthesis [26], successive ionic layer adsorption and reaction (SILAR) [27], electro-spinning technique [28], thermal oxidation method [29], spray pyrolysis [30], sputtering techniques [31], electrochemical deposition method [32] and biosynthesis techniques [33]. Moreover, the CuO based materials such as cauliflower-like nano CuO, Cr-doped CuO thin films and CuO-Cu2O@rGO nanocomposites synthesized by chemical deposition [34], radio frequency sputtering [35] and seed-mediated growth [36] techniques respectively have demonstrated the leading role of CuO materials in fabrication of electrodes for SC application. The CuO and the other transition metal oxide-based materials are promising in gas sensing technology. The worldwide researchers have reported the sensing of several gases like CO, petrol vapors (PV), green house gases, methanol, LPG and ethanol by utilizing the sensing materials like Fe2O3 embedded g-C3N4, LaCrO3-TiO2 composite, NiFe2O3, ZnO-CuO composite and Fe modified ZnO nanomaterials. [37,38,39,40,41,42]
Aimable et. al have reported that the precipitation of oxalates as precursors can produce a high-quality CuO with high surface area via the decomposition of copper oxalate precipitate. Also, the thermal decomposition of this precursor involves a small quantity of malachite which can afford a better-quality CuO material. In order to identify the effect of malachite on electrochemical properties of the CuO materials, another dicarboxylic acid i.e. adipic acid can be employed [43].
The present work emphasis on the CuO-1 and CuO-2 nanocrystallites which are prepared by thermal decomposition of their oxalate and adipate precursors respectively. The dicarboxylate precursors and the resultant CuO materials are thoroughly characterized by several sophisticated characterization techniques like TGA, SEM–EDS, XRD, IR and XPS analysis. The synthesized CuO materials have been further used to form composite with activated charcoal and explored as electrode materials for supercapacitor applications wherein the CuO-2 showed comparatively superior performance.
2 Experimental
2.1 Materials
Copper chloride (CuCl2.2H2O), methanol (CH3OH), oxalic acid (C2H2O4) and adipic acid (C6H10O4) used in this work which were of Analytical Reagent (AR) grade procured from SRL Chemicals, India Ltd.
2.2 Synthesis of CuO from dicarboxylate precursors
The dicarboxylate precursors were obtained using oxalic acid and adipic acid independently by dissolving the desired quantity of acids in 50 mL methanol followed by addition of 1.7 g CuCl2.2H2O in each round bottom flask. The reaction mixtures were continuously stirred for 6 h at room temperature using magnetic stirrer. The precipitated dicarboxylate precursors were filtered, washed with methanol and dried under IR lamp. The bluish solid precursors were crushed to fine powders using mortar and pestle. The fine powders were further calcined in quartz crucible for 6 h at 400 °C and 750 °C to obtain CuO-1 and CuO-2 materials respectively.
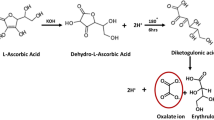
The required amounts of the reagents used to prepare the CuO materials are as summarized in Table 1 and the reactions involved in formation of oxalate and adipate precursors are as shown in Scheme 1.
2.3 Characterization of CuO materials
The Thermogravimetric Analysis (TGA) of CuO precursors was done using Aristan SDT-Q600 V 20.9 Build 20 instrument. The synthesized CuO materials were characterized by using Scanning Electron Micrograph-Energy Dispersive X-ray Spectroscopy (SEM–EDS, JEOL JSM-6360A), powder X-ray diffraction (Bruker D8 Venture Advance X-ray diffractometer with Cu Kα-microfocus and Mo Kα-fine focus), FTIR spectroscopy (Bruker Tensor II) and XPS X-ray Photo Spectrometer (Thermo Fisher Scientific Instruments, UK with X-Ray Source- Al K Alfa, 6 ma beam current and 12 kV).
2.4 Electrochemical studies
The Electrochemical measurements were carried out with the help of a traditional three-electrode system in a potentiostat (IVIUM VERTEX 1A, Netherlands). The working electrodes (WE) were fabricated by mixing the CuO-1 and CuO-2 active materials, acetylene black and PVDF binder in a weight ratio of 8:1:1. Initially, a mortar and pestle were used to mechanically combine the active material, conductive carbon and PVDF binder. Then, a few drops of N-methyl-2-pyrrolidine (NMP) solvent were added and an ink that resembled as slurry was produced by mixing the mixture once more. The ink was screen printed on one side of a dry Carbon paper of 1 cm × 1 cm area. Then carbon paper was dried in an oven at 60 °C for 12 h to evaporate the solvent completely. The resultant material was used as the working electrode and denoted as CuO-1/C and CuO-2/C for further supercapacitor application in combination with Platinum wire as the counter electrode (CE) and a calomel reference electrode (RE) using 1 M Na2SO4 as the supporting electrolyte. The mass deposited on carbon paper was approximately 0.6 mg as determined by an analytical balance. The schematic diagram of a typical three electrode system used for the electrochemical measurements is as shown in Fig. 1.
