Abstract
Theanine (Thea) is a unique metabolite in tea plants, but its physiological functions remain elusive. A low soil pH increases cadmium (Cd) availability, affecting the quality of tea plant products. In this study, we found that Thea reversed the Cd-induced reduction in free amino acid (FAA) and caffeine (CAF) in the young tea leaves, as well as the down-regulation in the expression of nitrate transporters CsNRT1.2 and CsNRT2.5, and genes responsible for the nitrogen (N) assimilation. We demonstrated that Thea could alleviate Cd-induced oxidative stresses and enhance photosynthesis. Moreover, an ATP-binding cassette (ABC) transporter, CsABCG11.2, could uptake distinct Cd substrates and the five major amino acids in tea plants. Heterologous expression of CsABCG11.2 in yeast indicated a competitive absorption between Cd and Thea in a concentration-dependent pattern. CsABCG11.2-overexpressing Arabidopsis plants exhibited increased sensitivity to Cd due to enhanced Cd concentration, accumulation in the shoots, and reduction in the primary root length. Exogenous application of Thea at environmentally regular levels attenuated the adverse effects of Cd-induced growth inhibition and chlorosis in CsABCG11.2-overexpressing Arabidopsis plants. Knockdown of CsABCG11.2 tea plants significantly lowered Cd levels in young shoots. Our results suggest that Thea plays beneficial roles in alleviating Cd stress directly or indirectly by modulating CsABCG11.2-mediated Cd uptake and translocation within plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is toxic and poses a major hazard to human health as it can be absorbed by crops and accumulate in the body through the food chain. (Åkesson et al. 2014; Nordberg et al. 2009). The safety limit of Cd residues is 1 mg·kg−1, according to the standard of the Ministry of Agriculture and Rural Affairs in China. Agricultural soil can be contaminated by Cd, and Cd bioavailability is increased because of soil acidification due to excessive fertilizer application, which results in elevated Cd accumulation in crops and ultimately poses a substantial threat to food safety and human health (Zhao et al. 2015; Wang et al. 2019b). Soil pH is a dominant factor in determining the availability of Cd in the soil solution and affecting plant uptake (Li et al. 2005; Du et al. 2013). Investigations in the major tea-producing regions of China revealed that the pH of most tea plantation soils ranges from 3.5 to 4.5, with some having a pH below 3.5, while the average acidification rate of tea plantation soils corresponds to a decrease in pH by 0.05 per year (Yan et al. 2020). Cd pollution in tea plantation soils would inevitably affect the composition quality of tea products; therefore, proper consideration should be given to this issue. Notably, the Cd content in the soil can be up to 9.5 mg·kg−1 in some areas (Lei et al. 2015). Cd taken up by roots is transferred to shoots, leaves, seeds, and other edible parts. Besides the toxicity to the environment and humans, Cd contamination also results in phytotoxicity with characteristic phenotypes of visible foliar damage such as chlorosis and necrosis, and root growth inhibition (Hua et al. 2022; Ismael et al. 2019; Meng et al. 2022).
Extensive research has been focused on the effect of Cd toxicity on photosynthesis. Cd has been shown to reduce photochemical efficiency and photosynthesis, resulting in the accumulation of toxic substances and reactive oxygen species (ROS), which are detoxified through the activity of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD) (Zhao et al. 2021). During the process of detoxification, the oxidation-mediated compounds and conjugates are compartmentalized by transmembrane-associated transporters, such as ATP-binding cassette members (Lee et al. 2005; Fu et al. 2019; Yang et al. 2021a). Identification of the genes responsible for Cd detoxification is an effective strategy for the mitigation of its phytotoxicity. These beneficial genes play crucial roles in Cd reduced accumulation and detoxification to further achieve food safety (Yan et al. 2016; Zhang et al. 2019).
