Abstract
The study focuses on developing the poly (vinyl alcohol) (PVA) nanocomposite films reinforced with Halloysite Nano-Tubes (HNT) which were surface functionalised with chitosan forming a Schiff base structure. The work was aimed to traverse its way in tissue engineering. Modification of halloysite with sea polysaccharide chitosan enhanced its ability to bind to the PVA matrix. The modification was assisted by FTIR, XRD, FESEM, TEM, AFM and DSC-TGA techniques. The films could withstand a higher temperature and exhibited high ranges of tensile strength and Young’s modulus. In addition, biocompatible studies such as in-vitro swelling, enzymatic degradation, water contact angle and hemolysis presented extremely well compatibilities proving it to be viable in physiological pH (in phosphate buffered saline). The cell adhesion and proliferation studies conducted on NIH3T3 mouse fibroblasts revealed the cell proliferation and tissue regeneration properties of the films. Both the tests performed for cell growth- Trypan blue dye exclusion and Acridine Orange Ethidium bromide assays showed a doubled rate of cell growth on the films which proves its biomedical nature.
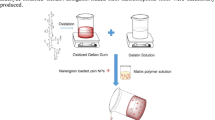
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the past years the world is shortly moving towards the threatful impact of non-biological plastics on living organisms, especially human beings. Many researches have given us the opportunity to shift this perspective to prepare materials with bio-friendliness. This era throws light on the involvement of biologically passive materials such as natural fillers, biopolymers, and many more that construct a possible way to be applied in biomedical field without harming the internal features. Improvement of physical and chemical properties of material is an essential feature for it to be applied in tissue engineering. Biocompatible materials made of polymers generally have the potential for tissue regeneration and are proven to be hemo- compatible as well and find their usages as implants or drug carriers [1]. The short-term behaviour of most of these biomaterials shrunk their sustainability and production.
The poly vinyl alcohol based polymeric materials are widely used as the wound healing scaffolds bearing antibacterial, anti-inflammatory and several other biomedical aspects [2, 3]. To go in an ease the sourcing of compounds that can combat this tiny disability and be enhanced with overall properties is being done worldwide [4, 5].
The naturally abundant aluminosilicate mineral which is explored for its enormous uses in the biomedical field is Halloysite nanotube (HNT) [6]. These tubular nanoclay are extensively functional, have good mechanical properties and most importantly are compatible with the living system. Halloysite nanotubes are naturally present and belong to the family of kaolin. These tubular clay have two different surfaces with layers bearing the inner aluminols and external silanols which are highly susceptible for surface functionalization [7]. The modification of the surface with biocompatible molecules still increases its excellent physiologically adaptable nature. Additionally, halloysite is been a reason for increasing the composite properties in several scaffolds and drug delivery systems made from polymers for wound healing, gene therapy and tissue engineering [8]. A very interesting study on the working of halloysite-carbon dots based multifunctional nano-vector has been the keen focus for gene therapy [9]. These natural fillers have also been known to enhance the intracellular delivery of antitumor agent antisense oligodeoxynucleotides.
The HNT exhibits rich mechanical properties and its incorporation to polymer base increases tensile strength of the bio as well as synthetic polymer in use [10]. This unique character was utilised for several kinds of biological-biomedical applications such as antimicrobial, drug carrier, derma patches, wound healers, bone transplants, biosensors and gene regulators [11,12,13]. A few studies to mention here, the microfluidic assembly of HNT and polymer is used in colon cancer therapy, the release of ofloxacin based on alkali activation and poly (N-isopropylacrylamide) grafted onto HNT for thermo-responsive curcumin release form the basis for its involvement in the in-vivo applications [14, 15]. Hence, HNT with modifiable surface stands as the best nanofillers for reinforcement into any polymer matrix.
In the decade carbohydrates of sea origin are extensively studied for their crucial role in getting adapted to physiological conditions along with being highly available and economic. On turning the pages of recent researches chitin and its derivatives come up with their tremendous usages in the synthesis of biocompatible polymer materials. Among such chitosan leads the group by significantly contributing exciting properties like biodegradability, non-toxicity, biocompatibility and antimicrobial nature for the development of composite materials. Due to the similarity in structure of chitosan to that of glycosaminoglycans (GAGs) which are found to be present in the extra cellular matrix of the cell makes it a promising biopolymer for cell growth applications [16]. Chitosan is been a centre of keen attention for its antimicrobial action due to the presence of positively charged amino group and also showed promising agent for metal binding [17].
Chitosan also seeped its presence in the drug delivery vehicles, gene therapy and many other tissue matrices [18]. This sea polysaccharide is an excellent agent which can be fabricated to any desirable material with other polymers to obtain the scaffolds and hence fits firmly in the biomedical field for multifunctional applications [19]. But, it alone suffers from a low sustainability issue and hence its composite with poly (vinyl alcohol) (PVA) by taking halloysite as a medium for reinforcement would give a better biomaterial [20]. Chitosan along with HNT has much more value because of its reliable applications in tissue regeneration and cell proliferation [21] [22]. It is very evident from the literature that chitosan holds its capacity to be covalently attached to the HNT’s surface and improve its way of incorporation onto the polymer surface in the preparation of polymer nanocomposite films. A well feasible reaction condition can be achieved for this synthesis.
The implantation or close-in action of any biomaterial is a rigorous process which assists much functionality. In this regard researchers believe that use of a biopolymer along with a highly biologically viable synthetic polymer would place a good combination for the preparation of biomaterials which in-turn increases the biodegradability of the polymer. The present research work is designed to modify the HNT’s external surface with chitosan by using a viable method with silane and glutaraldehyde and prepare its nanocomposite films using PVA as a matrix. All the key compounds described above (chitosan, halloysite nanotube and PVA) are thus used to recreate a network with retaining their actual properties. Furthermore, the as synthesised nanocomposite films are believed to show positive cell proliferation and adhesion studies in-vitro. Besides being a biocompatible synthetic polymer, poly (vinyl alcohol) is also eco-friendly and helps in managing medical wastes as well [23]. To our best knowledge the aim of our research work is certain to prepare an improved nanocomposite film which would act as a renewable and retainable biomaterial in all the physiological conditions and generates tissues for a re-growth.
2 Materials and methods
2.1 Materials
The HNT was obtained from Natural Nano Inc., USA. The well-established structure of HNT showed an inner lumen with 60 to70nm and an external coiled surface having 100-120 nm respectively. The marine polysaccharide chitosan (90% deacetylation, viscosity of 10-150 mPa.s), was purchased from Sisco Research Laboratories Pvt.Ltd. The silanizing agent 3-Aminopropyltriethoxysilane (APTES) and the cross linker glutaraldehyde (85%) were provided by Sigma Aldrich, India. Other chemicals used including Poly (vinyl alcohol) bearing a hydrolysing capacity of 89 mol%, molecular weight of 1,60,000, viscocity of 27-33Cp and degree of hydrolysis 86-89 mol% purchased from HIMEDIA were of analytical grade.
