Abstract
We hypothesized that ram epididymal tail sperm may be efficiently activated in homologous seminal plasma compared to tris and sperm-TALP. Eighty ejaculates were collectedfrom five healthy fertile rams by artificial vagina. Ejaculates with ≥ 3 mass motility and ≥ 70% initial motility score were considered and pooled. The seminal plasma was harvested by two-phase centrifugation (A-3000 g, 4 °C, 20 min; B-3600 g, 4 °C, 30 min). Sperm were collected by dissecting epididymal tail into 3 equal parts which were placed in three small (35 mm) petri dishes for activation in homologous seminal plasma (SP), sperm-TALP (TP) and tris buffer (TR). Sperm quality was assessed at 0, 24, 48 and 72 h of cold storage on the basis of motility, viability, HOST and acrosomal integrity. In addition to subjective assessment of motility, acrosomal integrity and viability were evaluated using molecular florescent probe combinations-fluorescein isothiocyanate (FITC) conjugated to peanut agglutinin (PNA) plus propidium iodide (PI) and carboxyflorescene diacetate (CFDA) plus propidium iodide, respectively. Motility, CFDA positive sperm (Viable) and HOST reacted sperm percentage were significantly higher (p < 0.05) for SP compared to both TP and TR at 48 and 72 h of cold storage. FITC-PNA negative sperm (Intact acrosomes) percentage did also differ significantly (p < 0.05) between SP, TP and TR at various hours of cold storage. In conclusion, homologous seminal plasma efficiently activated and preserved epididymal tail sperm compared to tris buffer and sperm-TALP. This study provides an opportunityto further explore the role of homologous seminal plasma in cryoprotection and fertilizing capacity of epididymal tail sperm.
Similar content being viewed by others
1 Introduction
Epididymal tail has a great potential to be used as a source of sperm in assisted reproductive technologies [1]. Spermatozoa retrieved from epididymal tail (Cauda epididymis) are motile and mature, and have produced live births [2, 3]. Therefore, there is considerable interest in recovering quality sperm from the epididymal tails of deceased animals of high genetic merit and endangered species/breeds for development of germplasm banks [4]. The recovery of motile and fertilizable sperm from the epididymal tail of an animal at death is a proven technique to preserve its germplasm and later on generate new chances for artificial breeding and revival of that genotype [5, 6]. Motile and viable sperm have been successfully recovered from epididymal tails stored either at room temperature or at 5 ℃ in boar [7], stallion [8], tom cat [9], bull [10], dog [11], and ram [12,13,14].
To enable fertilization, sperm must interact with a variety of factors present in the female reproductive tract which include proteins, hormones, endometrial epithelial cells, and immune cells. Many of these interactions are thought to involve proteins on the spermatozoa's outer membrane [15], derived mostly from protein-rich seminal plasma [16, 17]. Retrieving viable sperm from epididymal tail in synthetic buffers [18], inseminating them directly into the uterus [19] have resulted in successful births [2, 3] possibly indicating that seminal plasma is not at all required to affect fertilization. However, strong evidence in sheep indicate that insemination with ejaculated spermatozoa achieve considerably greater pregnancy rates than epididymal spermatozoa [20, 21], suggesting presence of a factor in ram seminal plasma which plays a crucial role in aiding sperm transport across the cervix. Besides this, seminal plasma in sheep contains a protein called RSVP-14 which is homologous to BSP in bull [22]. This protein has been reported to provide protection to ram spermatozoa during its cryostorage [23]. It binds to the plasma membrane of sperm [24] and helps in capacitation [25].
The seminal plasma contains a robust antioxidant enzyme system in the form of reduced glutathione (GSH), glutathione peroxidase (GSH-PX), catalase (CAT) and superoxide dismutase (SOD) which counter harmful effects of excess ROS [26]. Several studies [27,28,29] have reported the positive effect of seminal plasma supplementation on sperm motility, capacitation status, and ability to penetrate cervical mucus in frozen-thawed ram spermatozoa.
At the time of ejaculation, the epididymal tail sperm become activated on mixing with the seminal fluid secreted from accessory sex glands. This is called activated motility [30]. Contrary to this, under in vitro conditions, epididymal tail sperm remain deprived of important critical seminal plasma components and optimal activation of epididymal tail sperm from an animal after its death has always been a challenge. Therefore, optimal activation of epididymal tail sperm is crucial to its preservation and subsequent fertility.
To date, epididymal tail sperm of different species have been activated/retrieved in synthetic buffers such as tris, sodium citrate, powdered coconut water and sperm-TALP [12,13,14, 31,32,33] with varying results. Seminal plasma being natural secretion and loaded with a huge number of critically important components, activation/recovery of ram epididymal tail sperm in homologous seminal plasma may have a tremendous beneficial effect on the sperm activation and subsequent preservation at 4 °C. There are reports of its use as a supplement in various extenders. However, to the best of our knowledge, there is no published report on the use of homologous seminal plasma for activation of epididymal tail sperm in any of the species. In this endeavor, we conducted this experiment to investigate the epididymal tail sperm activation potential of homologous seminal plasma in comparison to sperm-TALP and tris buffer and their impact on sperm quality during its cold storage for 72 h.