3 Results and discussion
3.1 Thermogravimetric analysis (TGA)
In order to identify appropriate calcination temperature to obtain the desired monophasic stable CuO materials the oxalate (CuO-1) and adipate (CuO-2) precursors were subjected for TGA. The resultant thermograms obtained by TGA are as shown in Fig. 2.
A typical TGA curve of a copper oxalate (CuO-1) demonstrated a slow mass loss of approximately 0.8% within 50°C and 275 °C attributed to the loss of water content. The further sharp decomposition between 275 °C and 298 °C with 47.4 theoretical weight loss is due to formation of CuO-1 from oxalate precursor. Beyond 298 °C, a marked mass gain is observed, probably due to oxidation by traces of residual air in the furnace [44]. The oxalate precursor showed single stage decomposition and a stable CuO material was seen to be formed around 380 °C. Thus, the TGA analysis of oxalate precursor clearly suggested that 400 °C is a minimum possible temperature of synthesis of CuO-1.
The first stage of decomposition from 50 ℃ to 292 ℃ with 82.2% weight loss involves the dehydration with simultaneous decomposition of Cu adipate complex. In the second stage, beyond 276 ℃ temperature, the formation of stable CuO-2 was found at 641 ℃. This second stage weight loss may be attributed to desorption of the excess carbonate species from the CuO-2 surface. From TGA analysis of CuO-2 precursor, it was clear that the minimum calcination temperature for getting CuO-2 material is 650 °C [45].
3.2 SEM–EDS analysis
The micro-structural and morphological properties of CuO materials were investigated using Scanning Electron Microscopy (SEM) technique. The SEM images of the prepared CuO materials at different magnifications along with EDS spectra are shown in Fig. 3. The CuO-1 nanocrystallite material obtained from oxalate precursor showed fine flakes like morphology. Moreover, the CuO-2 nanocrystallite was found to be obtained in fine fiber like morphology. Thus, dicarboxylic acid has a definite role to play in deciding the morphology of resultant CuO materials. The CuO-2 material was found to be more superior as it can acquire more surface area than the flakes like morphology. The EDS spectra of both CuO-1 and CuO-2 showed presence of only Cu and O elements.
3.3 XRD analysis
The XRD patterns of the prepared CuO-1 and CuO-2 are shown in Fig. 4. The XRD pattern revealed a clear formation of monophasic CuO materials and all the 2θ peaks are in good agreement with monoclinic Cupric Oxide (CuO) structure. The Bragg peaks located at 32.56°, 35.40°, 38.70, 48.82°, 53.58°, 58.44°, 61.40°, 66.35°, 68.10°, 72.52° and 75.30° are representing (110), (−111), (111), (−202), (020), (202), (−113), (−311), (220), (311) and (004) hkl planes respectively. All these 2θ peaks are in perfect agreement with the JCPDS Card No. 80–0076 confirming Monoclinic CuO structure. Further it is clear from the XRD spectra that CuO-2 produced relatively high intensity signals than CuO-1 representing presence of more fine material with lower crystallite size [46].
The average crystallite size of the diffracted CuO-1 and CuO-2 materials was calculated using Debye Scherrer's equation using the full-width at half maximum (FWHM) 2Ɵ values at the highest intensity diffraction peak of the (−111) plane Monoclinic structure [47]. The calculated average crystallite size for the CuO-1 and CuO-2 were found to be 146 nm and 73 nm respectively which revealed that CuO-2 material is obtained with fine nanocrystalline form.
3.4 IR Spectra of CuO materials
The IR spectra of the synthesized CuO materials are shown in Fig. 5.
In the IR spectra of CuO materials, metal oxygen stretching bands prominently observed at 475 cm−1 and 531 cm−1. The IR band at 475 cm−1 and 531 cm−1 are due to the stretching vibration of the Cu–O which is in perfect agreement with CuO nanoparticles reported by Alghamdi et al. (475 cm−1 and 526 cm−1) which confirms the formation of cupric oxide associated with the Cu–O vibration of Monoclinic CuO [48]. Whereas, the IR band at 1129 cm−1 represents symmetric and asymmetric stretching vibrations of the O–C = O of the adsorbed species. This IR band at 1129 cm−1 is in perfect match with the IR band at 1130 cm.−1 reported by Alam et al. for nano CuO [49].
3.5 Electrochemical performance
The Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) analysis are used to examine the electrochemical characteristics of CuO nanocrystallites with a 1 M solution of Na2SO4 as an electrolyte. The morphology of the material can benefit the large surface area and porosity which is essential for material to show significant specific capacity [50]. Herein, the flakes and fiber-like morphology of CuO-1 and CuO-2 played important roles in the electrochemical reaction.
3.5.1 Cyclic voltammetry (CV)
The Fig. 6 displays the CV curves of both CuO-1/C and CuO-2/C working electrodes, measured at a scan rate of 10 mVs−1 at the potential range of −0.3 V to 0.8 V. Both electrode materials exhibited symmetric CV curves in the forward and reverse sweeps with different integrated area. It was clearly observed from CV curves that the transition between the Cu2+ and Cu1+oxidation states of CuO was responsible for the generation of a pair of redox peaks in the voltammogram response. During this electrochemical process, CuO becomes Cu2O by transitioning from its current oxidation state of Cu2+ to Cu1+ during reduction and vice versa for the oxidation process shown by Eq. 1.
