Abstract
Estuaries are crucial coastal ecosystems facing increased pressures from human activities. The Cross River Estuary in Nigeria is a vital tropical mesotidal estuary influenced by diverse anthropogenic and natural processes. This study investigated the distribution of foraminiferal assemblages in the estuary's sediments, alongside environmental parameters such as heavy metals, pH, depth, temperature, salinity, mean grain size, and dissolved oxygen. Field sampling involved collecting 55 bottom water and sediment samples, with laboratory analyses focusing on grain-size distribution, foraminiferal identification, and heavy metal concentrations. Results indicated that heavy metal concentrations, except for iron, were within permissible limits. A total of 287 benthic foraminiferal tests representing four species were identified. These were primarily agglutinated species, with Arenoparrella mexicana and Miliammina fusca being the most abundant. Foraminiferal distribution was influenced by sediment type, salinity, and pH, rather than heavy metal concentration. Principal Component Analysis (PCA) revealed weak relationships between foraminiferal species and environmental variables, indicating limited influence of mean grain size and heavy metals on foraminiferal distribution. The study highlights the adaptability of certain foraminiferal species to a wide range of environmental conditions and underscores the need for continuous monitoring of the estuarine ecosystem to assess the impacts of ongoing anthropogenic activities. The findings provide a baseline for future environmental assessments and the use of foraminifera as bioindicators in tropical estuarine environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Estuaries are important coastal ecosystems with active land–ocean interactions [1, 2]. They are influenced by natural processes, including waves, tides, and fluvial influx [3]. Estuaries serve as homes for many plants and animals and are often highly influenced by anthropogenic activities [4]. Globally, estuaries are increasingly under pressure from urban development. As industries continue to expand to meet the needs of growing coastal populations, estuarine systems are continually degraded by effluents resulting from a variety of human activities.
The population of the Cross River State increased from 1.9 million residents in 1991 to 4.4 million in 2022 [5]. The Cross River Estuary, which is one of the most important estuaries on the eastern coast of Nigeria, is a tropical mesotidal estuary that empties into the Atlantic Ocean through the Gulf of Guinea. The estuary is fed by several tributary rivers, including Calabar, Great-Kwa, Mbo, and Akpa-Yafe [6,7,8,9,10]. Semidiurnal tides generate flood and ebb currents. The Cross River estuary is a major navigation channel for vessels carrying crude oil and is a shipping route to the Nigerian Ports Authority and Export Processing Zone in Calabar. A wide range of boats are extensively used in the area for commercial fishing, maritime transportation, sand mining, and seafood trading. Agricultural activities are common along the banks of the channel. At present, municipal, agricultural, and industrial wastes are discharged through river tributaries into estuaries. Untreated wastes and effluents can alter the physical, chemical, and biological characteristics of an ecosystem [11]. With the increasing human population, these activities are degrading the estuary.
In this study, we investigated the distributions of foraminiferal assemblages in Cross River estuary sediments and compared them with environmental parameters, including heavy metals, pH, depth, temperature, salinity, mean grain size, and dissolved oxygen. Estuarine sediments act as sinks for many contaminants, including heavy metals [2, 12, 13]. These metals are often released from multiple sources, such as chemical weathering, agricultural waste, and industrial effluents [2, 13]. Foraminifera are single-celled protists with calcareous or agglutinated tests. They are sensitive to immediate environmental conditions and are widely used as modern and ancient analogs to identify paleoenvironments [9, 14,15,16,17,18]. Benthic foraminifera have been widely applied in environmental studies at spatial and temporal scales [14, 16,17,18,19,20,21,22,23,24] and are known to have good preservation potential, making them useful for reconstructing past ecosystem characteristics.
Several studies have been carried out on the use of foraminifera as bioindicators of pollution [4, 25,26,27]. Many studies have reported that polluted areas are typically associated with decreased foraminiferal density and diversity [12, 14, 28,29,30,31]. Langer et al. [32] studied shallow-water nearshore benthic foraminiferal assemblages in Gabon, West Africa, and attributed the high abundance and diversity of shallow-water nearshore benthic foraminiferal species to coastal sites that were not impacted by offshore drilling. The deteriorating health of estuarine environments in the Niger Delta region of Nigeria may be attributed to the effects of oil exploration and oil-related industrial activities. The geochemistry of mangrove swamps in the Niger Delta region of Nigeria was studied by Antia and Santa [33]. The authors observed increased concentrations of hydrocarbons in the easternmost coastal swamps of the Niger Delta (e.g., Cross River Estuary, Calabar River, Qua-Iboe River estuary, Great-Kwa River) despite the prevalence of oil spills in the western mangrove swamp areas (e.g., Forcados, Andoni and Nun estuaries). The higher hydrocarbon concentrations observed in the mangrove sediments of estuaries such as the Cross River estuary may be related to the eastward-flowing longshore currents, which transport oil spills to the easternmost coastal swamp areas as soon as they are flushed out of the western coastal swamps [33]. Fossil foraminifera are useful in paleoenvironmental interpretations of brackish and marine ecosystems, and their usefulness becomes more reliable when the distributions of modern analogs are well studied.