2.2 Methods
2.2.1 Preparation of chitosan functionalised halloysite nanotubes as nanofillers
The HNTs bear Si–OH on their outer layer which are highly susceptible for reactions with organic silanes and hence treated with APTES according to the previously used method in the literature [24]. Shortly, the procedure adopted begins with the drying of HNT at around 70–80 °C for 12 h to remove captured moisture. In the next step, APTES (3 ml) taken in 60 ml ethanol and stirred at 45 °C for 15 min followed by the addition of HNT. The HNT-APTES mixture was continued to stir mechanically for about 10–12 h at room temperature. The silanised HNTs were washed thoroughly with distilled water and vacuum dried at 60 °C. The as formed nanotubes are thus further treated with glutaraldehyde (85%) and chitosan till a light brown coloured solution is obtained. The process of centrifugation and drying is done. Advance to the use, chitosan solution was prepared in 1% acetic acid (50 ml of 1% acetic acid) and a known quantity of glutaraldehyde (about 8.5 ml) was added along with APTES-HNT. The mixture kept under stirring for about 12 h at 60 °C. The HNTs with externally attached chitosan are thus obtained. The as prepared powder of chitosan functionalised halloysite spirals were dried at 60 °C overnight ground into fine powder and sieved to obtain uniform particle size. The overall process is depicted in Fig. 1.
2.2.2 Fabrication of chitosan-glutaraldehyde-HNT (Chit-glut-HNT) embedded PVA films
The nanocomposite films were prepared by using the solution casting method. A fine powder of Chit-glut-HNT was added to the polymer matrix by weight percentage variance and the total volume of film solution was 100 ml. Advance to the use, nanofillers were sonicated in water so as to achieve uniform distribution in the matrix. A series of 1wt%, 3wt% and 5wt% PVA nanocomposite films were casted on petri dishes, sealed and dried in an oven for 7–8 h at 50 °C. This fabrication process uses double distilled water as the solvent. The Table 1 shows the different precursor weights taken for the preparation of films.
2.3 Characterisations
2.3.1 Fourier transformer infra-red spectroscopy (FTIR)
FTIR is used for the evaluation of different steps involved in the preparation of chitosan attached HNT and their nanocomposite films. An IR spectrometer working on the principle of Attenuated Total Reflectance (ATR) with the model Prestige-21 Shimadzu made in Japan was employed. The scanning range was 4000–400 cm−1. All together of 35 scans were done during the analysis to assess the precision.
2.3.2 Powder X-ray diffraction (PXRD)
Powder XRD was made use for acquisition of data to go with phase identification and specification of modification. Documentation of the data had done with a diffract meter using the copper-Kα source (wavelength, λ = 1.5406 A°). The voltage was 40 kV with a current of 15 mA. The instrument installed- a Rigaku Miniflex 600 bench top mode. The maintained scan rate was 2°min−1 (0−80° of 2θ range). The partly crystalline behaviour of films were studied by employing Scherer’s equation as,
And,
where Ac and Aa are the areas for crystalline and total (amorphous + crystalline) respectively and, β is the width at half maximum intensity, k is a constant = 0.9 and θ is Bragg’s angle.
2.3.3 Thermal studies (TGA–DSC)
The interaction of functionalised HNTs and their PVA films with time and temperature were described by the help of SDTQ600-WATERS, thermal analyser. The physical and chemical changes occurring in the test samples were set down using both TGA and DSC techniques. Testing of the powder and film samples were done at a slower rate of 10 °C per minute and the study was carried out till 800 °C in an inert atmosphere. The crystallinity (XC) of PVA films were determined by Eq. 3 [25].
where, \({\Delta \text{H}}_{\text{m}}\) = enthalpy for melting, \({\Delta \text{H}}_{\text{c}}\) = enthalpy of crystallisation, \({\Delta \text{H}}_{\text{m}}^{^\circ }\) = total heat content for crystalline PVA (138.60 J/g) and w = amount of PVA taken for the film forming solution [26]. All these parameters play an important role as the heat lost or gained during a reaction enables us to find the heat associated with a system [27].
2.4 Analysis of surface morphology
2.4.1 Atomic force microscopy (AFM)
This microscopic technique has the ability to demonstrate surface parameters with resolution on the order of nanometres. The roughened layers of samples were analysed by using an Innova Spin Atomic Force Microscope. The instrument working at a resonance frequency of 320 kHz in tapping mode was established for obtaining 3D and 2D images. The results showed good reproducibility and topological changes of PVA and reinforced material.
2.4.2 Field emission scanning electron microscopy—energy dispersive spectroscopy (FESEM-EDS)
Surface analysis is a necessary part of the investigation and is been carried out with the help of an electron microscope analyser (ZIESS-SIGMA) at 20 kV. The functionalised nanoclay as well as the polymer films has known to be non-conducting. To overcome this behaviour a 5 nm thick gold layer was sputtered and the measurements were done. Coupled action of SEM and EDS provides the elemental composition of samples.
2.4.3 Transmission electron microscope (TEM)
The surface properties of modified and unmodified HNTs differ in many ways. To evaluate the changes that have occurred on treatments with organic moieties a well resolved topological images are required. Hence one can attain at all tiny information by capturing the HNTs under TEM. A 60–300 kV low base FEI/Titan Themis instrument was made use for the analysis.
2.5 Mechanical properties
Evaluation of physical properties that a film exhibits on application of force was done using a Universal Testing Machine (UTM) (Hounsfield, Surrey, and UK). The films were cut into 10 × 2.5 cm size and analysed for testing the modulus, strength and the elasticity.
2.6 Study of hydrophilic/hydrophobic nature of the films.
The water absorption capacity of the films was quantised using a contact angle analyser. The study explains how well the water droplet spreads over a film surface of plane PVA and its nanocomposite film. Every film was exposed to a water drop sized 7µL and precise software usage.
2.7 Water uptake test of the films
Modified halloysite nanotube reinforced films and the pure PVA films were set to bulge in a media with pH 7.4 (PBS) [16]. The particular pH was chosen so that the films also adhere to physiological conditions. Clean and dry glass vials measuring about 7 ml were taken and 3 ml of saline was added to it followed by 1 × 1 cm test samples. Swelling ratios for all the samples after several intervals of time were calculated simultaneously using Eq. 4.
where, SW = swelling ratio, \({\text{W}}_{\text{a}}\)= weight of the film after swelling and \({\text{W}}_{\text{b}}\)= weight of the films before swelling respectively.