Equation 2 was used to compute the specific capacitance (Csp) from the CV curves,
where, “idV” is the current, “R” is the scan rate, “Δʋ” is the potential window and “m” is the mass of the active material.
The maximum specific capacitance (22.24 Fg−1) was achieved with a scan rate of 10 mV/s−1 for the CuO-2/C electrode. (Fig. 6) The achieved specific capacitance was high when compared to the CuO-1/C which comes out to be 4.15 Fg−1 which is six-fold lower in comparison with CuO-2/C. The reason behind the six-fold excess specific capacitance of CuO-2 lies in its fibrous morphology that offers more active surface area which can easily interact with excess supporting electrolyte than the flakes like CuO-1 nanocrystallites.
The fibrous CuO-2 showed an ideal pseudocapacitive behavior that can be confirmed from Fig. 7a–d. It displays cyclic voltammograms at various scan rates between 10 to 250 mVs−1. The increment in scan rate showed a gradual increase in the area of the CV graph along with oxidation and reduction current response demonstrating that the voltammetric current is directly proportional to the scan rate. Here, both of the graphs for oxidation and reduction current response with proportion to scan rate show linear regression coefficient (R2) values (0.98,0.97) and (0.99,0.98) respectively. However, an increase in the scan rate led to a decrease in the specific capacitance as shown in Fig. 8.
The specific capacitances of the both samples with different scan rate (10 to 250 mV s.−1) were calculated according to the CV curves and the results were collected in Fig. 8. This gradual decrease in the specific capacitance on the increment of the scan rate is detailed in Table 2. The decrease in capacitance is attributed to interior active sites that cannot fully support the redox transitions at higher scan rates [51]. This is most likely caused by the proton diffusion effect inside the electrode, indicating that at high scan rates, some part of the electrode surfaces is unreachable [52].
3.5.2 Galvanostatic charge–discharge (GCD):
The specific capacitance Csp (F/g) was also calculated from the galvanostatic charging/discharging (GCD) curve by using Eq. 3. [53]
where i (A) denotes the applied discharging current, Δt (S) is the time during discharging, m (g) is the mass of the deposited electrode material, and ΔV (V) is the potential window.
The galvanostatic charging/discharging curves of the CuO-1/C and CuO-2/C electrodes at a current density of 0.25, 0.5 and 1.0 A/g are shown in Fig. 9. The sample CuO-2/C shows the charging/discharging curves have more symmetrical properties than sample CuO-1/C. This reveals that the sample CuO-2/C has good electrochemical capacitive characteristics and superior reversible Faradaic reactions, implying excellent reversibility.
The specific capacitance of CuO-2/C at 0.25 A/g is as high as 58.07 F/g compared with current densities of 0.5 A/g and 1 A/g which comes out to be 49.8 F/g and 46.6 F/g. Herein, we have reported the specific capacitance of both CuO-1/C and CuO-2/C materials at current densities of 1 A/g and also compare the Csp values by GCD curves in Table 3.
We have further evaluated the energy and power densities of both samples using Eqs. 4 and Eqs. 5 [54].
Energy density, ED \(({Wh kg}^{-1})\)
Similarly, the power density PD \(({kW kg}^{-1})\) units are calculated using:
Here, \({C}_{sp}\) is specific capacitance calculated by the GCD curve, ΔV (V) is the potential window, and Δt (s) is the discharging time.
The coulombic efficiency ηc (%) is calculated at the applied current density of 1A/g using Eq. 6 [54]
Here, tc is the charging time and td is the discharging time.
The CuO-2/C displayed a high energy density of 1.59 Wh kg−1 achieved at a power density of 0.02 kW kg−1 which is better in comparison with CuO-1/C which exhibits a much lower energy density (0.36 Wh kg−1) and power density (0.025 kW kg−1). Moreover, the CuO-2/C exhibits ≈ 96.1% coulombic efficiency following 1000 cycles. Whereas, CuO-1/C lags in coulombic efficiency with only 51.8%. These results demonstrate the high reversibility of charge storage kinetics operating at the CuO-2/C electrode surface.
3.5.3 Electrochemical impedance spectral (EIS) analysis
Electrochemical Impedance Spectral (EIS) analysis is an extremely helpful technique for analyzing the basic behavior of the electrode materials for supercapacitor applications as well as analyzing the charge-transfer mechanism at the electrode/electrolyte contact. EIS measurements were made in the frequency range of 0.1 Hz to 100 kHz at a voltage of 10 mV AC. Figure 10 displays the Nyquist plots of the CuO-1/C and CuO-2/C electrodes, which are represented as a semicircle in the high frequency zone and a straight line in the low-frequency region. Rs stands for the resistance caused by ion transfer at the boundary between electrode material and electrolyte, which corresponds to the high-frequency semicircle's x-axis intercepts. Whereas Rct represents the difficulty in obtaining charge transfer during redox processes, is computed from the semicircle's diameter at high frequencies [55]. The straight line shows Warburg (W) impedance and depicts the diffusion of proton and electrolyte in active materials. As seen from Fig. 10, the semicircle of the Nyquist plot at the high frequency region should be caused by Faradaic reactions between Cu2+/Cu1+ redox couple [56, 57].