The distribution of foraminiferal assemblages in Nigerian coastal waters have been documented. Emeka et al. [9] studied the distribution patterns of foraminiferal assemblages in modern sediments of the Qua-Iboe River estuary and observed an abundance of agglutinated forms in the middle portions of the estuary where brackish-water conditions prevailed. The authors concluded that the distribution of foraminiferal species in the estuary is strongly influenced by salinity. Asseez et al. [34] studied patterns of foraminifera in the Ogun River estuary, and the authors identified salinity and rate of sedimentation as primary controlling factors. The distribution of foraminifera in the Bonny estuary, Niger Delta region of Nigeria, was studied by Dublin-Green [35]. The author considered low pH of the sediment as the major factor influencing the abundance of agglutinated tests in the upper and middle reaches of the estuary.
Given the ever-increasing human population and associated industrial development, our goal is to provide information on the ecological status and distribution pattern of foraminiferal assemblages in the Cross River estuary. Such information will be relevant in future monitoring of environmental changes in response to natural or anthropogenic impacts. A previous study of the ecology and distribution of foraminifera in the Cross River estuary was reported by Ramanathan [19]. The author reported two broad biotopes, the Arenoparella mexicana biotope, which is characteristic of the upper reaches of tidal waters, and the Ammonia tepida biotope, which is characteristic of the lower reaches of estuaries. Using a multidisciplinary approach (biological, sedimentological and geochemical), the objectives of our study are (1) to investigate the distribution and abundance of foraminifera in sediments of the Cross River estuary and (2) to elucidate the relationship between foraminifera and possible influencing environmental factors.
2 Methods
2.1 Field sampling
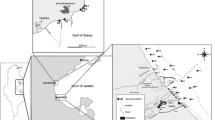
In December 2022, an outboard motor boat was utilized to collect 55 bottom water and sediment samples from georeferenced locations in the Cross River estuary (Fig. 1). Water samples were taken from the sediment–water interface with a Nansen bottom-water sampler. The bottom-water temperature and pH were measured in situ using a multiparameter sensor probe (Hanna HI 98130), while the salinity and dissolved oxygen content were measured in situ using a salinity-calibrated refractometer and a waterproof portable dissolved-oxygen meter (HI 198193). An echosounder was used to take depth readings at each georeferenced sample point. Using a Van Veen grab sampler, bottom sediment samples (top 0–2 cm) were taken from the channel. The surficial sediment was carefully scraped out of the sampler, placed in labeled plastic bags, preserved on ice, and transported to the laboratory.
2.2 Laboratory analysis
In the laboratory, wet sediment samples were thoroughly stirred in a mortar, in order to disaggregate them. They were subsampled into two 10 g fractions for grain-size and foraminiferal analyses, as well as heavy metal analyses, respectively.
2.3 Grain-size analysis
Each wet sediment sample was soaked in distilled water for 24 h. The disaggregated sludge was subsequently washed through a 63 µm sieve, separating mud from sand. This procedure was necessary to prevent the generation of secondary grain-sizes during disaggregation of dry samples. Also, foraminifera are typically sand-sized. The mud suspension was collected in a pan and oven dried at 40 °C, to recover the mud fraction. The sand fraction (> 63 µm) retained on the sieve was transferred into a Whatman No.1 Filter paper and air dried. The dry sediments were weighed using an electronic scale, and the dry sand fraction was sieved through a stack of sieves, following standard procedures as described by Folk [36]. The samples were retained for foraminiferal analysis.
2.4 Foraminiferal analysis
The weighed sand fractions (> 63 µm) were examined microscopically for the presence of foraminiferal tests following methods described by Carnahan et al. [12]. Each sample was poured into a petri dish and gently mixed for adequate representation of grain sizes before taking a small scoop into the counting tray [12]. Sediments were examined under a binocular microscope and, when possible, 100–300 foraminiferal specimens were picked from each sample and placed on a micropaleontological faunal slide. Identifications were based on relevant reference materials [38] and updated websites such as marinespecies.org and foraminifera.eu. The total assemblage (live and dead specimens) was used in this study. Foraminiferal abundance was expressed as number found per 10 g of dry sediment. Reworked specimens were identified by examining their preservation status, color, stratigraphic inconsistencies, as well as fragmentation and abrasion state.
Two way hierarchical clustering (Q and R-mode cluster analysis) was performed using PAST v. 4.17 on only samples with foraminiferal assemblages. Cluster analysis was based on Complete Linkage. All taxa present in the sample were used for the analysis. The Q-mode was conducted to define similarity, composition and abundance of foraminifera at each sample station while R-mode cluster analysis was performed to establish the relationship between foraminifera and environmental variables. Principal Component Analysis (PCA) was conducted using PAST v. 4.17 to explore relationships between foraminiferal species, heavy metals and bottom-water parameters. The spatial distribution map of foraminifera was plotted using ArcGIS v.10.8.1 (Esri Inc, Berkeley, California).
2.5 Heavy metal analysis
A total of 10 g of sediment was taken for heavy-metal (Cu, Fe, Cr, Zn, Pb, Cd) analyses at selected sampling stations. The moist sediment samples were air-dried for an hour, then gently crushed and sieved with a 2 mm mesh size sieve. Then 2 g of well ground sample was transferred into a 250 ml measuring beaker. Deionized water (50 ml) was added to the solution and stirred. The sample was treated with 12 ml concentrated HCl and 10 ml HNO3, then heated in a water bath until half the original volume was reached. The solution was allowed to cool and then filtered into a volumetric flask. The filtrate was diluted with 100 ml of deionized water and taken for heavy metals analysis. Heavy metals were measured using Atomic Absorption Spectrometer UNICAM model SOLAAR 969. The analytical procedures have been described elsewhere in detail [37].