2 Materials and methods
Animals were ethically handled throughout this trial. All chemicals used were of reagent grade. Florescence probes such as FITC-PNA (CAT No L7381–1 mg), PI (P4864–10 mL) and CFDA (C4916–25 mg) and antifading agent-DABCO (290734–100 mL) were procured from Sigma–Aldrich (St. Louis, MO, USA). Other chemicals were procured from Himedia Pvt. Ltd. India.
2.1 Study location, selection and management of the rams
The present study was carried out in Sperm biology laboratory which is situated at the foot hills of greater Himalayan mountainous region of Kashmir with latitude 34°5′ North and longitude 74° 48′ East and lies at an altitude of 1585 m above mean sea level. The temperature ranges between 20 and 33 °C during summer and – 5 to –15 °C during peak winter months. Five healthy crossbred breeding rams with proven fertility (2–5 years age) were selected from Mountain Research Centre for Sheep and Goat (MRCS&G), Faculty of Veterinary Science and Animal Husbandry, Shuhama during breeding season (September-December) when day light length is short (average 8 h/day). The rams were fed concentrate at the rate of 200 g/animal/day with 6 h of grazing during September and October and then from November to December were managed exclusively under intensive system. Intensive feeding included 500 g of concentrate/animal/day and hay (roughage) at the rate of 1 kg/animal/day. Molasses were also given at the rate of 15–30 g/animal/day. Water and salt were provided ad libitum.
2.2 Collection of semen and homologous seminal plasma (SP)
Semen ejaculates (n = 80) were collected by artificial vagina twice a week for sufficient amount of SP collection. Average ejaculate volume ranged between 0.7 and 1.1 ml. The average collection of SP from each ejaculate varied between 0.4 and 0.6 ml. Before semen collection, peri-prepucial area of all rams was thoroughly cleaned with normal saline and long hair was clipped to minimize contamination at the time of semen collection. All ejaculates were examined grossly for any abnormality followed by microscopic examination. Microscopically, mass activity and initial sperm motility of ejaculates were evaluated. Ejaculates with ≥ 3 mass motility score and ≥ 70% initial motility score qualified and thus were pooled. Homologous seminal plasma (SP) was obtained from pooled ejaculates after processing in two-phase centrifugations (A-3000 g, 4 °C, 20 min; B-3600 g, 4 °C, 30 min). To ensure that there was no sperm present in the SP, a drop of recovered HSP was examined under microscope. In case the sperm were spotted under microscope, a third –phase of centrifugation (3600 g, 4 °C, 30 min) was applied. To avoid microbial growth during its storage, Streptopenicillin @ 1 mg/ml was added to the SP samples and were stored at − 20 °C till further use.
2.3 Composition of tris buffer and sperm-TALP
Tris buffer contained 3.028 g tris (Hydroxymethyl amino methane), 1.70 g citric acid monohydrate, 1.25 g fructose and 100 mg strepto-penicillin dissolved in triple glass distilled water up to 100 mL. Sperm-TALP contained 2.886 g Sodium chloride, 0.115 g Potassium chloride, 0.21 g Sodium dihydrogen phosphate, 0.147 g Calcium chloride dehydrate, 0.1115 g Magnesium chloride, 1.85 g Lactic acid, 1.05 g Sodium hydrogen carbonate dissolved in triple glass-distilled water up to 500 mL.
2.4 Collection of testicles, in vitro quality check and experimental design
A total of 25 testicles were collected from freshly slaughtered rams from the local slaughter house and were transported in zip lock bags to the semen biology laboratory in an ice chest (4–5 °C). The testicles were processed within 2 hours of slaughter. The experimental design is depicted in the Fig. 1. After reaching at the laboratory, the weight of the testicles was measured with a weighing balance (CY-104, Citizon, India). Testicles ≥ 100 g were selected for subsequent processing. The mean weight of testicles was 150 ± 2.06. The connective tissue around each testicle was removed with the help of a scissor. The epididymal tails were separated from the testicles and their weight was also measured. The mean weight (g) of epididymal tails was 3.64 ± 0.19. To ensure that epididymal tails contain normal sperm, an in vitro sperm quality check was conducted. A small amount of epididymal fluid containing sperm was extracted with the help of 5 ml syringe and was put on a warm slide. A drop of tris buffer was added to it and a coverslip was placed on the mixture drop. After 2–3 min, it was examined at × 400 magnification of a phase contrast microscope (Eclipse E200, Nikon, Japan). Appearance of vigorous motile sperm with no major sperm abnormalities was considered as testicles being from a normal breedable male. Only 9 testicles (constituted 9 replicates) passed the quality check and were considered for this experiment. Epididymal tail sperm were activated by equally dissecting epididymal tail into 3 parts which were then placed in three small (35 mm) petri dishes designated as SP (Homologous seminal plasma), TP (sperm-TALP), and TR (Tris buffer) each containing 3 ml of respective activation/recovery fluids- SP, TP and TR which constituted three groups. These dishes were kept at 30 °C for 20 min to allow full sperm activation. After 20 min, the epididymal tail pieces were first squeezed and then discarded. Each sperm sample recovered in the dishes was kept in 15 ml centrifuge tubes. A small portion (100 µL) from each group was kept in respective designated 0.5 mL micro centrifuge tubes at 30 °C for pre-storage sperm quality evaluation (this was considered as quality at 0 h). The rest of the samples were stored at 4 °C for subsequent evaluation at 24, 48, and 72 h. The samples were held as such in lab for half an hour before placed in a refrigerator. It took 40 min to one hour to reach 4 °C so the samples were cooled slowly to avoid cold shock.