Moreover, Fig. 10 displays the Nyquist plot which contains three main regions of frequencies, generally noted as high-frequency, low-frequency and mid-frequency regions. The mid-frequency range reveals how quickly the electrolyte ions travel within the electrode material, whereas the low-frequency range provides the diffusion probability. The higher frequency area offers details on the electrode material's intrinsic resistance as well as the interfacial resistance of the current collector to the electrode material [58]. The obtained Nyquist plot is fitted to an equivalent circuit depicted in the inset of Fig. 10. Rs and Rct values for CuO-1/C and CuO-2/C both were compared and it was found that the CuO-2/C electrode has lower Rs (2.47 Ω) and Rct (1.01 Ω) values confirming a more suitable candidate for supercapacitor. These values for CuO-1/C were 4.33 Ω and 1.33 Ω for Rs and Rct respectively which has less impact. The CuO-2/C electrode has a lower solution resistance value which was caused by unique fiber-like crystallites that partially speed up ion and electron transmission. Meanwhile, the lower Rct value of the CuO-2/C electrode suggested that it has a superior ability to transport ions. All the above impedance data showed that the fiber like CuO-2/C electrode portrays better pseudo-capacitor performance.
3.5.4 Stability and reproducibility studies
Further, to investigate the stability of the CuO-2/C electrode, 1000 repeating cycles in 1 M Na2SO4 were recorded at 50 mVs−1 in the same potential window of earlier experiments. A retention capacity of 93.7% was observed at the 1000th cycle as depicted in Fig. 11, which confirmed the superior stability of the electrode for supercapacitor application.
The Fig. 12 shows the reproducibility of the CuO-2/C electrode which was examined with a series of five electrodes made by uniform procedure and at the same experimental conditions the current response was measured. The % RSD (Relative Standard Deviation) of the current response for five electrodes was found to be 0.18. The reproducibility results demonstrated that the CuO-2/C electrode is consistent in repeatability and it is a superior candidate for the supercapacitor electrode.
3.6 Comparison with the literature reports
The present electrochemical outcomes (CuO-2) are compared with the previous literature reports on CuO and other metal oxide-based materials. In a comprehensive correlation, although our material showed comparatively lower specific capacitance but it has good current density and better specific retention. The detailed compassion of the CuO-2 with previously reported electrode materials is given below in Table 4.
3.7 X-ray photoelectron spectroscopy (XPS) analysis
Finally, the XPS analysis of CuO-2 was performed for the survey of oxidation states Cu and O at sample surface. The XPS spectra of the CuO-2 material is as shown in Fig. 13.
The observed peaks in the complete survey XPS spectrum of CuO-2 designates the presence of only O and Cu species. The peaks positioned at 933.38 eV and 953.58 eV are associated with the 2p3/2 and 2p1/2 levels of Cu respectively. Satellite peaks seemed at the higher energy sides i.e. 943.38 eV and 941.36 eV which are representatives of the Cu 2p3/2. Whereas, the Cu 2p1/2 level is appeared at 961.78 eV ascertaining the presence of Cu in Cu2+ state of CuO. Also, the peak located at 529.35 eV is attributed to the O 1s level from the lattice oxygen (O2−) bound to Cu metal along with the shoulder peak at 531.70 eV resembles to the oxygen (O.2−) in the oxygen-deficient region signifying the existence of the oxygen vacancy [67].
4 Conclusions
The CuO-1 and CuO-2 nanocrystallites with flakes and fiber like morphology were successfully synthesized by calcination of their oxalate and adipate precursors respectively. The TGA studies confirmed the calcination temperatures for monophasic synthesis of CuO-1 and CuO-2 from their precursors as 400 °C and 650 °C respectively. All the XRD peaks of CuO-1 and CuO-2 were in perfect agreement with the JCPDS Card No. 80–0076 confirming Monoclinic CuO structures. The calculated average crystallite size for the CuO-1 and CuO-2 were found to be 146 nm and 73 nm respectively which supports the fine SEM morphology of CuO-2 material.
The IR spectra of the both CuO materials exhibited metal oxygen stretching bands at 475 and 531 cm−1, and carbonate species at surface (1129 cm−1) of only CuO-2 material. The GCD studies demonstrated that the specific capacitance of CuO-2/C is four-fold higher than CuO-1/C. Consequently, a specific capacitance of 46.6 F/g is obtained at a current density of 1 A/g with excellent rate capability by CuO-2/C. It also delivered an energy density of 1.59 Wh kg−1 at a power density of 0.02 kW kg−1 along with 96.1% coulombic efficiency. The EIS analysis showed that the CuO-2/C electrode has a lower Rs and Rct value confirming a more suitable candidate for supercapacitor application. The CuO-2/C electrode showed a retention capacity of 93.7% at the 1000th cycle confirming the superior stability as well as reproducibility for further supercapacitor application. The XPS spectra of CuO-2 exhibited the presence of Cu in Cu2+ oxidation state acerating the formation of monophasic CuO material. Thus, among the dicarboxylate precursors the adipate afforded CuO-2 material with superior electrochemical properties than CuO-1 obtained using oxalate precursor.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Yang H, Yang Y, Guo L. Renewable-biomolecule-based electrochemical energy-storage materials. Adv Energy Mater. 2017;7(23):1700663. https://doi.org/10.1002/aenm.201700663.