3 Results
The heavy metal concentrations in the sediment samples and the maximum permissible limits established by FEPA [39] are presented in Table 1. The mean values of Cu, Pb, Zn, Cd, Ni and Fe in the sediment were 0.26 mg/kg, 0.003 mg/kg, 0.42 mg/kg, 0.03 mg/kg, 0.52 mg/kg, and 0.93 mg/kg, respectively (Table 1). The mean concentrations of metals in the sediments decreased in the following sequence: Fe > Ni > Zn > Cu > Cd > Pb. The heavy metal concentrations in the sediment showed that the metal concentrations were low and within the maximum permissible limits established by the FEPA [39] except for Fe (mean = 0.93 mg/kg).
The depth, temperature, salinity, dissolved oxygen, and pH data recorded at the sampling stations are presented in Table 2. A maximum water depth of 11.8 m was recorded in the study area (Fig. 1). A total of 287 benthic foraminiferal tests representing four species were picked and identified in the bottom sediments of the Cross River estuary. Foraminifera were identified from 12 sampling stations. The number of species per sampling station ranged from 1 to 3, with a maximum of 3 species recorded at station 47 in the lower estuary. Foraminifera were recovered from water depths ranging between 0.7 and 10.3 m with bottom-water temperatures ranging between 28.9 and 31 °C. The pH varied from highly corrosive to near-normal shallow marine (5.5–7.8), and the salinity ranged from 0 ppt (oligohaline) to 15 ppt (polyhaline). The dissolved oxygen concentration varied between 0.5 and 10.8 mg/l. The sediments varied in terms of the proportions of sand and mud, with mud predominating in most of the samples (Appendix 1).
Foraminiferal tests were not recovered from sediments in the upper estuary (Fig. 2). Among 12 samples from the central and lower portions of the estuary, three agglutinated species (Miliammina fusca, Arenoparrella mexicana, Arenoparrella sp.) and one calcareous hyaline species (Ammonia parkinsoniana) were identified. The highest foraminiferal concentration (125 specimens/10 g dry sediment) was recorded at station 21 (Fig. 2), which was located on the western banks of the central channel near Parrot Island. This station is characterized by mangrove vegetation and a shallow water depth (< 1 m). The number of specimens per 10 g exceeded 10 at only four additional stations. Micrographs of foraminiferal species identified in the sediments of the estuary are shown in Fig. 3.
Foraminiferal abundance and species richness map of the Cross-River estuary (pie chart size indicates relative foraminiferal abundance in specimens per 10 g of sediment; see Table 2)
The Q-mode cluster analysis performed on foraminiferal assemblages produced two distinct clusters, A and B (Fig. 4). Cluster A consisted of eight samples (14, 18, 22, 29, 32, 34, 37, 47, 54) in which Arenoparrella mexicana, Ammonia parkinsoniana and Arenoparrella sp. were found. Arenoparrella mexicana constituted 72% of the total assemblage, with Ammonia parkinsoniana and Arenoparrella sp. constituting 26% and 0.2% respectively of the total. One species, Miliammina fusca, remained an outlier at sample station 21(Fig. 4). Cluster B consisted of three samples (27, 37, 42) in which Arenoparrella mexicana and Arenoparrella sp. were found. Arenoparrella mexicana constituted 87% of the total assemblage, with Arenoparrella sp. constituting 13% of the total. Based on R-mode cluster, two assemblages have been distinguished namely, Miliammina fusca (Group 1) and Arenoparrella mexicana (Group 2). Miliammina fusca assemblage shows a stronger relationship with mean grain size, temperature and heavy metals including Cu, Ni, Zn, Cd and Fe except Pb. Based on R-mode cluster, Arenoparrella mexicana assemblage shows better relationship with environmental variables including salinity, pH, dissolved oxygen, depth and Pb (Fig. 4). Other species including Arenoparrella sp. and Ammonia parkinsoniana are found within this assemblage.
In the present study, PCA was used to explore the relationships between foraminiferal taxa, heavy metal concentrations and bottom water parameters. Three principal components, 1,2 and 3, explained 74% of the variance in the data (Figs. 5, 6, Appendix 4). Principal component 1 (PC1), principal component 2 (PC2) and Principal component 3 (PC3) explained 42%, 18% and 14%, respectively, of the total variance (Fig. 6, Appendix 4). Principal component 1 (PC1) contained a high positive loading of A. mexicana, mean grain size, temperature, Zn, Fe, Ni; a negative loading of depth and dissolved oxygen; and small positive loadings of M. fusca, Arenoparrella sp. and heavy metals including Cd and Cu. Principal component 2 (PC2) contained a high positive loading of Arenoparrella sp. and A. mexicana; a negative loading of Cu, Cd, Ni, Fe and small positive loadings of temperature and Zn.. Principal component 3 (PC3) contained a high positive loading of Ammonia parkinsoniana; a negative loading of mean grain size and temperature; and small positive loadings of pH, salinity, dissolved oxygen and heavy metals including Ni and Fe. Results of Hierarchical cluster analysis generally agreed with those of PCA.It appears that the heavy metal concentration in the sediment did not significantly influence the distribution of foraminiferal species since they generally occurred in low concentrations. Compared to the other variables, M. fusca exhibited better relationships with mean grain size and temperature at PC1. Arenoparrella sp. and A. mexicana exhibited stronger relationships with salinity and pH at PC2 while Ammonia parkinsoniana exhibited better relationships with depth, DO and heavy metals including Pb, Cu and Cd at PC3 (Fig. 5). Small positive or negative values (small loadings) are indicative of weak relationships, while higher values (large loadings) indicate stronger relationships [40].