Amino acids are important metabolites in tea and are the main substances contributing to the umami and sweet taste of the tea infusion (Liu et al. 2016). Theanine (Thea) is a unique amino acid of tea, accounting for 50%–70% of total free amino acid (FAA). Amino acids are mainly synthesized in the tea roots and transported and distributed to the shoot (Zhang et al. 2020). The amino acid distribution profile in the young shoot determines the tea quality. Therefore, the biological processes of amino acid uptake and xylem loading in the tea roots and their distribution in the shoot by amino acid transporters significantly contribute to tea quality. Thus far, a number of amino acid transporters, including amino acid permeases (AAPs), cationic amino acid transporters (CATs), lysine-histidine-like transporters (LHTs) family members have been characterized in tea plants (Dong et al. 2020; Feng et al. 2018; Li et al. 2021; Zhang et al. 2020). Moreover, Thea was detected in the tea plantation soil, maybe originating from leftover plant residues (Sarwar et al. 2011). Similarly, in our group's research, Thea was detected in the tea plantation soil as a dominant amino acid component throughout the year (Fig. S1). Thea biosynthesis has been well characterized in plants, and it is regulated by many environmental factors, especially high salinity, high temperature, drought, nutrient condition, and light intensity (Wang et al. 2016; Li et al. 2018; Yang et al. 2020, 2021b). Therefore, the roles of Thea as a species-specific secondary metabolite that adapts tea plants to these abiotic stresses remain clearly established, and the findings should be of great significance for tea cultivation.
The ATP-binding cassette (ABC) transporters are ubiquitously distributed trans-membrane proteins that transport a wide range of substrates across different biological membranes (Rea 2007; Wilkens, 2015; Hwang et al. 2016). The functions of ABC transporters in plants are increasingly being characterized and elucidated (Gräfe and Schmitt 2021; Le Hir et al. 2013). For a long time, it was assumed that ABC importers were only present in prokaryotes and archaea, and ABC transporters were all exporters in eukaryotes. However, increased findings have described the importer function of plant ABC transporters (Terasaka et al. 2005; Kang et al. 2010). The ATP-binding cassette c (ABCC) subgroup members have been identified as Cd efflux transporters and Cd or Cd complexes are compartmentalized in the vacuoles to achieve detoxification (Park et al. 2012; Brunetti et al. 2015; Yang et al. 2021a). The role of the plasma membrane located AtABCG36/PDR8 has been demonstrated in Cd efflux from root cells (Kim et al. 2007). OsABCG43/PDR5 is a Cd-inducible transporter and confers tolerance to high Cd in yeast (Oda et al. 2011). OsABCG36 is required for Cd tolerance, mediating the export of Cd or Cd conjugates from the rice root cells, rather than contributes to reducing Cd accumulation in the shoots (Fu et al. 2019). Wang et al. (2019a, b) demonstrated that PtoABCG36 functions as a Cd extrusion pump, participating in enhancing tolerance to Cd through decreasing Cd content in plants. Overexpression of the strawberry FvABCC11 under Cd stress partially rescued the growth of Arabidopsis seedlings, suggesting that FvABCC11 enhances the tolerance of Arabidopsis to Cd (Shi et al. 2020). Le Hir et al. (2013) showed that Arabidopsis thaliana ATP-Binding Cassette G (AtABCG9), AtABCG11, and AtABCG14 are involved in lipid/sterol homeostasis regulation necessary for vascular bundle development in Arabidopsis. It has been hypothesized that, because of the sessile nature of plants combined with the highly variable growth environment, the expansion of the ABCG subfamily in plants was crucial and necessary to facilitate the efficient efflux of toxic compounds and various secondary metabolites (Gräfe and Schmitt 2021). However, the functions of many ABC transporters remain largely uncharacterized.