Larcher D, Tarascon JM. Towards greener and more sustainable batteries for electrical energy storage. Nat Chem. 2015;17:19–29. https://doi.org/10.1038/nchem.2085.
Winter M, Brodd RJ. What are batteries, fuel cells, and supercapacitors? Chem Rev. 2004;104(10):4245–70. https://doi.org/10.1021/cr020730k.
Sumboja A, Liu J, Zheng WG, Zong Y, Zhang H, Liu Z. Electrochemical energy storage devices for wearable technology: a rationale for materials selection and cell design. Chem Soc Rev. 2018;47:5919–45. https://doi.org/10.1039/C8CS00237A.
Wang F, Wu X, Yuan X, Liu Z, Zhang Y, Fu L, Zhu Y, Zhou Q, Wu Y, Huang W. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev. 2017;46:6816–54. https://doi.org/10.1039/C7CS00205J.
Jayalakshmi M, Balasubramanian K. Simple capacitors to supercapacitors—an overview. Int J Electrochem Sci. 2008;3:1196–217.
Majumdar D, Ghosh SJ. Recent advancements of copper oxide based nanomaterials for supercapacitor applications. Energy Storage. 2021;34: 101995. https://doi.org/10.1016/j.est.2020.101995.
Yadav MS, Sinha AK, Singh MN, Kumar A. Electrochemical study of copper oxide and activated charcoal based nanocomposite electrode for supercapacitor. Mater Today: Proc. 2021;46:5722–9. https://doi.org/10.1016/j.matpr.2021.02.134.
Naeem S, Abid A, Memon K, Bavluwala M, Shinde UP, Patil AV. A review of flexible high-performance supercapacitors for the internet of things (IoT) and artificial intelligence (ai) applications. Energy Thermofluids Eng. 2023;3:1–9.
An C, Zhang Y, Guo H, Wang Y. Metal oxide-based supercapacitors: progress and prospectives. Nanoscale Adv. 2019;1:4644–58. https://doi.org/10.1039/C9NA00543A.
Wu Z, Zhu Y, Ji X, Banks CE. Transition metal oxides as supercapacitor materials. In: Ozoemena KI, Chen S, editors. Nanomaterials in Advanced Batteries and Supercapacitors. Cham: Springer; 2016.
Naeem S, Shaikh AV, Rasool A, Husain D, Alam MT, Patil AV. Enhancing supercapacitor performance through electrodeposition of cobalt hydroxide- thin film: structural analysis, morphological characterization, and investigation of electrochemical properties. Ionics. 2024;30:399–405. https://doi.org/10.1007/s11581-023-05293-4.
Naeem S, Shinde UP, Patil AV. Cobalt hydroxide-based electrodes for supercapacitors: Synthesis, characterization, and electrochemical performance optimization. Energy Storage. 2023. https://doi.org/10.1002/est2.516.
Naeem S, Patil AV, Shaikh AV, Shinde UP, Husain D, Alam MT, Sharma M, Tewari K, Ahmad S, Shah AA, Syed AA, Ahmad A. A review of cobalt—based metal hydroxide electrode for applications in supercapacitors. Adv Mater Sci Eng. 2023;1:1133559.
Xu J, Gu P, Zhang J, Xue H, Pang H. Copper—based nanomaterials for high-performance lithium-ion batteries. Part Part Syst Charact. 2016;33(11):784–810. https://doi.org/10.1002/ppsc.201600150.
Chen A, Long H, Li X, Li Y, Yang G, Lu P. Controlled growth and characteristics of single-phase Cu2O and CuO films by pulsed laser deposition. Vacuum. 2009;83(6):927–30. https://doi.org/10.1016/j.vacuum.2008.10.003.
Wong TKS, Zhuk S, Masudy-Panah S, Dalapati GK. Current status and future prospects of copper oxide heterojunction solar cells. Materials. 2016;9(4):271. https://doi.org/10.3390/ma9040271.
Tomita R, Pu Z, Anpo M, Kamegawa T, Higashimoto S. Photochemical properties of copper oxide (CuO) influenced by work functions of conductive electrodes. Res Chem Intermed. 2019;45:5947–58. https://doi.org/10.1007/s11164-019-04012-x.
Majumdar D, Mandal M, Bhattacharya SK. Journey from supercapacitors to supercapatteries: recent advancements in electrochemical energy storage systems. Emergent Mater. 2020;3:347–67. https://doi.org/10.1007/s42247-020-00090-5.