4 Discussion
The environmental conditions recorded in the Cross River estuary during the study were consistent with the tropical setting and strong fluvial influence. Water temperatures were consistently warm, with a low temperature of ~ 29 °C recorded in the oligohaline (< 5 ppt) to mesohaline (5–10 ppt) middle estuary and high temperatures of 31 °C in the polyhaline (> 15 ppt) lower estuary. Because both pH and dissolved oxygen concentrations are strongly influenced by photosynthesis in warm, shallow waters, these parameters can vary greatly with time of day, with low values in the early morning and high values in the late afternoon. They can also be consistently low in organic-rich sediments that have accumulated below the depth of light penetration, which can be quite shallow (< 1–2 m) in such estuaries [16, 19]. Finally, they are influenced by mixing, especially in shallow waters. Fluctuations in bottom-water characteristics may be associated with changes in the tidal cycle. Flood currents transport seawater into estuaries, resulting in elevated pH and salinity conditions downstream of the channel.
Sandy sediments are present, consistent with the influence of fluvial and tidal currents and wave action. In less dynamic settings, organic-rich muds predominate, as is typical for such estuaries. Iron (Fe) was the most abundant metal in the sediment samples, while Pb had the lowest concentration. Fe was previously reported by Akpan and Thompson [41] as the most abundant metal in beach sediments along the Cross River. Similar findings have been reported by Ogri et al. [42] for coastal waters and sediments and Essien et al. [43] for sediments in the Cross River estuary mangrove swamp. Fe is introduced into estuaries from natural weathering of rocks and soil. Its richness in estuarine sediment may also be attributed to the local geology of the area and the presence of mangroves fringing the banks of the channel, which are associated with reducing environmental conditions. The Cross River flows through Fe-bearing basement rocks. An increased Fe content can be associated with the erosion of rocks in the area and incessant rain, which drains iron-rich sediments into the estuary. The values of all the metals analyzed were lower than the average shale value (Table 1), as reported by Turekian and Wedepohl [44].
Overall, very few foraminiferal specimens were found among the 55 samples collected from the Cross River estuary. A total of 12 specimens were identified (22%), and only four of those samples had more than 20 specimens per 10 g of sediment; only one sample (station 21) exceeded 100 per 10 g. Four species were found overall; in eight samples, only a single species was found. Two species were found in three samples, and three species were found in one sample. The highest number of specimens was found near Parrot Island (station 21). These specimens were found in very fine-grained sands in waters that were shallow (< 1 m), fresh (0 ppt), and corrosive (pH = 5.7) with low dissolved oxygen (2.2 mg/l).
The agglutinated Arenoparrella mexicana was the most widely distributed of the four species. It was found in ten samples ranging from Parrot Island in the middle estuary to Tom Shot Island in the lower section. This species made up 46% of the total foraminiferal assemblage (i.e., 131 specimens). Widely distributed in many brackish settings, this species has been documented in other estuaries of the Gulf of Guinea [19, 35]. Samples containing A. mexicana were recovered from muddy to very fine-grained sands under a wide range of conditions, including water depths ranging from 0.7 to 10.3 m, salinities ranging from 4 to 15 ppt, dissolved oxygen concentrations ranging from 2.2 to 10.7 mg/l, and pH values ranging from low to near-normal marine conditions (5.5–7.8). This species is clearly adaptable to a wide range of environmental conditions. Ramanathan [19] recognized A. mexicana as the dominant species of the upper Cross River estuary environment. The author reported low pH (5.8–7.0), fine-grained substrate and low salinity (2.33–27.7 ppt) as environmental factors that might have influenced the distribution of this species.
Similarly, the total number of Miliammina fusca, which is also an agglutinated species, was similar to that of A. mexicana, with 125 specimens composing 45% of the specimens recovered from the samples (Table 2). However, the distribution of M. fusca was much more limited. These specimens were recovered primarily from muddy sediment along the vegetated western banks of the central channel (stations 21 and 29), near Parrot Island. Specimens were found in samples from depths < 1 m at oligohaline (or nearly so) salinities (0–6 ppt) with low pH (< 6) and low dissolved oxygen (< 2.5 mg/l). The low pH of bottom waters is promoted by the abundance of organic-rich sediments from fringing mangrove swamps. M. fusca is cosmopolitan and has been recorded in habitats such as brackish estuaries, mangrove swamps and lower salt marshes [32, 45, 46]. Such habitats are stressful for species that produce calcareous tests, favoring agglutinated taxa [46]. Martins et al. [47] noted that M. fusca is often associated with organic enrichment. Ramanathan [19] observed an increase in the occurrence of this fungus with decreasing salinity in the Cross River and reported that M. fusca is the second most abundant species in the upper reaches of the Cross River estuary.
Only 17 specimens of the agglutinated Arenoparrella sp. (5.6%) (Table 2) were recovered from shallow water samples (< 1 m). They were found in muddy to very fine-grained sands in mesohaline (10–15) and corrosive (pH = 5.7–7) waters with low dissolved oxygen (2.2–2.3 mg/l) concentrations. A few Arenoparrella sp. were recorded at Parrot Island and along the western bank of the lower estuary.