2.8 Enzymatic activity on films
The commonly existing enzyme in body fluid and tissues is lysozyme with varying concentrations. In the investigation of degradation of chitosan and several biocompatible polymers lysozyme is used. Here, a 1 × 1 cm film sample was suspended in phosphate buffered saline maintaining the temperature at 37° C. The procedure was continued for 1, 3,5,9,11,14 and 21 days successively. During the study, solution was repeatedly extracted to get the appropriate results. Care was taken in the drying process of films (at 50°°C) and weighed. Equation 5 gives the degradation rate of the nanocomposite films.
where, \({M}_{1}\) and \({M}_{2}\) represent the difference in weight for initial and final weights.
3 Biocompatibility studies
3.1 Frangibility of red blood cells by hemocompatibility study
Chitosan and its derivatives along with halloysite have given a smooth route for their applicability in biomedical field by being viable towards red blood cells. In this regard, in-vitro tests for hemo-compatibility is more preferred over in-vivo as it is time consuming and requires animal life. The study involves collection of 4.5 ml of blood from a healthy human being and its centrifugation to separate the plasma. The commercially available saline (0.9%) is isotonic to erythrocytes’ sodium chloride and hence the former is used as the medium for haemolysis. Test solutions were made ready in PBS and incubated for whole day at 37° C. Every sample solution was mixed with an equal amount of compact RBCs (200 µl), stored at physiological temperature for 30 min and centrifuged. To this added 4 ml of normal saline to stop hemolysis and further kept in oven for one hour at the same temperature and centrifuged at a rate of 3000 rpm. A positive control having RBC in distilled water and negative control—RBC in normal saline were used appropriately and the amount of dead cells leaked into the resilient solution was tabulated at a wavelength of 540 nm for all the samples.
Here,\({\text{S}}_{\text{abs}}\),\({\text{N}}_{\text{abs}}\),\({\text{P}}_{\text{abs}}\) are the measured figures of absorbance for different weight percentage films and the controls used—negative and positive respectively.
3.2 Induction of cell proliferation
The mouse embryonic fibroblasts (NIH3T3) used for the cell adhesion and proliferation studies were acquired from the National Centre for Cell Sciences (NCCS), Pune, India. Based on their provenance, NIH3T3 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% Fetal Bovine Serum (FBS) and 1% antibiotic-antimitotic solution. Incubation of cell suspensions was done at 37° C in the humidified environment of 5% CO2.
3.2.1 Trypan blue dye exclusion method for cell viability
The chemosensitivity response is utilized to evaluate the ratio of cell mortality. The pigment penetrates the breached membrane of deceased cells, rendering them blue, while the viable cells remain intact and colorless. A culture medium containing NIH3T3 cells was prepared in a 24-well plate with a precise equivalence in the formation of uniformly sized nuclei (25,000 cells per well). Polymer nanocomposite films measuring 1 cm in length and width were introduced into the wells. Mouse embryonic cells exhibit strong adhesion properties, aiding in the assessment of cell viability. A specific volume of suspension culture mixed with an equal volume of dye was analyzed using a hemocytometer. A control solution, untreated by any of the aforementioned procedures, was included in the study using the same medium. Photomicrographs were captured using a Zeiss Axiocam attached to an inverted microscope (CarlZeiss, Germany) for both the treated and control groups.
3.2.2 Acridine orange ethidium bromide (AO-EB) fluorescent staining
It was employed in the determination of cell growth. AO is a highly membrane permeable and a cationic dye which gives visualization of both viable and non-viable cells when combined with ethidium bromide. The micro titre plates containing the cells were kept at 37°°C in a humidified atmosphere of CO2 for 24 h along with the polymer films in it. Later on, the cells were observed for apoptosis under green and red channels using a fluorescence imager. Cells were washed with PBS and stained using AO-EB mixture (2 µg/mL; 1:1 ratio) at 37°°C for 15 min under dark conditions. Later on, the cells were observed for apoptosis under different channels.
3.2.3 Statistical analysis
Data with Mean ± S.D. was carried through and documentations done in triplicates. GraphPad Prism version 9.0 was used to acquire statistically accepted values.
4 Results and discussions
4.1 Characterisations supporting the process of grafting chitosan to the external surface of HNT and its poly (vinyl alcohol) nanocomposite films
The surface of HNT bearing hydroxyls act as trapping agents for organic silanes since they are highly reactive towards oxygen. On the other hand glutaraldehyde is one of the most active agents for reaction with amino group and hence makes the process easier for covalent addition of chitosan. The spectra in Fig. 2A and B show the base peaks for pristine halloysite at 3696 cm−1 and 3624 cm−1 which certainly corresponds to the enormously present inner hydroxyl groups of Al–OH [28]. These peaks were found to be unaltered in the functionalised nanotubes though their intensity was decreased. The peaks at 3395 cm−1 and 2936 cm−1 could be attributed to the several OH and asymmetric stretching of CH2 of APTES [29]. The asymmetric deformation vibration of CH2 appeared as a weak band at 1246 cm−1 and 1228 cm-1 respectively in silanised and Chitosan functionalised HNT. The presence of new peaks at 1566 cm−1 and 1405 cm−1 are found to be assigned to deformation vibrations of NH2 group. The appearance of band 1647 cm−1 may be attributed to the water deformation vibration. The band corresponding to-NH stretching is observed at 1554 cm−1. Thus silanised HNTs were capable of tagging with glutaraldehyde and Chitosan covalently in the pH range of 3–7 and the reactions were almost irreversible [30]. The broadened peak from 3000 cm−1 to 3600 cm−1 in Chit-glut-HNT is ascribed to the increased number of OH groups upon addition of glutaraldehyde and chitosan. The peaks Along with these the peak for C = N bond formation appeared at 1639 cm−1 confirms the functionalization of HNT with Chitosan because it clarifies the imine bond (C = N) formation. The Fig. 2C also explains the poly (vinyl alcohol) composite films containing varying amounts of Chit-glut-HNT embedded onto the surface. The broad peaks observed at around 3300 cm−1 for pure PVA and composite films (1wt%, 3wt% and 5wt %) are solely caused by the stretching vibration of O–H that participate in hydrogen bonding. The alkyl stretching of C–H was verified with the bands appearing at 2922 cm−1 and 2852 cm−1. The sharp and low intense peak seen at 1731 cm−1 corresponds to C = O stretching vibration. Few tinier peaks observed at 1436 cm−1 and 835 cm−1 are ascribed to the C–H bending and rocking vibrations respectively. Along with these absorption bands also appeared at 1245 cm−1 and 1096 cm−1 are associated with C–C as well as C–O groups of PVA [31]. From the graph it is evident that reinforcement of Chit-glut-HNT into the PVA matrix helps in increasing the intermolecular bonding between the matrix and the nanofillers. Additionally, one can see the hydroxyl band decreases in its intensity due to unavailability of free O–H groups on increasing the weight percentage of HNTs. A possible interaction pathway of PVA and modified HNTs is presented in Fig. 3.