Yassine M, Fabris D. Performance of commercially available supercapacitors. Energies. 2017;10(9):1340. https://doi.org/10.3390/en10091340.
Jabbar SM. Synthesis of CuO Nano structure via Sol-Gel and precipitation chemical methods. Al-Khwarizmi Eng J. 2016;12:126–31. https://doi.org/10.22153/kej.2016.07.001.
Hench LL, West JK. The sol-gel process. Chem Rev. 1990;90(1):33–72. https://doi.org/10.1021/cr00099a003.
Ahmad T, Chopra R, Ramanujachary KV, Lofland SE, Ganguli AK. Canted antiferromagnetism in copper oxide nanoparticles synthesized by the reverse-micellar route. Solid State Sci. 2005;7(7):891–5. https://doi.org/10.1016/j.solidstatesciences.2004.11.029.
Hai Z, Zhu C, Huang J, Liu H, Chen J. Controllable synthesis of CuO Nanowires and Cu2O crystals with shape evolution via γ-irradiation. Inorg Chem. 2010;49(16):7217–9. https://doi.org/10.1021/ic101143u.
Wang H, Xu JZ, Zhu JJ, Chen HY. Preparation of CuO nanoparticles by microwave irradiation. J Cryst Growth. 2002;244(1):88–94. https://doi.org/10.1016/S0022-0248(02)01571-3.
Suslick KS. Sonochemistry. Science. 1990;247(4949):1439–45. https://doi.org/10.1126/science.247.4949.1439.
Shinde SK, Dubal DP, Ghodake GS, Kim DY, Fulari VJ. Morphological tuning of CuO nanostructures by simple preparative parameters in SILAR method and their consequent effect on superconductors. Nano-Struct. Nano-Objects. 2016;6:5–13. https://doi.org/10.1016/j.nanoso.2016.01.004.
Vidhyadharan B, Misnon II, Aziz RA, Padmasree KP, Yusoff MM, Jose R. Superior supercapacitive performance in electrospun copper oxide nanowire electrodes. J Mater Chem. 2014;A2:6578–88. https://doi.org/10.1039/C3TA15304E.
Liu J, Xue D. Thermal oxidation strategy towards porous metal oxide hollow architectures. Adv Mater. 2008;20(13):2622–7. https://doi.org/10.1002/adma.200800208.
Saravanan V, Shankar P, Mani GK, Bosco J, Rayappan BJ. Growth and characterization of spray pyrolysis deposited copper oxide thin films: Influence of substrate and annealing temperatures. Anal Appl Pyrol. 2015;111:272–7. https://doi.org/10.1016/j.jaap.2014.08.008.
Kelly PJ, Arnell RD. Magnetron sputtering: a review of recent developments and applications. Vacuum. 2000;56(3):159–72. https://doi.org/10.1016/S0042-207X(99)00189-X.
Dhanasekaran V, Mahalingam T, Chandramohan R, Rhee JK, Chu JP. Electrochemical deposition and characterization of cupric oxide thin films. TSF. 2012;520(21):6608–13. https://doi.org/10.1016/j.tsf.2012.07.021.
Nwanya AC, Ndipingwi MM, Mayedwaa N, Razanamahandry LC, Ikpo CO, Waryo T, Ntwampe SKO, Malenga E, Fosso-Kankeu E, Ezema FI, Iwuoha EI, Maaza M. Maize (Zea mays L.)fresh husk mediated biosynthesis of copper oxides: Potentials for pseudo capacitive energy storage. Electrochim. 2019;301:436–48. https://doi.org/10.1016/j.electacta.2019.01.186.
Zhang H, Zhang M. Synthesis of CuO nanocrystalline and their application as electrode materials for capacitors. Mater Chem Phys. 2008;108(2–3):184–7. https://doi.org/10.1016/j.matchemphys.2007.10.005.
Durai G, Kuppusami P, Arulmani S, Anandan S, Shaik KP, Kheawhom S. Microstructural and electrochemical supercapacitive properties of Cr- doped CuO thin films: effect of substrate temperature. J Energy Research. 2021;45(14):20001–15. https://doi.org/10.1002/er.7075.
Poramed W, Wattana T, Attaphol K, Chaiwat P, Sujittra D, Samuk P, Pairot M, Pornjuk S, Chaval S, Chesta RJ. Characterization and electrochemical properties of CuO-Cu2O@rGO nanocomposite synthesized by a seed-mediated growth process. Phys Chem Solids. 2022;163: 110540. https://doi.org/10.1016/j.jpcs.2021.110540.
Koli PB, Birari MD, Ahire SA, Shinde SG, Ingale RS, Jadhav PI. Ferroso-ferric oxide(Fe3O4) embedded g-C3N4 nanocomposite sensor fabricated by photolithographic technique for environmental pollutant gas sensing and relative humidity characteristics. Inorg Chem Commun. 2022;146: 110083. https://doi.org/10.1016/j.inoche.2022.110083.