2.5 Sperm quality evaluation
Sperm plasma and acrosome membrane integrity and their proper function are essential for sperm metabolism, capacitation, oocyte binding and acrosome reaction [34]. The most basic sperm attributes to affect successful fertilization include sperm motility, plasma and acrosomal membrane integrity. The most common sperm variables determined at farm or semen station level include motility, plasma membrane and acrosome integrity which are considered enough [35]. Thus we also considered only basic parameters to study the activating potential and quality during cold storage.
2.6 Sperm motility
Sperm motility was subjectively assessed as per the method of Mortimer [36]. A drop of a sperm sample was placed on a clean grease free slide kept on a warmer stage (37 °C) and a cover slip was put over the drop and then examined at × 400 magnification of a phase contrast microscope (Nikon eclipse 200, Japan). The number of spermatozoa that moved in forward direction in various fields was estimated. Three independent researchers evaluated progressive motility of each sample and average of the three values was considered as the final value for sperm motility.
2.7 Sperm viability using molecular probe CFDA + PI
Sperm viability was determined using molecular probes-Carboxyflorescene diacetate (CFDA) and propidium iodide (PI) as per the method of Harrison and Vickers [37] with some modifications. Firstly, working concentrations of CFDA (0.5 mg/ml) and PI (0.3 mg/mL) were prepared. To make stock solution (25 mg/mL) of CFDA, 1 mL of dimethyl sulphoxide (DMSO) was added to the vial containing 25 mg powdered CFDA at room temperature (20–22 °C) under dark conditions. Working concentration (0.5 mg/mL) was prepared by diluting an aliquot of stock solution with DMSO using C1V1 = C2V2 formula. PI stock is available as 10 mg/mL and working concentration (0.3 mg/mL) was prepared by diluting an aliquot of stock with PBS (pH = 7.2). First semen sample was washed twice in TALP by centrifugation @ 90 g for 3 min. To 40µL of suspension, 15µL of CFDA working solution was added in 0.5 mL microcentrifuge tube under dark condition and incubated at 37 ℃ for 15 min. Then 2 µL PI working solution was added to the mixture and the resulting mixture was again incubated for 5 min. Finally 100 µL of TALP was added to the tube for washing at 90 g for 5 min. Supernatant was discarded and a thin smear was prepared from the sediment on a clean grease-free slide. Air dried smear was examined under epifluorescence microscope (Magnus MLXi21 EPILED, India). The microscope was connected to the computer through Magcam MU2A with 2.3 MP 1/1.19″ CMOS sensor (Magnus, India) operated through Magvision software installed on a computer. Fluorescence was studied using blue (excitation wavelength of 480 nm) and green filters (excitation wavelength of 535 nm). Sperm cells that emitted green fluorescence under blue filter were considered as CFDA positive (live sperm-Fig. 2) and those emitted red fluorescence on green filter were considered as CFDA negative (dead sperm). More than 400 sperm were counted in different fields (10–12) and percentage of CFDA positive sperm cells was determined.
2.8 Acrosomal status using molecular probe (FITC-PNA)
The acrosomal status was evaluated by using FITC-PNA molecular probe (fluorescein isothiocyanate conjugated to peanut agglutinin) as per the method of Singh et al. [38] with some modifications. Briefly, working concentration of FITC-PNA (0.04 mg/mL) and PI (0.3 mg/mL) were prepared. First semen sample was washed twice in TALP by centrifugation @ 90 g for 3 min. The sediment was resuspended in TALP and 40 µL of suspension (10 million sperm) was taken into a 0.5 mL microcentrifuge tube and 15 µL of FITC-PNA working solution was added to it under dark condition and incubated at 37 ℃ for 15 min. Then 2 µL PI working solution was added to the mixture and was again incubated for 5 min. The stain mixture was washed twice in 100 µL of TALP by centrifugation at 90 g for 5 min. Supernatant was discarded and a thin smear was prepared from the sediment on a clean grease-free slide. Air dried smear was examined under epifluorescence microscope similarly as in CFDA plus PI staining. Sperm with green fluorescence on head tip (Fig. 3) were counted as FITC-PNA positive sperm (damaged acrosome) while as sperm with no green fluorescence on head tip were counted as FITC-PNA negative sperm (intact acrosomes). More than 400 sperm were counted in different fields and acrosome intact percentage was determined.