The XRD studies further support the modification process of HNT with organic molecules and biopolymer chitosan and has the values indexed in JCPDS card No. 29-1487. The as shown spectra in Fig. 4. A clarifies the formation of Chit-glut-HNT. Chitosan is known to exhibit its peaks at 2θ values of 11, 20 and 22° respectively. The reflections corresponding to plane halloysite fall at 12.5° which clearly stands for its 001 plane [32]. Along with these peaks HNT also borrows other regions of X-ray diffraction pattern at 19.47°, 24.41°, 34.74°, 37.86°, 54.57°; 62.14° and 73.41° are corresponding to (100), (002), (110), (003), (210), (300) planes of its crystalline structure [33]. The peak at 22° in case of silanized HNT was seen to be more intense than that of found in HNT alone and is because of the additional atoms present in the plane. After grafting the polysaccharide chitosan onto silanized halloysite using glutaraldehyde the crystalline nature of chitosan increases [34]. X ray diffraction pattern of Chit-glut-HNT also reveals the mixture of different compounds being together by showing crystal lattice transformations. Figure 4B is attributed to the diffraction pattern of PVA films containing grafted HNTs. It is evident from the diffraction patterns that semi crystalline nature of PVA is explained with a peak appearing at 19.50° and it consistently shifts towards higher 2θ value after incorporation of nanofillers. PVA is semicrystalline and the peaks at 2θ value of 19.50° and 21.8° are for the (101) planes of the crystal respectively. Usually in neat PVA the second (101) plane is observed at 20.6°, but due to the modification process this peak gradually shifts from its original value. The d-spacing values for PVA films surge up with the increasing amount of fillers. This change assists the involvement of molecules of matrix with chitosan functionalised halloysite (Table 2). The percentage of crystallinity though increases for the nanocomposite films; it is the highest for the film with 1wt% of nanofillers. This could be due to the uniform distribution of the nanofillers in the matrix.
Thermo gravimetric analysis of the chitosan functionalised halloysites and their nanocomposite films are pictured in Fig. 5. A, C and B indicates the derivative curve for the modification process. Chitosan alone suffers from a drawback of having highly sensitive action towards heat. Additionally it is believed to withstand a temperature of 200–220°°C [35]. Although, a thermally stable substance like HNT can re-modify the heat bearing capacity of this biopolymer and push it high for thermo-acceptance [36]. The weight loss nature of films is illustrated in Table 3A. A vital change in mass of HNT was observed at 50–125°°C and 410–610°°C range. The degradation seen in this range are believed to be owing to the dehydration process from the surface [37]. The dehydroxylation of the residual structure of Al–OH groups is observed at 400–600°°C. The band at 350–520°°C is corresponding to the additional decomposition of the organic part of the silane molecule [38]. The TGA thermo gram for chit-HNT showed three different weight loss patterns in three different ways ranging from 50 to 610° C. Majority of weight loss for Chit-glut-HNT falls in the 420–620°°C range. This degradation resulted from the removal of more acetyl units.
The decrease in melting temperature was compared for both the nanocomposite films and neat PVA which clearly indicates the interaction of chitosan with that of matrix. On moving from 1-5wt% films, the enthalpy of melting as well as enthalpy of crystallization does show linear laps. The extended intermolecular hydrogen bonding between chitosan and PVA decreases the decomposition temperature of the films. The observed DSC parameters are listed in Table 3B. When we look at the crystallinity of the polymer PVA and its nanocomposites the percentage gradually increases. This observation could also be made using DSC technique. But the results though show the similar variation the values may not be exactly the same. This might be due to the fact that XRD involves a very depth analysis in microns as compared to DSC. It is also been noted that for thermal studies the sample required is more and the result depends on its bulk sensitivity. Due to these reasons we observed different percentage of crystallinity values for the PVA nanocomposite films using XRD and DSC techniques. As it is very evident from the table contents for a DSC the crystallinity gradually increases from 23.97% for pure PVA to 26.64% for 5wt% film.
The DTA curve given (Fig. 5E) represents the differential temperature of the nanocomposite films Td. The values corresponding to the weight loss changes are monitored and the DTA curve shows that differential temperature decreases as the nanofiller’s concentration increases in the matrix. The major weight loss occurring in the region of 290–390°°C is confirmed by this graph. Compared to TGA, differential thermogravimetry provides more prominent result for the decomposition of the compound. As it is visible from the spectra that for PVA alone Td is observed at 331.57°°C whereas for 1wt% 320.84°°C and this further increases to 325.92°°C and 327.99°°C for 3wt% and 5wt% respectively.
4.1.1 Morphological studies (FESEM, TEM, AFM)
Field Emission Scanning Electron microscopy (FESEM) of nano structural halloysites and their modified versions were studied for a careful evaluation of topological changes occurring in the functionalization of HNT as well as fabrication of polymer nanocomposite films. The results explain the microstructural analysis of attachment of chitosan onto the HNT’s surface by analysing the composition of the nanoclay. The FESEM performed alon with EDS helps to correlate the surface alterations occurred during the functionalization process of halloysite nanotubes. In Fig. 6.the soft and plane surface of nanotube is seen (A) which alters itself upon modification with silane (B) and chitosan (C) respectively on the surface [39]. These externally bonded molecules give rise to a more roughened surface for the halloysite nanotube. As visualised from the pictures, HNT shows a broadened and altered external surface due to the adherence of chitosan with silane. The chitosan grafted tubular nanoclays exhibit a relatively larger structures than that of pristine halloysites [40]. The process of addition of chitosan onto the silane containing tubes was further confirmed by the EDX (Energy Dispersive X-ray) data collected. Thus the study provides a set of conclusions ascertaining the covalent addition of chitosan to HNT. The poly (vinyl alcohol) and its Chit-glut-HNT composite films were also analysed for betterment in the understanding of reinforcement and surface changes. Figure 6. also explains the uniform distribution of Chit-glut-HNTs on PVA matrices with their increasing weight percentages (F,G, H and I). The film containing 5wt% of Chit-glut-HNTs reveal that after a certain weight percentage the nanofillers tend to coagulate and perturbation is seen in their distribution.