The distribution of foraminifera within the studied estuary channel has rarely been studied. The only calcareous hyaline species, Ammonia parkinsoniana, constituted only 4% (11 specimens) of the total assemblage (Table 2) and was recovered from depths < 2 m. A few specimens were recorded from samples taken from a sand bar near Parrot Island (Stations 14) and on Tom Shot Island within the lower estuary (Station 47). The specimens were found mainly in fine-grained sands in mesohaline (5–14 ppt) water with a very low pH (5.6–5.8). Dissolved oxygen varied widely between sites where this species was found (2.2–10.8 mg/l). Ammonia parkinsoniana may have been transported by tidal currents into the estuary. Ammonia spp. are known to tolerate varying environmental conditions [46] and have been identified as environmental stress indicators [15]. They are common in habitats near marine to mesohaline environments where fluvial and tidal currents interact [21, 45]. Several species of Ammonia have been associated with muddy sands [16].
Porcelaneous taxa were not found in the estuary, probably because of the combination of low pH and low salinity conditions, which favored the dominance of agglutinated taxa. Ramanathan [19] recognized 11 species (Milammina fusca, Ammonia tepida, Arenoparella mexicana, Ammoastuta inepta, Ammotium salsum, Haplophragmoides subinvolutum, Ammobaculities sp., Elphidium excavatum, Protelphidium anglicum, Quinqueloculina sp., and Globigerionoides ruber) for the Cross River, with up to 85 specimens per gram of sediment. Of the 11 species, Miliammina fusca, Ammonia tepida and Arenoparella mexicana were the most common living species. Ramanathan [19] reported A. mexicana and M. fusca as the most abundant species in the upper estuary and A. tepida as the most common species in the estuary, recording higher frequencies in the lower reaches of the tidal waters.
In contrast, we found only four benthic foraminifera species overall, with three found only at Station 47 on the western bank of the channel near Tom Shot Island. This environment is characterized by shallow water (< 1 m). Species were found in fine sands in waters that were mesohaline (14 ppt) or corrosive (pH = 5.5) with low dissolved oxygen (2.2 mg/l). Agglutinated foraminifera overwhelmingly dominated (96%) the study area. Agglutinated foraminifera have been previously shown to dominate marginal marine environments [45].
Species richness in the estuary is low. The declines in overall abundance and species richness over time may be due to the low salinity conditions in the estuary [7, 8]. This difference may be attributed to the high precipitation and increased influx of fresh water from adjoining tributaries into the Cross River estuary, as historical weather and climate data in the Cross River state show that the average rate of precipitation has increased over the years [48]. The bottom water pH was slightly acidic at most of the stations where foraminiferal species were recovered. The observed low pH may have adversely influenced the dissolution of calcareous taxa. Several authors have found that low pH can induce dissolution of foraminiferal test [49,50,51]. Salinity has been reported as one of the primary controlling factors for the distribution of foraminiferal species [9, 19, 22]. Similar trends of low faunal diversity have been observed in other estuaries and tidal rivers within the Gulf of Guinea [9, 19, 24, 34, 35, 52].
5 Conclusion
This study provides a detailed analysis of the distribution of foraminiferal assemblages in the Cross River estuary and their relationship with environmental parameters such as heavy metals, pH, depth, temperature, salinity, mean grain size, and dissolved oxygen. Our findings indicate that the foraminiferal population in this estuary is relatively sparse, with only four species identified—Miliammina fusca, Arenoparrella mexicana, Arenoparrella sp., and Ammonia parkinsoniana. The agglutinated species, M. fusca and A. mexicana, were the most abundant and widely distributed, suggesting their adaptability to a wide range of environmental conditions typically characterized by low pH, low salinity, and low dissolved oxygen levels.
The study reveals that heavy metal concentrations in the sediment were generally low and within permissible limits, except for iron, which was more abundant. Heavy metal concentrations in sediment showed minimal influence on the distribution of foraminiferal species, indicating that other factors, such as salinity, pH, and grain size, play more crucial roles in determining the presence and distribution of these microorganisms.
The limited number and low diversity of foraminiferal specimens suggest that the estuarine environment is subject to conditions that may not be conducive to the proliferation of foraminiferal species. This can be attributed to the strong fluvial influence, low salinity, and low pH levels found in the estuary. The presence of mangrove vegetations at the banks of the channel further contributes to the challenging conditions for calcareous species, favoring the dominance of agglutinated forms.
Overall, this study underscores the importance of foraminiferal assemblages as indicators of environmental conditions in estuarine ecosystems. The data obtained provide a baseline for future monitoring and can help in understanding the impacts of natural and anthropogenic changes on the Cross River estuary. Continued research and monitoring are essential to assess the long-term health of this vital ecosystem and to inform conservation and management strategies aimed at mitigating the adverse effects of human activities and natural processes.
Data availability
The authors have provided the data set used to support the conclusions of the current study in the appendices of the report. The corresponding author can provide any additional data sets related to the current work upon reasonable request.
References
Antia VI, Emeka NC, Ntekim EEU, Amah EA. Grain size distribution and flow measurements in Qua-Iboe River Estuary and Calabar Tidal River, S.E. Nigeria. Eur J Sci Res. 2012;67(2):223–39.
Zhao K, Fu W, Qiu Q, Ye Z, Li Y, Tunney H. Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma. 2019;337:453–62. https://doi.org/10.1016/j.geoderma.2018.10.004.