2.9 HOS test (hypo-osmotic swelling test) for functional sperm plasma membrane integrity
The HOS test was performed as per the method described by Vasquez et al. [39]. Briefly 10 µL of a sperm sample was added to 150 µL of the HOS medium (75 mOsm/kg: Sodium citrate 0.367 g and fructose 0.675 g dissolved in 100 ml of distilled water) in a 0.5 mL microcentrifuge tube, which was incubated at 37 °C for 15 min. After incubation, 10 µL of the mixture was placed on a clean, grease-free slide with a cover slip over it and examined at × 400 magnification. Sperm with swollen or coiled tails were counted as HOST reacted sperm (Fig. 4) indicating functionally intact sperm plasma membrane. More than 200 spermatozoa were counted to determine the percentage of HOST reacted spermatozoa.
2.10 Statistical analysis
The data generated were first examined for normal distribution. All the percentage data for each parameter were not normally distributed and were square root transformed. Sperm motility, viability, HOST reacted sperm and percent intact acrosome at different time points of cold storage within groups and between treatment groups at each time point were analyzed using one-way ANOVA. The difference between the means was determined by Tukey’s HSD tests (Post hoc test). The p ≤ 0.05 was considered as statistically significant.
3 Results
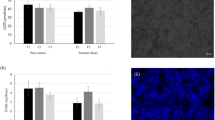
The sperm motility percentage (Fig. 5a) was significantly (p < 0.05) higher for SP (64.33 ± 4.80–48 h; 52.50 ± 2.14–72 h) group compared to both TP (51.66 ± 2.10–48 h; 40.83 ± 3.00–72 h) and TR groups (52.50 ± 4.03–48 h; 41.66 ± 3.33–72 h) at 48 and 72 h of cold storage. Further, it declined significantly (p < 0.05) from 0 to 72 h of cold storage within all the groups (Fig. 5b). The CFDA positive sperm (Viable sperm) percentage (Fig. 6a) for SP group (72.39 ± 0.62) was also significantly (p < 0.05) higher than TP (67.13 ± 1.29) and TR group (66.89 ± 1.65) at 48 and 72 h of cold storage (63.37 ± 2.19-SP, 56.13 ± 1.60-TP, 58.18 ± 1.49-TR). However, it declined significantly (p < 0.05) from 0 to 72 h of cold storage in all the groups except in SP group where no significant difference was observed from 0 to 24 h of cold storage (Fig. 6b). Again HOST reacted sperm percentage (Fig. 7a) for SP group (65.10 ± 1.55) was significantly (p < 0.05) higher at 48 h than TP (59.95 ± 0.24) and TR (61.50 ± 1.11) and also at 72 h of cold storage (55.09 ± 1.17-SP, 46.53 ± 0.78-TP, 50.16 ± 0.16-TR). Further HOST reacted sperm percentage declined significantly (p < 0.05) from 0 to 72 h of cold storage in all the groups (Fig. 7b). FITC-PNA negative sperm percentage (intact acrosome, Fig. 8a) in SP group was significantly (p < 0.05) higher than TP and TR groups at 0 (91.66 ± 0.90-SP, 88.13 ± 1.02-TR), 48 (75.54 ± 0.70-SP, 74.49 ± 1.07-TP) and 72 h (68.27 ± 1.15-SP, 61.97 ± 1.35-TP, 62.91 ± 1.53-TR) of cold storage. Further it declined significantly (p < 0.05) from 0 to 72 h of cold storage in all the groups (Fig. 8b).
a Sperm motility percentage of epididymal tail sperm samples activated in homologous seminal plasma (SP), sperm-TALP (TP) and Tris (TR) at different time points of cold storage. a,bDifferent alphabets indicate significant (p < 0.05) difference between means. b Sperm motility trend from 0 to 72 h of cold storage within each treatment group/activation fluid (SP, TP, TR). a,b,c,dDifferent alphabets indicate significant (p < 0.05) difference between means
a CFDA positive sperm (Viable sperm) percentage in epididymal tail sperm samples activated in homologous seminal plasma (SP), sperm-TALP (TP) and Tris (TR) at different time points of cold storage. a,bDifferent alphabets indicate significant (p < 0.05) difference between means. b CFDA positive sperm trend from 0 to 72 h of cold storage within each treatment group/activation fluid (SP, TP, TR). a,b,c,dDifferent alphabets indicate significant (p < 0.05) difference between means
a HOST reacted sperm percentage in epididymal tail sperm samples activated in homologous seminal plasma (SP), sperm-TALP (TP) and Tris (TR) at different time points of cold storage. a,bDifferent alphabets indicate significant (p < 0.05) difference between means. b HOST reacted sperm trend from 0 to 72 h of cold storage within each treatment group/activation fluid (SP, TP, TR). a,b,c,dDifferent alphabets indicate significant (p < 0.05) difference between means
a FITC-PNA negative sperm (Intact acrosome) percentage in epididymal tail sperm samples activated in homologous seminal plasma (SP), sperm-TALP (TP) and Tris (TR) at different time points of cold storage. a,bDifferent alphabets indicate significant (p < 0.05) difference between means. b FITC-PNA negative sperm (Intact acrosome) trend from 0 to 72 h of cold storage within each treatment group/activation fluid (SP, TP, TR). a,b,c,dDifferent alphabets indicate significant (p < 0.05) difference between means
4 Discussion
Male epididymal duct secretions gradually alter the proteins and lipids on the sperm membrane; however, spermatozoa become mixed with a different set of surface-remodeling substances produced by the accessory sex glands during ejaculation [40]. These proteins together with antioxidant agents are responsible for protection of sperm during cold and cryostorage and enhancement of quality may result due to interaction between molecules on the surface of spermatozoa and in seminal plasma [41, 42]. With this concept, numerous studies [43, 44] have been conducted on supplementation of seminal plasma in the extenders with an aim to improve the quality of the sperm during preservation. But activation of epididymal tail sperm in homologous seminal plasma has not been tried yet. Taking lead, the present experiment was designed to find how efficiently SP activate epididymal tail sperm in comparison to sperm-TALP and tris buffer and its impact on sperm quality during its cold storage for 72 h.