Amino acid metabolism is an integral part of nitrogen metabolism in higher plants. Transcriptome data of tea plants treated with different nitrogen concentrations revealed a low N-responsive gene, Camellia sinensis ATP-Binding Cassette G 11.2 (CsABCG11.2), exhibiting a relatively high expression level in the young shoots and extremely low expression levels in the tea plant roots. The involvement of ABCG transporters in the transport of heavy metals has been reported by numerous studies (Fu et al. 2019; Oda et al. 2011; Wang et al. 2019a). In addition, CsABCG11.2 expression, mediated by Cd stress, was increased by almost 20-fold in tea roots and threefold in shoots, respectively. CsABCG11.2 is considered to be a broad-spectrum substrate transporter homologue. Considering the simultaneous presence of amino acids and Cd in the tea plantation soilplantation, whether tea plants possess adaptive measures to selectively uptake beneficial amino acids and exclude Cd remains unknown but deserves further investigation. It is currently unknown how Cd affects nitrogen metabolism for tea plants and whether there is an adaptive mechanism via nitrogen metabolism involved in Cd tolerance. Therefore, little is known about how CsABCG11.2 coordinately transports Cd and amino acids within tea plants. In this study, we investigated the effect of Cd on tea plants' growth and tea quality, and we sought to elucidate how Thea affects tea plant responses to Cd. The findings indicated that the exogenous application of Thea alleviated Cd uptake and translocation in tea plants through the regulation of CsABCG11.2.
Materials and methods
Gene expression analysis
Total RNA was extracted from the samples using the Quick RNA Isolation Kit (Huayueyang, Beijing, China). The gDNA Eraser was used to eliminate DNA contamination, and reverse transcription was performed using TRUEscript RT Kit (Aidlab, Beijing, China). Quantitative PCR was performed with the SYBR Green qPCR Mix (Aidlab, Beijing, China) on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems). The expression of each gene was calculated using the 2−∆∆ method (Livak and Schmittgen 2001), with the genes of Camellia sinensis Glyceraldehyde 3-phosphate Dehydrogenase (CsGAPDH) and AtGAPDH, as internal control genes for the tea plant and Arabidopsis, respectively. Each biological replicate was collected from at least two independently grown groups of plants. Primer sequences are listed in Table S1.
Cloning of CsABCG11.2 and generation of transgenic Arabidopsis thaliana
The coding sequence (CDS) of CsABCG11.2 was amplified from the tea plant cultivar Shuchazao cDNA, confirmed by sequencing, and subsequently cloned into a pTOPO vector. Then, it was ligated into the pBin35SRed vector with primers containing SmaI/XhoI sites. The resulting constructs were then introduced into Agrobacterium tumefaciens strain GV3101. Finally, Arabidopsis wild-type Col-0 was transformed with the pBin35SRed: CsABCG11.2 construct to generate overexpression lines. Three independent homozygous T3 lines with high expression levels of CsABCG11.2 were selected for further analysis. All primers used in vector construction are listed in Table S1.
Plant materials, growth conditions, and treatments
Forty-eight one-year-old tea seedlings were hydroponically cultivated in each tank in nutrient solution. The growth conditions were 22℃/18℃ and 16 h/8 h light/dark. The nutrient solution was renewed every five days. The solution was initially at half-strength for ten days. The plants were then cultured at a full-strength nutrient solution to accelerate the development of new roots. The full-strength solution was prepared as described in our earlier study (Huang et al. 2020). Tea seedlings were hydroponically cultured for Cd treatments. The solution was supplemented with CdCl2 to final concentrations of 0, 5, 10, 15, 20, 50 and 80 µM. The young leaves were collected 15 days after treatment to measure the FAA, caffeine (CAF), and tea polyphenols (TP) contents.
In a separate experiment, the tea seedlings were treated with Cd (0, 10, 40, and 80 µM) for 6 h to determine the effect of Cd on the CsABCG11.2 expression profile. For the temporal and spatial expression analysis, the tea plants were treated with 40 µM CdCl2 for 6 h and 24 h, respectively. Samples from the first developing leaves from the top (L1), mature leaves (L2), and roots (Root) were collected.
Tea seedlings were subjected to the following treatments: control, Cd (80 µM CdCl2), and combined treatment of Cd and Thea (80 µM Cd and 50 µM Thea), with at least 30 seedlings assessed in each group. Three weeks later, young leaves were collected to determine the malondialdehyde (MDA) content and the antioxidant enzyme activities of CAT, SOD, and POD. The transcript levels of genes associated with Thea synthesis and nitrogen assimilation in the young leaves and newly developed roots of tea seedlings were also measured.