The transmission electron microscopy (TEM) analysis of the samples containing chitosan embedded onto the surface of halloysite nanotubes was done in order to boost the outcome of FESEM. The as obtained microscopic images predict the attachment of chitosan onto the silanised surface of nanotube. Additionally, the tubular representation of halloysite clay nanotubes is also visible and we can easily distinguish the internal lumen with that external roughened surface. Upon modification the HNTs show an enlargement in inter surficial gap due to the entangled silanes and chitosan moieties (Fig. 6D, E) clearly indicates the morphological changes occurred onto the surface of HNT before and after functionalization of chitosan.
Atomic force microscopic studies of the prepared poly (vinyl alcohol) nanocomposite films are visible in Fig. 7. The critical evaluation of the surface morphology reveals the incorporation of chitosan containing halloysites on the PVA turning it to be a non-planar one. The pictures emphasise on the attachment of HNTs and they appear as peaks with white and light brown colours [41]. The average roughness (Ra) and root mean square roughness (Rq) of the plane PVA and that of composite films were studied and compared for the overall changes occurring on the surface of polymer film upon addition of HNT [42]. The plane PVA bears a root mean square roughness and an average roughness of about 0.712 nm and 0.908 nm respectively. These two parameters experience a surge in their values upto 5.45 nm (Ra) and 8.42 nm (Rq) for the film with 5wt% of Chit-glut-HNT. This essential increase helps in cell adhesion and proliferation by providing sufficient amount of spaces for the cells to grow with.
4.1.2 Mechanical analysis
Employing an UTM helps us to interconnect the nanocomposite films with their mechanical properties. The tensile strength and Young’s modulus of pure PVA increases from 7.038 MPa to 13.162 MPa and 47.43 MPa to 106.857 MPa respectively for 5wt% polymer nanocomposite film. These enhancements in properties are observed after the incorporation of Chit-glut-HNT into the matrix. The well dispersed nanotubes in the polymer cause an increase in other mechanical properties too. The stress versus strain graph also indicates the increase in strength for the incorporated halloysite based PVA films than the PVA alone. The flexibility of the films have been found to be increased with the surging amount of the nanofillers in them. This makes the HNTs as a better reinforcing material for enhancing the mechanical properties of the polymer matrix. The elongation at break of PVA was observed to be 29.97%, but for a 5wt% film it is 34.55%. All these findings indicate a trend toward the characteristics of the integrated halloysite-infused biopolymer chitosan and its interaction with the polymer matrix. In corelation to the results obtained through the AFM analysis as well the increased roughness of the films also adds up the space for accumulation of cells during the cell adhesion. Also, the roughness created on the film surface due to the reinforcement of functionalised HNTs forms the basis for holding the matrix together with further increased attachments and hence enhancing the mechanical properties. These findings collectively provide confidence in the suitability of the fabricated polymer films for tissue regeneration, given their substantial mechanical resilience. The aforementioned details are visually represented in Fig. 8a–d. For the analysis, an extension with a 1KN load and a speed of 1 mm/min was employed.
4.2 Determination of hydrophilic/hydrophobic nature of the film samples
4.2.1 Water contact angle
The cell adhesion and proliferation in turn depend on the hydrophilicity of polymer films. Water contact angle enables us to deal with the water absorbing capacity of polymers. All the images presented in Fig. 9A are aligned with the standard deviations [43]. On comparison of water contact angle of pure PVA and composite films it was found that the latter ones possess a slightly higher value due to the covalent interaction of chitosan functionalised HNTs. The angles were within the limit of hydrophilicity with PVA showing 40.1°, and composite films show 52.2°, 61.6°, and 74.3° respectively for 1wt%-5wt% of nanofillers. These reports give a certain agreement with the literature [44]. Moreover, the values also accompany the results that of AFM by showing increased roughness upon reinforcement of Chit-glut-HNT.
4.2.2 Swelling study
To add more weight to the WCA observations, swelling properties of PVA alone and its nanocomposite films were also taken into account. A 1 × 1 cm film sample was immersed in a phosphate buffered saline solution (PBS) for several hours and noted down the water intake capacity. Swelling ratios regulate the films nature for hydrophilic or hydrophobic. The Fig. 9B. represents the time variant swelling ratios of the prepared nanocomposite films. The inbuilt natural property of PVA with its structure having more hydroxyl groups paves the way for increased hydrophilicity. The advantage of such property further reflects in its applicability towards cell growth and management. Plane PVA being hydrophilic has showed a swelling ratio of 322 ± 0.4 in duration of 30 min [45]. The swelling ratio for the nanocomposite films increases and for a film with 5wt% of nanofillers at 180 min it is 512 ± 0.8 (maximum). At this point we observed that the PVA was almost completely swelled and it was unable to swell further and started decomposing.
4.3 In vitro studies for determination of biocompatibility of prepared nanocomposite films
4.3.1 Enzymatic degradation
In accordance to the need for approval of the films towards tissue engineering lyso-enzyme degradation activity was performed [46]. The physiological condition of human body was achieved by using PBS throughout the experimental procedure [47]. More cautiously a considerable change in the degradation rate was seen for films with Chito-glut-HNT than that for PVA alone. This effective mortification of films is mainly caused by the internal actions of chitosan functionalised halloysite embedded onto the PVA surface. A mentionable result shows PVA as highly recommendable polymer as it bears a degradation rate (%) of about 7.0 ± 0.24 on day 1. A remarkable increase in degradation is seen for films containing varying amounts of Chit-glut-HNT. The unspoken truth behind this may be attributed to the interaction of chitosan and poly(vinyl alcohol) also lysozyme’s nature of fractionating hydroxyls groups [48]. The variation in the degradation percentage of films is depicted in Fig. 10. It’s significantly clear that PVA is hydrophilic polymer and is been put up with huge biocompatibility by the addition of modified HNTs [49].
4.3.2 Hemocompatibility assay
The uninvited effects of synthesised polymer films have to be analysed before prioritizing its applications. The usability of polymeric biomaterials in bodily applications needs a hemolysis of either 0% or less than 5% as mentioned in national biological safety [50]. Before beginning to test, the films were preserved in phosphate buffered saline at 37 °C for 24 h. Furthermore, 2 types of control were taken—positive control (distilled water) and negative control (physiological saline) [51]. Figure 11A schematically shows haemolytic percentage of nanocomposite films and that of plane PVA with different amount (%) of Chit-glut-HNT. The results were almost agreed with the above mentioned fundamental fact and PVA alone arose with a 3.201% (± 0.021) of haemolysis. This haemolytic percentage almost moves towards the lowest with the chit-glut-HNT addition to PVA matrix. Thus obtained results are very promising and all the three filler containing films show a lower haemolytic percentage of 0.202 ± 0.008 (1wt%), 0.408 ± 0.022 (3wt%) and 0.402 ± 0.032 (5wt%). Hence, the prepared films are highly compatible with the bodily fluid. The functionalization of halloysite with chitosan in-turn subside the slightly lying toxicity of halloysite when compared to previous results [26].