Here we report significantly higher sperm motility, CFDA positive sperm (Live sperm), FITC-PNA negative sperm (Intact acrosome) and HOST reacted (functionally viable) sperm percentage in SP compared to TALP (TP) and tris (TR). Efficient activation and subsequent better sperm quality during cold storage might indicate better interaction of epididymal tail sperm with the constituents of seminal plasma during recovery than when sperm are recovered directly in TR and TP. Expectedly such an interaction does not exist in TP and TR. When sperm are directly exposed to seminal plasma as in SP group, they become mixed with a different set of surface-remodeling substances present in it produced by the accessory sex glands during ejaculation. These proteins together with antioxidant agents could be responsible for efficient activation and protection of sperm during cold and cryostorage [41, 42, 45]. There are no published data to compare the present findings directly, however, supplementation of seminal plasma in extenders have been reported to improve sperm quality even incomplete interaction with the components of seminal plasma due to dilution effect [46]. Yoval-Montemira et al. [43] and Neuhauser et al. [47] reported increase in motility with the addition of seminal plasma to tris at 10% level indicating beneficial effect of seminal plasma components on sperm attributes. The beneficial effect can also be explained on the fact that SP contains protective proteins and robust antioxidant system that prevent sperm from oxidative stress and cryocapacitation [48, 49]. Studies have also been conducted species-wise on the beneficial effect of homologous SP or heterologous SP on sperm attributes. It was found that homologous seminal plasma benefit ram spermatozoa more than bull spermatozoa after its supplementation to extender [50]. This indicates that this effect is quite prevalent in sheep. Nongbua et al. [51] also investigated the effect of adding homologous or heterologous bovine seminal plasma to seminal plasma free sperm samples before freezing on sperm quality after thawing. The authors reported that addition of 5% homologous seminal plasma offered beneficial effect on sperm quality compared to 5% heterologous seminal plasma obtained from high fertility bulls. These findings are in agreement with the findings of our study. Beneficial effect of homologous seminal plasma obtained from high fertile bulls on motility characteristics of bull cauda epididymal sperm has also been reported by Holden et al. [52]. These findings also support our results as homologous SP obtained from high fertile rams in our study also efficiently activated the epididymal sperm and had profound effect on the quality of epididymal tail sperm during cold storage. Nikolovski et al. [53] also reported better sperm quality with combination of glutathione and homologous seminal plasma (20%). Our results are also corroborate well with the findings of Fernando-gago et al. [54] who reported that 50% of seminal plasma supplementation is most efficient concentration compared to 0 and 10% for maintaining the sperm quality in boar during thawing. The significant outcome in this study is that ram epididymal tail sperm could be efficiently activated in homologous seminal plasma, stored and then may be processed/extended before using in various assisted reproductive techniques for better outcome or for prolonged storage. In conclusion, activation of ram epididymal tail sperm in homologous seminal plasma (SP) may be adopted for high quality sperm recovery for efficient short and long term preservation. This study being the first report has opened new avenues of research in this direction and provides an opportunity to further explore the role of seminal plasma in cryoprotection and enhanced fertilizing abilities of epididymal tail sperm.
Data availability
Data sets generated during this study will be made available at the request.
References
Luvoni GC, Morselli MG. Canine epididymal spermatozoa: a hidden treasure with great potential. Reprod Domest Anim. 2017;52:197–201. https://doi.org/10.1111/rda.12820.
Santiago-Moreno J, Toledano-Díaz A, Pulido-Pastor A, Gómez-Brunet A, López-Sebastián A. Birth of live Spanish ibex (Capra pyrenaica hispanica) derived from artificial insemination with epididymal spermatozoa retrieved after death. Theriogenology. 2006;66(2):283–91. https://doi.org/10.1016/j.theriogenology.2005.11.012.
Ocampo LC, DelaRosa ICJ, Lofranco JOC, Ortiz JGM, Ocampo MB. Live birth after artificial insemination using cryopreserved epididymal sperm recovered from the cauda epididymis of slaughtered non-descript bucks in the Philippines. Int J Agric Technol. 2021;17(6):2183–96.