The seeds of Arabidopsis wild type (WT) and CsABCG11.2-overexpressing lines (OE1/OE2/OE3) were sterilized and germinated on 1/2 Murashige Skoog media with the four treatments: control, 20 μM Thea, 100 μM Cd, and combined-treatment of 20 μM Thea and 100 μM Cd. After a 2-week culture in an incubator (22℃, 16 h light, 180 µmol·m−2·s−1, 20℃, 8 h dark), the seedlings were harvested, and the primary root length was determined.
Four-week-old WT and overexpressing (OE) Arabidopsis lines were transferred to a 1/2-strength nutrient solution for 5 days, followed by another 10 days in a full-strength solution in hydroponics, as previously described (Huang et al. 2020). Then, the plants were divided into five treatment groups, which corresponded to 40 μM Cd, 50 μM Thea, 10 μM Thea, 40 μM Cd + 50 μM Thea, and 40 μM Cd + 10 μM Thea in the solution. After 9 days, the rosette leaves of WT and three OE lines were separately collected to determine the antioxidant enzyme activities and Cd concentration.
In addition, two-week-old WT and OE Arabidopsis lines seedlings were divided into the following four treatment groups: control, 40 μM Cd, 20 μM Thea, 40 μM Cd + 20 μM Thea. A month later, their growth performance was recorded, and the Cd concentration was determined to examine the percentage of shoot-Cd accumulation.
Generation of CsABCG11.2-knock down tea plants and treatments
Tobacco rattle virus (TRV)—based virus-induced gene silencing (VIGS) was carried out to generate CsABCG11.2-knock down tea plant lines for the functional characterization of CsABCG11.2. The plasmid Tobacco Rattle Virus-Camellia sinensis Phytoene Desaturase (pTRV2-CsPDS1) transformed plants showed visualized TRV spread in more than 60% of treated tea plants based on the leaf bleaching phenotype on the inoculated and non-inoculated leaves two months post agroinfiltration (Fig. S2). A panel of pTRV2 vectors was constructed for pTRV2-CsABCG11.2 as described by Zhou et al. (2021), including the citrus tristeza virus P20, cucumber mosaic virus 2b (C2b) and tomato aspermy virus 2b (T2b) to improve the silencing efficiency of the target gene. These vectors were co-expressed with pTRV1 in young tea plant leaves (the second and third leaf from the top) of tea seedlings by agroinfiltration. Briefly, a 375 bp CsABCG11.2 gene fragment was amplified using gene-specific primers (Table S1). The resulting fragment was subsequently cloned into a pTRV2 destination vector, which was then transformed into the GV3101 strain. Tea plants inoculation and transformation for VIGS characterization were performed for the second leaf from the top.
Six days after agroinfiltration, agroinfiltrated leaves and non-infiltrated leaves at similar positions in tea plant seedlings were collected to identify CsABCG11.2-knock downplants. Subsequently, ten days later, along with the control plant lines transformed with empty vector (TRV1-TRV2 infiltration lines), the CsABCG11.2-knock down tea plants were grown for three weeks in different solutions containing 40 uM Cd, 40 mM Cd with 50 uM Thea, and a control solution. Young leaves (the second and third developing leaves from the top) and mature leaves (developed for over two months) of the treated tea seedlings were harvested and oven-dried at 80°C to constant weight to determine their Cd concentration. Three individual plants were bulked to form a biological replicate, and three biological replicates were assessed in total (9 plants).
Determination of Fv/Fm, lipid peroxidation, and antioxidant enzyme activities
To assess the effects of Cd and Thea, The tea plants were placed in the dark for 30 min to measure the maximum photochemical efficiency of photosystem II (Fv/Fm). and. for the measurements were performed in the second leaf from the top using a computer-operated machine of imaging-PAM chlorophyll fluorimeter (IMAGING-PAM/L; Heinz Walz, Effeltrich, Germany) as described by Li et al. (2015).
MDA content was determined as an indicator of membrane lipid peroxidation (Hodges 1999). The SOD (Giannopolitis and Ries. 1977), POD, and CAT (Cakmak and Marschner. 1992) antioxidant activities were determined as previously reported. All the above measurements were performed in both tea plants and Arabidopsis leaves.