Shinde VS, Kapadnis KH, Patil AP, Koli PB. Designing of LaCrO3- TiO2 nanocomposites p:n heterojunction-based sensor material for the selective detection of volatile petrol vapors (PV) and CO2 gas vapors. J Indian Chem Soc. 2022;99(3): 100367. https://doi.org/10.1016/j.jics.2022.100367.
Koli PB, Kapadnis KH, Deshpande UG, Tupe UJ, Shinde S, Ingale RS. Fabrication of thin film sensors by spin coating using sol-gel LaCrO3 perovskite material modified with transition metals for sensing environmental pollutants, greenhouse gases and relative humidity. Environ Chall. 2021;3: 100043. https://doi.org/10.1016/j.envc.2021.100043.
Koli PB, Kapadnis KH, Deshpande UG. Nanocrystalline—modified nickel ferrite films: an effective sensor for industrial and environmental gas pollutant detection. J Nanostructure Chem. 2019;9:95–110. https://doi.org/10.1007/s40097-019-0300-2.
Shinde RS, Khairnar SD, Patil MR, Adole VA, Koli PB, Deshmane VV, Halwar DK, Shinde RA, Pawar TB, Jagdale BS, Patil AV. Synthesis and characterization of ZnO/CuO nanocomposites as an effective photocatalyst and gas sensor for environmental remediation. J Inorg Organomet Polym Mater. 2022;32:1045–66. https://doi.org/10.1007/s10904-021-02178-9.
Waghchaure RH, Adole VA, Jagdale BS, Koli PB. Fe3+ modified zinc oxide nanomaterial as an efficient, multifaceted material for photocatalytic degradation of MB dye and ethanol gas sensor as part of environmental rectification. Inorg Chem Commun. 2022. https://doi.org/10.1016/j.inoche.2022.109450.
Aimable A, Torres Puentes A, Bowen P. Synthesis of porous and nanostructured particles of CuO via a copper oxalate route. Powder Technol. 2011;208(2):467–71. https://doi.org/10.1016/j.powtec.2010.08.044.
Lamprecht E, Watkins GM, Brown ME. The thermal decomposition of copper(II) oxalate revisited. Thermochim Acta. 2006;446(1–2):91–100. https://doi.org/10.1016/j.tca.2006.03.008.
Raste MN, Jagtap RM, Pardeshi SK. Optical second harmonic generation studies of pure and glycine-doped bisthiourea copper/cadmium adipates. J Mater Sci: Mater Electron. 2022;33:10785–99. https://doi.org/10.1007/s10854-022-08060-9.
Cheng P, Zheng D, Zhang Y, Chen X, Chen T, Zhang T, Wu F. Enhancing charge separation and photocurrent response of CuO nanocone array by CuO/PC61BM hybrid bulk heterojunction. Materials Lett. 2022;315: 132005. https://doi.org/10.1016/j.matlet.2022.132005.
Jagtap RM, Kshirsagar DR, Khire VH, Pardeshi SK. Facile fabrication of porous La doped granular nanocrystallites and their catalytic evaluation towards thermal decomposition of ammonium perchlorate. J Solid State Chem. 2019;276:194–204. https://doi.org/10.1016/j.jssc.2019.05.001.
AlhusaikiAlghamdi HM. Structural, morphological, optical, and electrical characteristics of polyethylene oxide/chitosan-copper oxide nanoparticles for optoelectronic applic Opt. Mater. 2022;134: 113101. https://doi.org/10.1016/j.optmat.2022.113101.
Alam MW, Aamir M, Farhan M, Albuhulayqah M, Ahmad MM, Ravikumar CR, Dileep Kumar VG, Ananda Murthy HC. Green synthesis of Ni-Cu-Zn based nanosized metal oxides for photocatalytic and sensor applications. Crystals. 2021;11(12):1467. https://doi.org/10.3390/cryst11121467.
Saravanakumar B, Radhakrishnan C, Ramasamy M, Kaliyaperumal R, Britten AJ, Mkandawire M. Surfactant determines the morphology, structure and energy storage features of CuO nanostructures. Results Phys. 2019;13: 102185. https://doi.org/10.1016/j.rinp.2019.102185.
Shinde S, Dhaygude H, Kim DY, Ghodake G, Bhagwat P, Dandge P, Fulari V. Improved synthesis of copper oxide nanosheets and its application in development of supercapacitor and antimicrobial agents. J Ind Eng Chem. 2016;36:116–20. https://doi.org/10.1016/j.jiec.2016.01.038.
Dubal DP, Gund GS, Lokhande CD, Hozle R. Decoration of spongelike Ni(OH)2 nanoparticles onto MWCNTs using an easily manipulated chemical protocol for supercapacitor. Appl Mater Int. 2013;5:2446–54. https://doi.org/10.1021/am3026486.
Mathis TS, Kurra N, Wang X, Pinto D, Simon P, Gogotsi Y. Energy storage data reporting in perspective-guidelines for interpreting the performance of electrochemical energy storage systems. Adv Energy Mater. 2019;9(39):1902007. https://doi.org/10.1002/aenm.201902007.
Wang Y, Song Y, Xi Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev. 2016;45(21):5925–50. https://doi.org/10.1039/C5CS00580A.