Martínez-Fresneda L, Castaño C, Bóveda P, Tesfaye D, Schellander K, Santiago-Moreno J, García-Vázquez FA. Epididymal and ejaculated sperm differ on their response to the cryopreservation and capacitation processes in mouflon (Ovis musimon). Sci Rep. 2019;9(1):15659. https://doi.org/10.1038/s41598-019-52057-0.
Mota Filho AC, Silva HVR, Nunes TGP, de Souza MB, de Freitas LA, de Araújo AA, da Silva LDM. Cryopreservation of canine epididymal sperm using ACP-106c and TRIS. Cryobiology. 2014;69(1):17–21. https://doi.org/10.1016/j.cryobiol.2014.04.013.
Vernocchi V, Morselli MG, Consiglio AL, Faustini M, Luvoni GC. DNA fragmentation and sperm head morphometry in cat epididymal spermatozoa. Theriogenology. 2014;82(7):982–7. https://doi.org/10.1016/j.theriogenology.2014.07.014.
Kikuchi K, Nagai T, Kashiwazaki N, Ikeda H, Noguchi J, Shimada A, Soloy E, Kaneko H. Cryopreservation and ensuing in vitro fertilization ability of boar spermatozoa from epididymides stored at 4 C. Theriogenology. 1998;50(4):615–23. https://doi.org/10.1016/S0093-691X(98)00166-6.
Muradás PR, Weiss RR, Kozicki LE, Granemann LC, Santos IW, Pimpão CT. Some viability parameters from equine spermatozoa harvested by artificial vagina and by epididymal tail washing. Arch Vet Sci. 2006;11(3):69–74. https://doi.org/10.5380/avs.v11i3.7420.
Tittarelli C, Savignone CA, Arnaudín E, Stornelli MC, Stornelli MA, de la Sota RL. Effect of storage media and storage time on survival of spermatozoa recovered from canine and feline epididymides. Theriogenology. 2006;66(6–7):1637–40. https://doi.org/10.1016/j.theriogenology.2006.01.021.
Martins CF, Driessen K, Costa PM, Carvalho-Neto JO, De Sousa RV, Rumpf R, Dode MN. Recovery, cryopreservation and fertilization potential of bovine spermatozoa obtained from epididymides stored at 5 C by different periods of time. Anim Reprod Sci. 2009;116(1–2):50–7. https://doi.org/10.1016/j.anireprosci.2008.12.018.
Toyonaga M, Kaihara A, Tsutsui T. The quality of cryopreserved sperm collected from feline caudal epididymides stored at room temperature. J Vet Med Sci. 2011;73(10):1395–8. https://doi.org/10.1292/jvms.11-0052.
Lone FA, Islam R, Khan MZ, Sofi KA. Effect of transportation temperature on the quality of cauda epididymal spermatozoa of ram. Anim Reprod Sci. 2011;123(1–2):54–9. https://doi.org/10.1016/j.anireprosci.2010.10.012.
Lone FA, Islam R, Khan MZ, Sofi KA. Effect of different egg yolk-based extenders on the quality of ovine cauda epididymal spermatozoa during storage at 4° C. Reprod Domest Anim. 2012;47(2):257–62. https://doi.org/10.1111/j.1439-0531.2011.01847.x.
Ahmed T, Islam R, Malik AA, Lone FA, Shikari AB. Cryopreservation of ram cauda epididymal spermatozoa using different buffers and sugar combinations. J Anim Res. 2019;9(6):927–33. https://doi.org/10.3095/2277-940X.06.2019.22.
Tecle E, Gagneux P. Sugar-coated sperm: unraveling the functions of the mammalian sperm glycocalyx. Mol Reprod Dev. 2015;82(9):635–50. https://doi.org/10.1002/mrd.22500.
Caballero I, Parrilla I, Almiñana C, Del Olmo D, Roca J, Martínez EA, Vázquez JM. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod Domest Anim. 2012;47:12–20. https://doi.org/10.1111/j.1439-0531.2012.02028.x.
Soleilhavoup C, Tsikis G, Labas V, et al. Ram seminal plasma proteome and its impact on liquid preservation of spermatozoa. J Proteomics. 2014;109:245–60. https://doi.org/10.1016/j.jprot.2014.07.007.
Chaveiro A, Cerqueira C, Silva J, Franco J, da Silva FM. Evaluation of frozen thawed cauda epididymal sperms and in vitro fertilizing potential of bovine sperm collected from the cauda epididymal. Iran J Vet Res. 2015;16(2):188.
Monteiro GA, Papa FO, Zahn FS, Dellaqua JA Jr, Melo CM, Maziero RR, Avanzi BR, Alvarenga MA, Guasti PN. Cryopreservation and fertility of ejaculated and epididymal stallion sperm. Anim Reprod Sci. 2011;127(3–4):197–201. https://doi.org/10.1016/j.anireprosci.2011.08.002.
López-pérez A, Perez-clariget R. Ram seminal plasma improves pregnancy rates in ewes cervically inseminated with ram semen stored at 5 °C for 24 hours. Theriogenology. 2012;77(2):395–9. https://doi.org/10.1016/j.theriogenology.2011.08.013.