Schröder-Adams CJ. Estuaries of the past and present: a biofacies perspective. Sed Geol. 2006;190:289–98. https://doi.org/10.1016/j.sedgeo.2006.05.008.
Alve E. Benthic foraminiferal responses to estuarine pollution: A review. J Foramin Res. 1995;25:190–203. https://doi.org/10.2113/gsjfr.25.3.190.
National Population Commission of Nigeria. 2022. Retrieved from https://www.citypopulation.de/en/nigeria/admin/NGA009/. www.nationalpopulation.gov.ng.
Emeka NC, Antia VI, Ukpong AJ, Amah EA, Ntekim EEU. A study on the sedimentology of tidal rivers: Calabar and Great Kwa, S. E. Nigeria. Eur J Sci Res. 2010;47(3):370–86.
Emeka CN, Emeka VI, Akpan EB, Essien NU, Nwosu FM. Dry season physicochemical characteristics of a tropical meso-tidal estuary: cross River estuary, Southeast Nigeria. Global J Geol Sci. 2023;21(2):183–200.
Emeka CN, Emeka VI, Odey EKA, Ambo AA, Edem GO. Wet season physicochemical characteristics of the Cross River estuary, Southeast, Nigeria. Global J Geol Sci. 2023;21(2):149–66.
Emeka VI, Nyong EE, Emeka CN, Ukpong AJ. Distribution of foraminiferal assemblages in contemporary bottom sediments of qua-Iboe River Estuary, Southeast Nigeria. J Foramin Res. 2023;53(2):97–108. https://doi.org/10.2113/gsjfr.53.2.97.
Emeka CN, Emeka VI, Ebong ED, Ojong RA, Chidozie CP. Dynamics in a tropical meso-tidal river: Great Kwa River, southeastern Nigeria (Gulf of Guinea). J Sediment Environ. 2023;8:1–17. https://doi.org/10.1007/s43217-023-00151-9.
Akpan E, Ekpe U, Ibok U. Heavy metal trends in the Calabar River, Nigeria. Environ Geol. 2002;42:47–51. https://doi.org/10.1007/s00254-001-0479-6.
Carnahan EA, Hoare AM, Hallock P, Lidz BH, Reich CD. Distribution of heavy metals and foraminiferal assemblages in sediments of Biscayne Bay, Florida, USA. J Coastal Res. 2008;24:159–69. https://doi.org/10.2112/06-0666.1.
Jayaraju N, SundaraRajaReddy BC, Reddy KR. Anthropogenic metal pollution in surface sediments of the Tambaraparni River Estuary. Chem Ecol. 2011;27(4):337–50. https://doi.org/10.1080/02757540.2011.570752.
Alve E. Benthic foraminifera in sediment cores reflecting heavy-metal pollution in Sorfjord, Western Norway. J Foramin Res. 1991;21(1):1–19. https://doi.org/10.2113/gsjfr.21.1.1.
Boltovskoy E, Wright R. Recent Foraminifera. Netherlands: Junk Publisher; 1976. p. 515.
Murray JW. Ecology and paleoecology of benthic foraminifera. Longman, Harlow, Essex, England, Longman Scientific and Technical. New York, Wiley, 397. 1991. https://doi.org/10.4324/9781315846101.
Itam AE, Emeka VI, Emeka CN. Foraminiferal study of nkporo shale exposures, Calabar Flank (SE Nigeria): age and depositional environment. J Sediment Environ. 2019;4:369–78. https://doi.org/10.12957/jse.2019.46129.
Kaushik T, Ghosh A, Thirumalai M, Das I. Srinivasania Sundarbanensis gen. et. Sp. Nov., a new agglutinated benthic foraminifer from the world’s largest Mangrove Ecoregion, the Sundarbans, India. J Foramin Res. 2021;51(2):81–91. https://doi.org/10.2113/gsjfr.51.2.81.
Ramanathan RM. Ecology and distribution of foraminifera in Cross River estuary and environs off Calabar, Nigeria. J Mining Geol. 1981;18(1):154–62.
Debenay JP. Recent foraminiferal assemblages and their distribution relative to environmental stress in Paralic Environments of West Africa (Cape Timiris to Ebrie Lagoon). J Foramin Res. 1990;20(3):267–82.
Hayward BW, Sabaa AT, Grenfell HR. Benthic foraminiferan and the late Quaternary (last 150ka) paleooceanographic and sedimentary history of the Bounty Trough east of New Zealand. Paleogeogr Paleoclimatol Paleoecol. 2004;211(1–2):59–93. https://doi.org/10.1016/j.paleo.2004.04.007.
Hayward BW, Hollis CJ. Brackish foraminifera in New Zealand: a taxonomic and ecologic review. Micropaleontology. 1994;40:185–222.
Nigam R, Saraswat R, Kurtarkar SR. Laboratory experiments to study the effects of salinity variations on benthic foraminiferal species—Pararotalia nipponica (Asano). J Geol Soc India. 2006;67:41–6.
Ukpong AJ, Ikediasor CK, Emeka CN, Emeka VC. Tidal influence on foraminifera distribution in a typical meso-tidal River: A case study of the Great-Kwa River, Southeastern Nigeria. Int J Sci Eng Res. 2015;6:889–97.
Alve E, Nagy J. Estuarine foraminiferal distribution in Sandebukta, a branch of the Oslo fjord. J Foramin Res. 1986;16:261–83.