CsABCG11.2 heterologous expression in yeast
The yeast strain Δycf1 deficient in Cd transport into the vacuoles was used for the functional characterization of CsABCG11.2. CsABCG11.2 was cloned into the vector pYES2 (Invitrogen) within the KpnI and XhoI sites, and the recombinant vector was transformed into the yeast strain Δycf1. The yeast transformants harboring the recombinant vector and empty vector were individually cultured in an SD-U/Glu liquid medium until the OD600 value reached 1.0. Then, the yeast cells were diluted in a tenfold gradient with sterile water (20 to 200 μL) and spotted on an SD-U/Gal induction medium containing 0, 5, 10, 15, 20, or 40 μM CdCl2. The plates were incubated at 30℃ for 3 d to evaluate yeast growth performance. The growth of yeast cells carrying different plasmids was monitored in a liquid SD-Ura medium containing 0, 5, and 10 μM CdCl2 in the presence of galactose.
The yeast strain 22∆10α (Besnard et al. 2016) was transformed with a pYES2 vector carrying CsABCG11.2 or a pYES2 empty vector. The 23344c wild-type strain was also assessed. The yeast cells were grown in a liquid synthetic defined (SD) medium (Yeast Nitrogen Base without nitrogen, Gibco, USA), 2% glucose, 5 g/L (NH4)2SO4. They were harvested by centrifugation when OD600 reached 1, then washed twice with sterile water and diluted to OD600 value of 1. Further, a serially diluted cell suspension was spotted on the SD agar medium containing either individual amino acids or (NH4)2SO4 as the sole nitrogen source. Plates were incubated at 30℃ to record the yeast growth performance. Yeast uptake assays were performed as previously described by Su et al. (2004).
A single yeast colony carrying CsABCG11.2 was inoculated in the SD-URA liquid medium to an OD600 value of 0.6. Yeast cells were collected and re-suspended in SD-URA /Gal liquid medium. Then, CdCl2 was supplied at 20 μM, as well as a gradient of Thea at 0.1, 0.5, 1, 2, 4, and 8 mM, and then the corresponding media were incubated in a 220 r/min shaker for 1 h. The yeast cells were washed twice with ice-cold EDTA solution (10 μM, pH 5.0) and then washed twice with ddH2O, dried at 80℃ overnight for Cd concentration measurement after accurate weighing.
Histochemical β-glucuronidase (GUS) activity assays of CsABCG11.2
The promoter of CsABCG11.2 (-2329 bp) was amplified by PCR using primers listed in Table S1. The promoter was then introduced into a promoter-less and HindIII/BamHI-digested pBI121 vector upstream of the GUS-reporter-gene. The proCsABCG11.2:GUS transgenic lines were screened and verified. Subsequently, 3-week-old plants were cultured for 12 h in a Hoagland solution along with three treatments of 50 μM Thea, 40 μM Cd, and the combined treatment of 50 μM Thea and 40 μM Cd to detect GUS expression. GUS activity was visualized using a GUS histochemical assay kit (GT0391, Huayueyang Biotechnology, Beijing, CO., LTD).
Subcellular localization of CsABCG11.2
The CDS of CsABCG11.2 was inserted into the transient expression vector pCAMBIA-2300-C-EGFP (BamHI an XbaI) and was driven by a35S promoter. The construct was transformed into Agrobacterium tumefaciens strain GV3101 and used to infect tobacco (Nicotiana benthamiana) leaves, with the pCAMBIA-2300-C-EGFP vector used as the control. A vector containing the mCherry-labeled CBL1n-ORF was co-transformed as a plasma membrane-localized marker. After transformation, fluorescence signals of the transfected tobacco leaves were scanned under a confocal laser scanning microscope (Leica TCS SP8, Lecia Camera AG, Wetzlar, Germany).
Measurement of Cd concentration
The plant tissues or yeast cells were dried to constant weight and then wet-digested after accurate weighing as previously described with minor modifications (Guan et al. 2019). Briefly, samples were immersed in nitric acid overnight at room temperature and then digested at 120℃ until the digests were clear, which were then diluted with ddH2O. Cd determination was performed in all samples using an Inductively Coupled Plasma-Optical Emission Spectrometer (iCAP™ 7200 ICP-OES, Thermo Fisher Scientific, Waltham, USA).