4.3.3 Cell proliferation analysis by Trypan blue dye exclusion
The test deals with the fundamental fact of plasma membrane being semipermeable to the external moieties. The living cells have an unbroken and an intact plasma membrane that denies to let in foreign substances such as dyes into the protoplasm [52]. The established guideline advises suspending the test specimens in the observation medium (NIH3T3 fibroblast cell suspension) for a specific duration, during which dye exclusion is assessed (observations may also rely on the clarity of the analyst) [53]. Here, mitochondria play a central role in cell proliferation and modulation of cell metabolism. The oxidative rate of mitochondria needs to be depressed for cell proliferation [54].The NIH3T3 fibroblasts are the mesenchymal cells that produce connective tissues and is therefore extensively studied for tissue regeneration properties[55].The viable cell count of PVA and its nanocomposite films are listed in Fig. 12 along with its pictorial representation. Cell proliferation process begins with the cells exhibiting spindle shape and rapid constitution of their small dominion occurs over the surface of films [56]. The amplification in the growth of cells on polymer film specimens were of notable interest as it the process was observed for all composition films.
In particular, the number of cells gradually increased from 1wt% films to 5wt% which is given in Table 4. These sequel are mainly due to the increased concentration of chitosan functionalised HNTs on PVA surface. The visualization of NIH3T3 cell proliferation on the poly(vinyl alcohol)/Chit-glut-HNT nanocomposite films revealed enhanced filopodia formation, which significantly contributes to cell proliferation and maintains a favorable cellular environment. The observed significant variations in cell multiplication of fibroblasts predominantly in Chit-glut-HNT/PVA films are attributed to the increased surface roughness of samples. Because these rough surfaces act as capturing voids for cells and their growth. All these aspects lead the way to the relevance of Chit-glut-HNT/PVA nanocomposites in tissue engineering.
4.3.4 Cell adhesion studies by AO-EB assay
Apoptosis assists programmed cell death in metazoans that bring about multiple impairments in the cells. Cell proliferation is a requisite for the assessment of biological acceptance of materials. Also it acts as a consequence of its basic requirement in cell’s well-being [57]. In cell adhesion cell junction proteins play an important role in which it helps to hold the cells together and facilitates adhesion. In most animal cells glycoproteins characterised to play the role of cell junctions [58]. In the present study, a clear distinction was made between pure polyvinyl alcohol (PVA) and its nanocomposite films concerning cell adhesion, as assessed through Acridine Orange Ethidium Bromide (AOEB) staining [54]. Figure 13 illustrates fluorescent images of polymer nanocomposite films incorporating the stains in three distinct channels. It is evident from these images that the composite films exhibit enhanced cell growth. This observation holds true across a range of film compositions, from 1wt% to 5wt%, further establishing their potential as promising biomaterials.
The green and merged channels reveal an absence of cell death, as evidenced by the absence of red or orange staining. These experimental findings strongly indicate that the prepared nanocomposite films are highly conducive to tissue formation. Furthermore, the observed biocompatible properties can be attributed to the reinforced characteristics of chitosan and halloysite nanotubes (HNTs), in combination with the favorable properties of polyvinyl alcohol (PVA).
5 Conclusions
The current study highlights and validates the natural propensity of clay minerals to undergo surface functionalization. These modified nanoclays serve as essential fillers for incorporating biologically compatible materials like the biopolymer chitosan. Conversely, the bio-polymer chitosan, known for its high biocompatibility, enables a feasible combination with halloysite nanotubes (HNT). The environmentally friendly method employed for preparing nanocomposite films enhances the reproducibility of the study.
The addition of HNT has improved the mechanical properties of poly(vinyl alcohol) matrix. As the percentage of Chit-glut-HNT increases, the films exhibit increased hydrophilicity. Notably, a film containing 5wt% nanofillers exhibits the highest swelling ratio, approximately 512 ± 0.8%, compared to other values. This hydrophilic nature acts as a nurturing medium for the cells to get attached on. The in vitro analysis of the nanocomposite films reveals a significant increase in cell count, with a value of 0.186 ± 0.003 for the 5wt% film, surpassing that of plain PVA (0.134 ± 0.002). The findings of cell adhesion activity (AOEB), cell proliferation (Trpan blue dye) and hemolysis, collectively indicating the suitability of Chit-glut-HNT-containing polyvinyl alcohol nanocomposite films for a wide range of physiological conditions. The test samples have demonstrated substantial cell growth, leading to the formation of tissue colonies composed of NIH3T3 fibroblasts. However, caution must be exercised to prevent excess nanoclay, which can lead to agglomeration and uneven distribution within the polymer matrix.
Data availability
Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Khan MUA, Stojanović GM, Rehman RA, et al. Graphene oxide-functionalized bacterial cellulose-gelatin hydrogel with curcumin release and kinetics: in vitro biological evaluation. ACS Omega. 2023;8:40024–35. https://doi.org/10.1021/acsomega.2c06825.
Khan R, Aslam Khan MU, Stojanović GM, et al. Fabrication of bilayer nanofibrous-hydrogel scaffold from bacterial cellulose, PVA, and gelatin as advanced dressing for wound healing and soft tissue engineering. ACS Omega. 2024;9:6527–36. https://doi.org/10.1021/acsomega.3c06613.
Al-Arjan WS, Khan MUA, Almutairi HH, et al. pH-responsive PVA/BC-f-GO dressing materials for burn and chronic wound healing with curcumin release kinetics. Polymers (Basel). 2022. https://doi.org/10.3390/polym14101949.
Khan MUA, Razak SIA, Hassan A, et al. Multifunctional arabinoxylan-functionalized-graphene oxide based composite hydrogel for skin tissue engineering. Front Bioeng Biotechnol. 2022;10:1–12. https://doi.org/10.3389/fbioe.2022.865059.
Khan MUA, Aslam MA, Bin Abdullah MF, et al. Recent perspective of polymeric biomaterial in tissue engineering– a review. Mater Today Chem. 2023. https://doi.org/10.1016/j.mtchem.2023.101818.
Ghalei S, Hopkins S, Douglass M, et al. Nitric oxide releasing halloysite nanotubes for biomedical applications. J Colloid Interface Sci. 2021;590:277–89. https://doi.org/10.1016/j.jcis.2021.01.047.