Hu X, Nan H, Liu M, Liu S, An T, Tian H. Battery-like MnCo2O4 electrode materials combined with active carbon for hybrid supercapacitors. Electrochim Acta. 2019;306:599–609. https://doi.org/10.1016/j.electacta.2019.03.166.
Ghenaatian HR, Mousavi MF, Rahmanifar MS. High performance hybrid supercapacitor based on two nanostructured conducting polymers: self-doped polyaniline and polypyrrole nanofibers. Electrochim Acta. 2012;78:212–22. https://doi.org/10.1016/j.electacta.2012.05.139.
Li Q, Lu X-F, Xu H, Tong Y-X, Li G-R. Carbon/MnO2 doubled-walled nanotube arrays with fast ion and electron transmission for high-performance supercapacitors. Appl Mater Int. 2014;6(4):2726–33. https://doi.org/10.1021/am405271q.
Angelin MD, Rajkumar S, Merlin JP, Xavier AR, Franklin M, Ravichandran AT. Electrochemical investigation of Zr-doped ZnO nanostructured electrode material for high-performance supercapacitor. Ionics. 2020;26(11):5757–72. https://doi.org/10.1007/s11581-020-0368-8.
Xu J, Wu L, Liu Y, Zhang J, Liu J, Shu S, Kang X, Song Q, Liu D, Huang F, Hu Y. NiO-rGO composite for supercapacitor electrode. Surf Int. 2020;18: 100420. https://doi.org/10.1016/j.surfin.2019.100420.
Guo XL, Li G, Kuang M, Yu L, Zhang YX. Tailoring kirkendall effect of the KCu7S4 microwires towards CuO@MnO2 core-shell nanostructures for supercapacitors. Electrochim Acta. 2015;174:87–92. https://doi.org/10.1016/j.electacta.2015.05.157.
Racik KM, Guruprasad K, Mahendiran M, Madhavan J, Maiyalagan T, Victor Antony Raj M. Enhanced electrochemical performance of MnO2/NiO nanocomposite for supercapacitor electrode with excellent cycling stability. J Mater Sci Mater Electron. 2019;30:5222–32. https://doi.org/10.1007/s10854-019-00821-3.
Zhang Y, Guo WW, Zheng TX, Zhang YX, Fan X. Engineering hierarchical Diatom@CuOr@MnO2 hybrid for high performance supercapacitor. Appl Surf Sci. 2018;427:1158–65. https://doi.org/10.1016/j.apsusc.2017.09.064.
Heng B, Qing C, Sun D, Wang B, Wang H, Tang Y. Rapid synthesis of CuO nanoribbons and nanoflowers from the same reaction system, and a comparison of their supercapacitor performance. RSC Adv. 2013. https://doi.org/10.1039/C3RA42869A.
Zhang YX, Li F, Huang M. One -step hydrothermal synthesis of hierarchical MnO2- coated CuO flower-like nanostructures with enhanced electrochemical properties for supercapacitor. Mater Lett. 2013;112:203–6. https://doi.org/10.1016/j.matlet.2013.09.032.
Patake VD, Joshi SS, Lokhande CD, Oh-Shim J. Electrodeposited porous and amorphous copper oxide film for application in supercapacitor. Mater Chem Phys. 2009;114(1):6–9. https://doi.org/10.1016/j.matchemphys.2008.09.031.
Endut Z, Hamdi M, Basirun WJ. Pseudocapacitive performance of vertical copper oxide nanoflakes. Thin Solid Films. 2013;528:213–6. https://doi.org/10.1016/j.tsf.2012.09.084.
Jan T, Raheem S, Sawant SV, Manolikar TV, Sakate SS, Pardeshi SK, Jagtap RM, Rizvi MA. Photocatalytic evaluation of CuO and ZnO crystallites synthesized hydrothermally using binary eugenol/iso-eugenol mixtures: isomer effects on the capping propensity of biogenic agents. New J Chem. 2024;48(11):5040–59. https://doi.org/10.1039/d3nj05237k.
Acknowledgements
Authors are thankful to ‘Central Sophisticated Analytical Instrumentation Facility’ (CSAIF), Progressive Education Society’s Modern College of Arts, Science and Commerce (Autonomous), Shivajinagar, Pune, 411005, India for providing characterizations of the materials.
Funding
There is no any funding agency to mention for accomplishing this research work.
Author information
Authors and Affiliations
Contributions
R. M. J. conceptualization/design of the research work and data analysis. S. V. S., T. V. M., M. D. B. collected and analysed the experimental data of specified segments. M. D. B., S. V. S. and T. V. M., S. S. S. prepared and formatted the figures/tables. R. M. J. and S. S. S. wrote the manuscript. S. K.P. reviewed and edited the final version of the manuscript. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
No any conflicts of interest/competing interests to mention.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sawant, S.V., Manolikar, T.V., Babar, M.D. et al. Oxalate and adipate mediated fabrication of CuO nanocrystallites for their electrochemical and supercapacitance studies. Discov. Chem. 1, 6 (2024). https://doi.org/10.1007/s44371-024-00006-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44371-024-00006-w