Antia VI. Foraminifera as bioindicators for monitoring coastal pollution and environmental change: a study of recent sediments in Cross River estuary and Qua-Iboe River estuary, Southeast Nigeria: PhD Thesis, Department of Geology, University of Calabar, Nigeria. 2021, p. 188.
Schafer CT. Monitoring nearshore marine environments using benthic foraminifera: some protocols and pitfalls. Micropaleontology. 2000;46(1):161–9.
Yanko V, Ahmad M, Kaminski MA. Morphological deformities of benthic foraminiferal tests in response to pollution by heavy metals: Implication for pollution monitoring. J Foramin Res. 1998;28(3):177–200.
Samir AM, El-Din AB. Benthic foraminiferal assemblages and morphological abnormalities as pollution proxies in two Egyptian bays. Mar Micropaleontol. 2001;41(3–4):193–227. https://doi.org/10.1016/S0377-8398(00)00061-X.
Armynot du Châtelet E, Gebhardt K, Langer MR. Coastal pollution monitoring: foraminifera as tracers of environmental perturbation in the port of Boulogne-sur-Mer (Northern France) Neues Jahrbuch Fur Geologie Und Palaontologie-Abhandlungen. NJGPA. 2011;262(1):91–116. https://doi.org/10.1127/0077-7749/2011/0187.
Elshanawany R, Naiel B, Fawzy M. Distribution of benthic foraminiferal assemblages and heavy metals as a characterization of the environment in Lake Edku, Egypt. Egypt J Aquatic Biol Fisher. 2019;23(1):105–33. https://doi.org/10.21608/ejabf.2019.38330.
Langer MR, Fajemila OT, Mannl S. Assemblages of recent intertidal mangrove foraminifera from the Akanda National Park, Gabon: sea level proxies preserved in faunal assemblages. Neues Jahrb Geol Palaontol Abh. 2016;281(3):327–38.
Antia EE, Santa B. Geochemistry of mangrove swamps of Niger Delta region of Nigeria: Nigeria Geological Survey Agency, Bulletin No. 48, Government of the Federal Republic of Nigeria, 2013, pp. 284.
Aseez LO, Fayose EA, Omatsola ME. Ecology of the Ogun River estuary.y Multivariate analysis of planktonic foraminiferal assemblage of Southwestern Nigeria’s surface continental shelves’ sediments. Eur J Sci Res. 1974;54:84–101.
Dublin-Green C. The foraminiferal fauna of Bonny Estuary, Niger Delta: a baseline study. Technical Paper of the Nigerian Institute of Oceanography and Marine Research. 1990;64:1–27.
Folk RL. Petrology of sedimentary rocks. Austin: Hemphill Publishing Company; 1980. p. 184p.
Ogundele LT, Owoade OK, Olise FS, Hopke PK. Heavy metals in industrially emitted particulate matter in Ile-Ife, Nigeria. Environ Res. 2017;156:320–5.
Loeblich AR, Tappan H. Foraminiferal genera and their classification. Van-Nostrand-Reinhold, New York, 1987, pp. 970. https://doi.org/10.1007/978-1-4899-5760-3.
FEPA. Guidelines and standards for environmental pollution control in Nigeria. Nigeria: Federal Environmental Protection Agency. Government Press, Abuja; 2003.
Attia OEA, Ghrefat H. Assessing heavy metal pollution in the recent bottom sediments of Mabahiss Bay, North Hurghada, Red Sea, Egypt. Environ Monit Assess. 2013;185:9925–34. https://doi.org/10.1007/s10661-013-3302-4.
Akpan IO, Thompson EA. Assessment of heavy metal contamination of sediments along the Cross River Channel in Cross River State, Nigeria. IOSR J Environ Sci Toxicol Food Technol. 2021;2(5):20–8.
Ogri OR, Malu SP, Ubwa ST. Distribution of heavy metals in coastal waters and sediments of Cross River, South-South Niger delta of Nigeria. Int J Appl Environ Sci. 2011;6(3):251.
Essien JP, Antai SP, Olajire AA. Distribution, seasonal variations and ecotoxicological significance of heavy metals in sediments of Cross River Estuary Mangrove Swamp. Water Air Soil Pollut. 2009;197:91–105. https://doi.org/10.1007/s11270-008-9793-x.
Turekian KK, Wedepohl KH. Distribution of elements in some major units of the earth’s crust. Geol Soc Am Bull. 1961;72:175–92. https://doi.org/10.1130/0016-7606(1961)72[175:doteis]2.0.co;2.
Sen Gupta BK, editor. Modern foraminifera. Kluwer: Dordrecht. 1999; pp. 371.
Murray JW. Ecology and applications of benthic foraminifera. Cambridge: Cambridge University Press; 2006. p. 426.
Martins MVA, Silva F, Laut LLM, Frontalini F, Clemente IMMM, Miranda P. Response of benthic foraminifera to organic matter quantity and quality and bioavailable concentrations of metals in Aveiro Lagoon (Portugal). PLoS ONE. 2015;10(2): e0118077. https://doi.org/10.1371/journal.pone.0118077.
Cross River weather. 2020. https://tcktcktck.org/cross-river/October-2020.
Saraswat R, Kouthanker M, Kurtarkar SR, Nigam R, Naqvi SWA, Linshy VN. Effect of salinity induced pH/alkalinity changes on benthic foraminifera: a laboratory culture experiment. Estuar Coast Shelf Sci. 2015;153:96–107. https://doi.org/10.1016/j.ecss.2014.12.005.