Liu M, Shen Y, Ao P, et al. The improvement of hemostatic and wound healing property of chitosan by halloysite nanotubes. RSC Adv. 2014;4:23540–53. https://doi.org/10.1039/c4ra02189d.
Bertolino V, Cavallaro G, Lazzara G, et al. Halloysite nanotubes sandwiched between chitosan layers: Novel bionanocomposites with multilayer structures. New J Chem. 2018;42:8384–90. https://doi.org/10.1039/c8nj01161c.
Massaro M, Barone G, Biddeci G, et al. Halloysite nanotubes-carbon dots hybrids multifunctional nanocarrier with positive cell target ability as a potential non-viral vector for oral gene therapy. J Colloid Interface Sci. 2019;552:236–46. https://doi.org/10.1016/j.jcis.2019.05.062.
Amir Afshar H, Ghaee A. Preparation of aminated chitosan/alginate scaffold containing halloysite nanotubes with improved cell attachment. Carbohydr Polym. 2016;151:1120–31. https://doi.org/10.1016/j.carbpol.2016.06.063.
Naumenko E, Fakhrullin R. Halloysite nanoclay/biopolymers composite materials in tissue engineering. Biotechnol J. 2019;14:1–11. https://doi.org/10.1002/biot.201900055.
Khan S, Kumar V, Roy P, Kundu PP. TiO2 doped chitosan/hydroxyapatite/halloysite nanotube membranes with enhanced mechanical properties and osteoblast-like cell response for application in bone tissue engineering. RSC Adv. 2019;9:39768–79. https://doi.org/10.1039/c9ra08366a.
Liu M, Dai L, Shi H, et al. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mater Sci Eng C. 2015;49:700–12. https://doi.org/10.1016/j.msec.2015.01.037.
Li W, Liu D, Zhang H, et al. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017;48:238–46. https://doi.org/10.1016/j.actbio.2016.10.042.
Satish S, Tharmavaram M, Rawtani D. Halloysite nanotubes as a nature’s boon for biomedical applications. BJGP Open. 2019;6:1–16. https://doi.org/10.1177/1849543519863625.
Sheik S, Sheik S, Nairy R, et al. Study on the morphological and biocompatible properties of chitosan grafted silk fibre reinforced PVA films for tissue engineering applications. Int J Biol Macromol. 2018. https://doi.org/10.1016/j.ijbiomac.2018.05.019.
Varma AJ, Deshpande SV, Kennedy JF. Metal complexation by chitosan and its derivatives: A review. Carbohydr Polym. 2004;55:77–93. https://doi.org/10.1016/j.carbpol.2003.08.005.
Yang H, Chen Y, Chen Z, et al. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH-responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomater Sci. 2017;5:1001–13. https://doi.org/10.1039/c7bm00043j.
Szymańska E, Winnicka K. Stability of chitosan—a challenge for pharmaceutical and biomedical applications. Mar Drugs. 2015;13:1819–46. https://doi.org/10.3390/md13041819.
Jayakumar A, Radoor S, Nair IC, et al. Lipopeptide and zinc oxide nanoparticles blended polyvinyl alcohol-based nanocomposite films as antimicrobial coating for biomedical applications. Process Biochem. 2021;102:220–8. https://doi.org/10.1016/j.procbio.2020.12.010.
Liu M, Wu C, Jiao Y, et al. Chitosan-halloysite nanotubes nanocomposite scaffolds for tissue engineering. J Mater Chem B. 2013;1:2078–89. https://doi.org/10.1039/c3tb20084a.
da Amaral S, et al. Cytotoxic effect of crude and purified pectins from Campomanesia xanthocarpa Berg on human glioblastoma cells. Carbohydr Polym. 2019;224: 115140. https://doi.org/10.1016/j.carbpol.2019.115140.
Mandru M, Bercea M, Gradinaru LM, et al. Polyurethane/poly(vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur Polym J. 2019;118:137–45. https://doi.org/10.1016/j.eurpolymj.2019.05.049.
Bulbul YE, Uzunoglu T, Dilsiz N, et al. Investigation of nanomechanical and morphological properties of silane-modified halloysite clay nanotubes reinforced polycaprolactone bio-composite nanofibers by atomic force microscopy. Polym Test. 2020;92: 106877. https://doi.org/10.1016/j.polymertesting.2020.106877.
Shabeena M, Kouser S, Prabhu A, et al. Biocompatible pectin-functionalised-halloysite loaded poly(vinyl alcohol) nanocomposite films for tissue engineering applications. J Drug Deliv Sci Technol. 2023;82: 104320. https://doi.org/10.1016/j.jddst.2023.104320.
Kouser S, Sheik S, Prabhu A, et al. Effects of reinforcement of sodium alginate functionalized halloysite clay nanotubes on thermo-mechanical properties and biocompatibility of poly (vinyl alcohol) nanocomposites. J Mech Behav Biomed Mater. 2021. https://doi.org/10.1016/j.jmbbm.2021.104441.
Virtanen S, Vartianen J, Setälä H, et al. Modified nanofibrillated cellulose-polyvinyl alcohol films with improved mechanical performance. RSC Adv. 2014;4:11343–50. https://doi.org/10.1039/c3ra46287k.
He Y, Kong W, Wang W, et al. Modified natural halloysite/potato starch composite films. Carbohydr Polym. 2012;87:2706–11. https://doi.org/10.1016/j.carbpol.2011.11.057.
Zhang D, Hegab HE, Lvov Y, et al. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. Springerplus. 2016. https://doi.org/10.1186/s40064-016-1682-y.
Li B, Shan CL, Zhou Q, et al. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar Drugs. 2013;11:1534–52. https://doi.org/10.3390/md11051534.
Zhang J, Wang Q, Wang A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite superabsorbent composites. Carbohydr Polym. 2007;68:367–74. https://doi.org/10.1016/j.carbpol.2006.11.018.
Cavallaro G, Milioto S, Konnova S, et al. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl Mater Interfaces. 2020;12:24348–62. https://doi.org/10.1021/acsami.0c05252.
Karami Z, Moini Jazani O, Navarchian AH, et al. Well-cured silicone/halloysite nanotubes nanocomposite coatings. Prog Org Coatings. 2019;129:357–65. https://doi.org/10.1016/j.porgcoat.2019.01.029.
Yuan P, Southon PD, Liu Z, et al. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J Phys Chem C. 2008;112:15742–51. https://doi.org/10.1021/jp805657t.
Khalili R, Zarrintaj P, Jafari SH, et al. Electroactive poly (p-phenylene sulfide)/r-graphene oxide/chitosan as a novel potential candidate for tissue engineering. Int J Biol Macromol. 2020;154:18–24. https://doi.org/10.1016/j.ijbiomac.2020.03.029.