Rickard JP, Pini T, Soleilhavoup C, Cognie J, Bathgate R, Lynch GW, Evans G, Maxwell WM, Druart X, de Graaf SP. Seminal plasma aids the survival and cervical transit of epididymal ram spermatozoa. Reprod. 2014;148(5):469–78. https://doi.org/10.1530/REP-14-0285.
Bergeron A, Villemure M, Lazure C, Manjunath P. Isolation and characterization of the major proteins of ram seminal plasma. Mol Reprod Dev. 2005;71(4):461–70. https://doi.org/10.1002/mrd.20310.
Del Valle I, Casao A, Pérez-Pé R, Holt WV, Cebrián-Pérez JA, Muiño-Blanco T. Seminal plasma proteins prevent detrimental effects of ram sperm cryopreservation and enhance the protective effect of lecithin. Biochem Anal Biochem. 2017;6:319. https://doi.org/10.4172/2161-1009.1000319.
Souza CEA, Rego JPA, Lobo CH, Oliveira JTA, Nogueira FC, Domont GB, Moura AA. Proteomic analysis of the reproductive tract fluids from tropically-adapted Santa Ines rams. J Proteomics. 2012;75(14):4436–56. https://doi.org/10.1016/j.jprot.2012.05.039.
Leahy T, Rickard JP, Bernecic NC, Druart X, de Graaf SP. Ram seminal plasma and its functional proteomic assessment. Reprod. 2019;157(6):R243–56. https://doi.org/10.1530/REP-18-0627.
Dacheux JL, Belghazi M, Lanson Y, Dacheux F. Human epididymal secretome and proteome. Mol Cell Endocrinol. 2006;250(1–2):36–42. https://doi.org/10.1016/j.mce.2005.12.022.
Graham JK. Effect of seminal plasma on the motility of epididymal and ejaculated spermatozoa of the ram and bull during the cryopreservation process. Theriogenology. 1994;41(5):1151–62. https://doi.org/10.1016/S0093-691X(05)80037-8.
Maxwell WMC, Johnson LA. Physiology of spermatozoa at high dilution rates: the influence of seminal plasma. Theriogenology. 1999;52(8):1353–62. https://doi.org/10.1016/S0093-691X(99)00222-8.
Leahy T, Marti JI, Evans G, Maxwell WMC. Seasonal variation in the protective effect of seminal plasma on frozen–thawed ram spermatozoa. Anim Reprod Sci. 2010;119(1–2):147–53.
Quill TA, Garbers DL. Sperm motility activation and chemoattraction. In: Hardy DM, editor. Fertilization. Cambridge: Academic Press; 2002. p. 29–55.
Buranaamnuay K. Sperm-TALP: an alternative extender for retrieving and diluting epididymal sperm in the domestic cat. Reprod Domest Anim. 2013;48(6):912–7. https://doi.org/10.1111/rda.12185.
Olaciregui M, Gil L, Montón A, Luño V, Jerez RA, Martí JI. Cryopreservation of epididymal stallion sperm. Cryobiology. 2014;68(1):91–5. https://doi.org/10.1016/j.cryobiol.2013.12.009.
De Sousa BB, Izzo RG, Silva HVR, Nunes TGP, Brito BF, da Silva TFP, da Silva LDM. Recovery and cryopreservation of epididymal sperm from domestic cat using powdered coconut water (ACP-117c) and Tris extenders. Cryobiology. 2020;92:103–8. https://doi.org/10.1016/j.cryobiol.2019.11.042.
Brito LFC, Barth AD, Bilodeau-Goeseels S, Panich PL, Kastelic JP. Comparison of methods to evaluate the plasmalemma of bovine sperm and their relationship with in vitro fertilization rate. Theriogenolog. 2003;60(8):1539–51. https://doi.org/10.1016/S0093-691X(03)00174-2.
Maside C, Recuero S, Salas-Huetos A, Ribas-Maynou J, Yeste M. Animal board invited review: an update on the methods for semen quality evaluation in swine—from farm to the lab. Animal. 2023;17(3):100720. https://doi.org/10.1016/j.animal.2023.100720.
Mortimer D, Shu MA, Tan R. Standardization and quality control of sperm concentration and sperm motility counts in semen analysis. Hum Reprod. 1986;1(5):299–303. https://doi.org/10.1093/oxfordjournals.humrep.a136409.
Harrison RAP, Vickers SE. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J Reprod Fert. 1990;88(1):343–52. https://doi.org/10.1530/jrf.0.0880343.
Singh RK, Kumaresan A, Chhillar S, Rajak SK, Tripathi UK, Nayak S, Malhotra R. Identification of suitable combinations of in vitro sperm function test for the prediction of fertility in buffalo bull. Theriogenology. 2016;86:2263–71. https://doi.org/10.1016/j.theriogenology.2016.07.022.
Vasquez J, Florentini EA, Camarago LA, Gonalez J, Valdivia M. Hypo osmotic swelling test in ram (Ovis aries) spermatozoa. Livest Sci. 2013;157:618–22. https://doi.org/10.1016/j.livsci.2013.08.023.
Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell. 2015;163(5):1225–36. https://doi.org/10.1016/j.cell.2015.10.029.
Catalán J, Yánez-Ortiz I, Tvarijonaviciute A, González-Aróstegui LG, Rubio CP, Barranco I, Yeste M, Miró J. Seminal plasma antioxidants are related to sperm cryotolerance in the horse. Antioxidants. 2022;11(7):1279. https://doi.org/10.3390/antiox11071279.
Kowalczyk A. The role of the natural antioxidant mechanism in sperm cells. Reprod Sci. 2022;29(5):1387–94. https://doi.org/10.1016/j.anireprosci.2009.12.010.
Yoval-Montemira MF, Castro C, Ake-Villanueva R, Magaña-Monforte JG, Bottini-Luzardo M, Segura-Correa JC (2020) Seminal plasma inclusion during boar semen freezing improves post-thaw sperm quality in the tropics. Livest Res Rural 32(11).https://www.lrrd.org/lrrd32/11/jose.s32183.html
Green C, Rickard JP, de Graaf SP, Crean AJ. From one ejaculate to another: transference of sperm traits via seminal plasma supplementation in the ram. Biology. 2020;9(2):33. https://doi.org/10.3390/biology9020033.
Rodríguez-Martínez H, Kvist HU, Ernerudh J, Sanz Calvete LJJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(1):11–22. https://doi.org/10.1111/j.1600-0897.2011.01033.x.
Peris-Frau P, Soler AJ, Iniesta-Cuerda M, et al. Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int J Mol Sci. 2020;21(8):2781. https://doi.org/10.3390/ijms21082781.
Neuhauser S, Dörfel S, Handler J. Dose-dependent effects of homologous seminal plasma on motility and kinematic characteristics of post-thaw stallion epididymal spermatozoa. Andrology. 2015;3(3):536–43. https://doi.org/10.1111/andr.12003.
Muiño-Blanco T, Pérez-Pé R, Cebrián-Pérez J. Seminal plasma proteins and sperm resistance to stress. Reprod Dom Anim. 2008;43:18–31. https://doi.org/10.1111/j.1439-0531.2008.01228.x.
Rodriguez-Martinez H, Martinez EA, Calvete JJ, Peña Vega FJ, Roca J. Seminal plasma: relevant for fertility? Int J Mol Sci. 2021;22:4368–96. https://doi.org/10.3390/ijms22094368.
Höfner L, Luther AM, Waberski D. The role of seminal plasma in the liquid storage of spermatozoa. Anim Reprod Sci. 2020;220:106–290. https://doi.org/10.1016/j.anireprosci.2020.106290.
Nongbua T, Al-Essawe EM, Edman A, Johannisson A, Morrell JM. Effect of adding heterologous versus homologous bovine seminal plasma prior to cryopreservation on bull sperm quality after thawing. Zygote. 2018;26(5):388–94. https://doi.org/10.1017/S0967199418000394.
Holden SA, Fernandez-Fuertes B, Murphy EM, Lonergan P, Fair S. Effect of seminal plasma from high-and low-fertility bulls on cauda epididymal sperm function. Reprod Fertil Dev. 2017;29(12):2457–65. https://doi.org/10.1071/RD17136.
Nikolovski M, Dovenska M, Ilievska K, et al. Homologous seminal plasma and glutathione promote pre-capacitation motility and structural stability of cryopreserved ram spermatozoa. Maced Vet Rev. 2019;42(2):169–79. https://doi.org/10.2478/macvetrev-2019-0022.
Fernández- Gago R, Álvarez-Rodríguez M, Alonso ME, González JR, Alegre B, Domínguez JC, Martínez-Pastor F. Thawing boar semen in the presence of seminal plasma improves motility, modifies subpopulation patterns and reduces chromatin alterations. Reprod Fertil Dev. 2017;29(8):1576–84. https://doi.org/10.1071/RD15530.
Acknowledgements
The authors would like to thank in charge Mountain Research Centre for Sheep and Goat for sparing fertile cross bred rams for harvesting of quality homologous seminal plasma.
Funding
No specific funding was received for this research.
Author information
Authors and Affiliations
Contributions
Farooz A. Lone conceptualized and supervised the whole experiment, contributed to study design, drafted the intellectual content and reviewed. Rameez Ali Dar contributed to the study design and intellectual content. Pawan Preet Singh, Gh. Rasool Bhat conducted the experiment and generated data. Muzamil Abdullah contributed to the experimental design and manuscript reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no competing interests.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Animal Ethics Committee, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-Kashmir, India Vide No: AU/FVS/IAEC-17/236, dated: 16th of January 2023. The present experiment on animals fully complied with the Basel declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, P.P., Dar, R.A., Lone, F.A. et al. Homologous seminal plasma efficiently activates epididymal tail sperm compared to traditional tris buffer and sperm-TALP in sheep. Discov Anim 1, 2 (2024). https://doi.org/10.1007/s44338-024-00002-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44338-024-00002-5