Statistical analysis
Data were analyzed using the IBM SPSS Statistics 26 program. One-way ANOVA followed by Tukey's test was performed to identify significant differences.
Results
Cd affects the FAA content of tea plants in a Cd-concentration-dependent manner
To investigate the effect of Cd on tea plant growth and FAA content, the hydroponically cultured tea plants under different treatments were compared. The plant Cd levels in the different treatments, 5 μM, 10 μM, 15 μM, 20 μM, 50 μM, 80 μM Cd, ranged from naturally normal to toxic. Fifteen days after treatments, there was no significant difference in the growth performance except for root browning after exposure to 50 μM and 80 μM Cd. Notably, the FAA content in the young shoots increased with Cd levels in the solution from 0 to 10 μM. It gradually decreased when plants were exposed to 10 μM to 20 μM Cd levels and sharply decreased in the 20 μM to 80 μM Cd treatments (Fig. 1a). An increased or decreased expression of the Camellia sinensis Thea Synthesis genes, CsTS1 and CsTS2, was observed following the low Cd and high Cd treatments, respectively (Fig. 1b), which was consistent with the changes in FAA content with increasing Cd concentration. Further, the expression levels of the key genes related to nitrogen assimilation, Camellia sinensis Glutamate Dehydrogenase (CsGDH1/2), Camellia sinensis Glutamine Synthetase (CsGS1/2) and Camellia sinensis Ferredoxin-dependent Glutamate Synthase (CsFd-GOGAT1) were not significantly affected by low levels of Cd, in contrast, they were all significantly inhibited under high Cd concentrations (Fig. 1c). These results showed that the FAA contents in young tea plant shoots were affected by Cd and were Cd level dependent. Considering Thea as the main component of FAA, it was assumed that tea plants exhibit an adaptive mechanism by enhancing gene expression related to Thea biosynthesis to adapt to Cd stress.
Effects of cadmium (Cd) on the level of free amino acids in the young shoots (a), expression levels of Camellia sinensis Theanine Synthetase genes (CsTS1 and CsTS2) in the young shoots (b), and expression of genes related to nitrogen assimilation (Camellia sinensis Glutamate Dehydrogenases, CsGDH1 and CsGDH2, Camellia sinensis Glutamine Synthetases, CsGS1 and CsGS2, Camellia sinensis Ferredoxin-dependent Glutamate Synthase, CsFd-GOGAT1) in the young shoots of tea plants (c). A hydroponic experiment with tea plants grown in 5 μM to 80 μM Cd levels was conducted for 15 days. Different lowercase letters indicate a significant difference at P < 0.05 by Duncan's multiple range test
Exogenous Thea reduces oxidative stress induced by Cd
The physiological role of Thea in Cd stress tolerance remains unknown. It is well known that Cd stress impairs plant photosynthesis. The sensitivity of tea plants to Cd was investigated by comparing the photosynthesis parameters of the plants grown in a hydroponic solution with the following treatments: 50 µM Thea, 80 µM Cd, combined treatment of 80 µM Cd and 50 µM Thea, and control (with no addition of Cd and Thea, CK). After four weeks of growth, young leaves of tea seedlings were used to determine the Fv/Fm to assess the effects of Cd stress on photosystem II. As expected, based on pseudocolor imaging of chlorophyll fluorescence in tea leaves, Cd stress drastically reduced the Fv/Fm by 39.9% (Fig. 2a, b, c). It is noted that exogenous Thea application did not significantly affect Fv/Fm compared to CK, while the Fv/Fm value was significantly increased under the combined treatment of 80 µM Cd and 50 µM Thea when compared to Cd treatment alone (Fig. 2c).