Venkatesan J, Qian ZJ, Ryu B, et al. Preparation and characterization of carbon nanotube-grafted-chitosan—natural hydroxyapatite composite for bone tissue engineering. Carbohydr Polym. 2011;83:569–77. https://doi.org/10.1016/j.carbpol.2010.08.019.
Massaro M, Riela S, Cavallaro G, et al. Eco-friendly functionalization of natural halloysite clay nanotube with ionic liquids by microwave irradiation for Suzuki coupling reaction. J Organomet Chem. 2014;749:410–5. https://doi.org/10.1016/j.jorganchem.2013.10.044.
Zhang Y, Ouyang H, Chwee TL, et al. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res Part B Appl Biomater. 2005;72:156–65. https://doi.org/10.1002/jbm.b.30128.
Zhai R, Zhang B, Wan Y, et al. Chitosan-halloysite hybrid-nanotubes: Horseradish peroxidase immobilization and applications in phenol removal. Chem Eng J. 2013;214:304–9. https://doi.org/10.1016/j.cej.2012.10.073.
Molaei A, Yousefpour M. Preparation of Chitosan-based nanocomposites and biomedical investigations in bone tissue engineering. Int J Polym Mater Polym Biomater. 2019;68:701–13. https://doi.org/10.1080/00914037.2018.1493683.
Adeli H, Khorasani MT, Parvazinia M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int J Biol Macromol. 2018. https://doi.org/10.1016/j.ijbiomac.2018.10.115.
Films B, Mousa M, Dong Y. The role of nanoparticle shapes and structures in material characterisation of polyvinyl alcohol (PVA). Polymers. 2020. https://doi.org/10.3390/polym12020264.
Ceylan S. Propolis loaded and genipin-crosslinked PVA/chitosan membranes; characterization properties and cytocompatibility/genotoxicity response for wound dressing applications. Int J Biol Macromol. 2021;181:1196–206. https://doi.org/10.1016/j.ijbiomac.2021.05.069.
Ramakrishnan RK, Wacławek S, Černík M, Padil VVT. Biomacromolecule assembly based on gum kondagogu-sodium alginate composites and their expediency in flexible packaging films. Int J Biol Macromol. 2021;177:526–34. https://doi.org/10.1016/j.ijbiomac.2021.02.156.
Taylor P, Khoo WS, Ismail H, Ariffin A. International journal of polymeric materials and tensile, swelling, and oxidative degradation properties of crosslinked polyvinyl alcohol / chitosan / halloysite nanotube composites tensile, swelling, and oxidative degradation properties of crosslinke. Int J Polym Mater. 2013. https://doi.org/10.1080/00914037.2012.719133.
Gaaz TS, Sulong AB, Akhtar MN, et al. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules. 2015. https://doi.org/10.3390/molecules201219884.
You A, Be MAY, In I. Enzymatic degradation of chitosan blend for tissue engineering application. 2019; https://doi.org/10.1063/1.5116944
Banerjee A, Chatterjee K, Madras G, et al. Enzymatic degradation of polymers : a brief review enzymatic degradation of polymers: a brief review. Mater Sci Technol. 2017. https://doi.org/10.1179/1743284713Y.0000000503.
Shah R, Stodulka P, Skopalova K, Saha P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers. 2019. https://doi.org/10.3390/polym11122094.
Gharibi R, Kazemi S, Yeganeh H, Tafakori V. International Journal of Biological Macromolecules Utilizing dextran to improve hemocompatibility of antimicrobial wound dressings with embedded quaternary ammonium salts. Int J Biol Macromol. 2019;131:1044–56. https://doi.org/10.1016/j.ijbiomac.2019.03.185.
Zhang H. Development and blood compatibility assessment of electrospun polyvinyl alcohol blended with metallocene polyethylene and plectranthus amboinicus (PVA/mPE/PA ) for bone tissue engineering. Int J Nanomed. 2018. https://doi.org/10.2147/IJN.S151242.
Protocol B. Trypan Blue Exclusion Test of Cell Viability. CP Immunol. 1997. https://doi.org/10.1002/0471142735.ima03bs21.
Almeida VG, Pinto MCX, Moura FAG. Trypan blue exclusion assay by flow cytometry. Braz J Med Biol Res. 2014;47:307–15.
Arciuch VGA, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxidants Redox Signal. 2012;16:1150–80. https://doi.org/10.1089/ars.2011.4085.
Rahimi AM, Cai M, Hoyer-Fender S. Heterogeneity of the NIH3T3 fibroblast cell line. Cells. 2022. https://doi.org/10.3390/cells11172677.
Zhou WY, Guo B, Liu M, et al. Poly ( vinyl alcohol )/Halloysite nanotubes bionanocomposite films: properties and in vitro osteoblasts and fibroblasts response. J Biomedical Materials Res. 2009. https://doi.org/10.1002/jbm.a.32656.
García-rodríguez MC, Carvente-juárez MM, Altamirano-lozano MA. Antigenotoxic and apoptotic activity of green tea polyphenol extracts on hexavalent chromium-induced DNA damage in peripheral blood of CD-1 Mice : analysis with differential acridine orange/ethidium bromide staining. 2013:
Vahedi P, Moghaddamshahabi R, Webster TJ, et al. The use of infrapatellar fat pad-derived Mesenchymal stem cells in Articular cartilage regeneration: a review. Int J Mol Sci. 2021;22:1–13. https://doi.org/10.3390/ijms22179215.
Acknowledgements
The authors express gratitude to Directorate of Minorities, government of Karnataka for awarding Minority fellowship to carry out research and to VGST project provided by Govt. of Karnataka. I would like to express my immense thanks to Dr.Saraswathi P.Masthi, Principle Investigator, SERB project No. SB/EMEQ-213/2014, Department of chemistry, Karnataka Science college, Dharwad, 580001, Karnataka, India to help us record mechanical properties using UTM.
Author information
Authors and Affiliations
Contributions
Shabeena M: Designing the study, overall interpretation and analysis of all data and drafting the complete manuscript. Sabia Kouser:Interpretation of data and careful evaluation of manuscript. G. K. Nagaraja: Guiding the research work and approval of the version of the manuscript to be published. Ashwini Prabhu: Guiding the biological studies and data analysis. Deepali Warale: Visualization and interpretation of data and manuscript. D. J. Manasa: Visualisation of data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shabeena, M., Kouser, S., Prabhu, A. et al. Unravelling the tissue regenerative nature of marine polysaccharide chitosan embedded halloysite reinforced poly (vinyl alcohol) nanocomposite films. Discov Polym 1, 3 (2024). https://doi.org/10.1007/s44347-024-00004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44347-024-00004-2