Charrieau LM, Filipsson HL, Nagai Y, Kawada S, Ljung K, Kritzberg E, et al. Decalcification and survival of benthic foraminifera under the combined impacts of varying pH and salinity. Mar Environ Res. 2018;138:36–45. https://doi.org/10.1016/j.marenvres.2018.03.015.
Daviray M, Geslin E, Risgaard-Petersen N, Scholz V, Fouet M, et al. Potential impacts of cable bacteria activity on hard-shelled benthic foraminifera: a prelude to implications for their interpretation as bioindicators or paleoproxies. 2023. Biogiosciences. https://doi.org/10.5194/bg-2023-169.
Essienumoh UEN. Sedimentation and foraminiferal ecology of Qua-Iboe estuary and its adjoining Creeks, Ibeno. Cross River State, Nigeria (1987). Thesis. Department of Geology, University of Calabar, Nigeria. 1987.
Kornfeld MM. Recent littoral foraminifera from texas and louisiana. Contributions from the Department of Geology of Stanford University. 1931;1(3):77–93. Available online at https://babel.hathitrust.org/cgi/pt?id=mdp.39015035458358&view=1up&seq=131&skin=2021. p. 86.
Andersen HV. Two new genera of Foraminifera from recent deposits of Louisiana. J Paleontol. 1951;25:31–4.
Brady GS, Robertson D. XXVI.—The Ostracoda and Foraminifera of tidal rivers. With an analysis and descriptions of the Foraminifera. In: Brady HB, editor. Annals and Magazine of Natural History. 1870; 6(34):273–309. Available online at https://www.biodiversitylibrary.org/part/67991#/summary, p. 286.
Orbigny ADd'. Foraminifères. In: de la Sagra R, editor. Histoire physique, politique et naturelle de l'ile de Cuba. A. Bertrand. 1–224. Available online at https://books.google.pt/books?id=KpVeAAAAcAAJ&pg. 1839.
Acknowledgements
We thank Moses, Kakpo, Emmanuel, and Williams for their assistance during sample collection. Special thanks to Prof. Dr. Wolfgang Kuhnt for foraminifera imaging, and to Professor Pamella Hallock for her patience in critically reviewing the manuscript and providing useful suggestions. We appreciate all the reviewers of this article for their painstaking effort, which greatly improved the quality of the research. Thanks also to all staff of the Faculty of Oceanography and Department of Geology, University of Calabar, Nigeria, for their technical support throughout the course of this study.
Funding
This work was funded by the Agate Project.
Author information
Authors and Affiliations
Contributions
Victoria Emeka: Conceptualization; Funding acquisition; Resources; Methodology; Investigation; Validation; Supervision; Project administration; Writing—Original draft preparation and editing. Chimezie Emeka: Investigation; Methodology; Data analysis; Writing—Review and Editing. Asukwo Itam: Methodology, Investigation; Writing—Review and Editing. Eyo Etim Nyong: Resources; Investigation; Writing—Review and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research did not involve human subjects, so clinical trial registration is not applicable.
Consent for publication
The authors certify that this manuscript is our original unpublished work, has not been published elsewhere, and is not under consideration by another journal. All the authors have approved the manuscript and agree with its submission.
Competing interests
The authors hereby declare that there are no known conflicts of interest, financial or personal relationships, which may have appeared to influence the work as reported in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
See Table 3.
Appendix 2
See Table 4.
Appendix 3
Taxonomic description of foraminiferal species (taxonomic citations are included in the reference list).
Arenoparrella mexicana [53].
Classification.
Chromista (Kingdom) Harosa (Subkingdom) Rhizaria (Infrakingdom) Foraminifera (Phylum) Globothalamea (Class) Textulariana (Subclass) Lituolida (Order) Trochamminina (Suborder) Trochamminoidea (Superfamily) Trochamminidae (Family) Arenoparellinae (Subfamily) Arenoparrella (Genus) mexicana (Species).
Arenoparrella sp. [54]
Classification.
Chromista (Kingdom) Harosa (Subkingdom) Rhizaria (Infrakingdom) Foraminifera (Phylum) Globothalamea (Class) Textulariana (Subclass) Lituolida (Order) Trochamminina (Suborder) Trochamminoidea (Superfamily) Trochamminidae (Family) Arenoparellinae (Subfamily) Arenoparrella (Genus).
Miliammina fusca [55]
Classification.
Chromista (Kingdom) Harosa (Subkingdom) Rhizaria (Infrakingdom) Foraminifera (Phylum) Tubothalamea (Class) Miliolida (Order) Miliamminidae (Family) Miliammina (Genus) fusca (Species).
Ammonia parkinsoniana [56]
Classification.
Chromista (Kingdom) Harosa (Subkingdom) Rhizaria (Infrakingdom) Foraminifera (Phylum) Globothalamea (Class) Rotaliana (Subclass) Rotaliida (Order) Rotalioidea (Superfamily) Ammoniidae (Family) Ammoniinae (Subfamily) Ammonia (Genus) parkinsoniana (Species).
Appendix 4
See Table 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Emeka, V.I., Emeka, C.N., Itam, A.E. et al. Factors influencing foraminiferal assemblages in coastal sediments of the Cross River Estuary, Southeast Nigeria, Gulf of Guinea. Discov Oceans 1, 15 (2024). https://doi.org/10.1007/s44289-024-00017-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44289-024-00017-6