Effects of exogenous application of theanine (Thea) and Cadmium (Cd) on the maximum photochemical efficiency of photosystem II (Fv/Fm), oxidative stress, and antioxidant enzyme activities in the young shoots of tea plants. a Phenotypes indicative of tea plant tissue damage. The purple-blue color indicates a normal state of the photosynthetic apparatus, whereas the green and yellow colors indicate damage to photosystem II caused by Cd stress. b Pseudocolor images of tea leaves illustrate the Fv/Fm under different treatments. The color gradient scale at the bottom indicates the magnitude of the fluorescence signal represented by each color, ranging from 0 (black) to 1 (purple). c Fv/Fm values. d Malondialdehyde (MDA) content. e Superoxide dismutase (SOD) activity. f Peroxidase (POD) activity. g Catalase (CAT) activity. Data are the means of a minimum of three replicates (± standard deviation, SD). Different letters indicate significant differences between treatment groups at P < 0.05
The effect of exogenous Thea treatment on the MDA levels in the tea leaves was assessed. Significantly reduced MDA levels were observed in the tea leaves under the combined 80 µM Cd and 50 µM Thea treatment compared to the Cd treatment alone, suggesting that the Thea treatment alleviated the oxidative stress stimulated by Cd (Fig. 2d). The SOD, CAT, and POD antioxidant enzyme activities were determined to assess the mechanism by which Thea is involved in the alleviation of Cd-generated oxidative stress. The activities of CAT, SOD, and POD were increased by 49.45%, 83.64%, and 57.70% under Cd treatment, respectively. Notably, the activities of antioxidant enzymes were further increased by 19.94%, 24.75%, and 9.8% under the combined Cd and Thea treatment, respectively, compared with those under Cd treatment alone (Fig. 2e, f, g). This indicated that Thea significantly increased the activities of SOD and CAT but had a minimal impact on POD activity. Therefore, under Cd stress conditions, the application of Thea activates the antioxidant system of tea plants, which benefits ROS scavenging and MDA content reduction.
Exogenous Thea reduces Cd uptake and increases FAA levels under Cd stress
The tea seedlings treated with 80 μM Cd showed phytotoxicity symptoms, including chlorosis, yellowing, and shedding in the young leaves (Fig. 3a). Tea seedlings treated with 50 μM Thea showed no significant differences in growth compared with the control plants. Tea seedlings grown in 80 μM Cd and 50 μM Thea exhibited an obviously increased growth than those grown in 80 μM Cd (Fig. 3a). Cd content in the tea plant shoots was at a basal level (~ 0.35 μg·g−1) under the 50 μM Thea and control treatments, and no significant differences were observed. As expected under the 80 μM Cd treatment, Cd concentration was 2.16 μg·g−1, significantly higher than the control. Interestingly, the Cd levels (1.24 μg·g−1) of plants in the combined treatment of 80 μM Cd and 50 μM Thea were significantly reduced compared to the 80 μM Cd treatment (Fig. 3b).
Exogenous theanine (Thea) reduces the uptake of cadmium (Cd) and improves the tea quality of tea plants under Cd stress. a Phenotypes of shoots and roots of tea seedlings under different treatments (Control, 50 μM Thea, 80 μM Cd2+, 80 μM Cd2+ + 50 μM Thea). b Cd content in the young leaves. c Free amino acid (FAA) content in the young leaves. d tea polyphenols (TP) content in the young leaves. e Caffeine (CAF) content in the young leaves. Data are means of three replicates (± standard deviation, SD). Different letters indicate significant differences between treatment groups at P < 0.05
The levels of quality components in the tea leaves, such as FAA, TP, and CAF, were further determined. No significant difference in FAA, TP, and CAF levels in tea leaves was observed between the 50 μM Thea and the control treatment. On the other hand, the levels of FAA and CAF were markedly increased under the combined treatment of 80 μM Cd and 50 μM Thea compared to the 80 μM Cd treatment (Fig. 3c, d, e). Therefore, exogenous Thea effectively alleviated the adverse impact of Cd stress on the growth of tea seedlings via increased photosynthesis, reduced Cd uptake, and improved tea quality.
Exogenous Thea upregulates the genes associated with Thea synthesis and nitrogen assimilation in the presence of Cd
Our previous transcriptome analyses demonstrated that the treatments of mixed amino acids-N (containing 30 μM Thea) induced the expression of the genes that are involved in heavy metal uptake and transport in plants (Fig. S3). In this study, we found that exogenous Thea increased the activity of antioxidant enzymes and reduced the MDA levels in tea plants under Cd stress (Fig. 2). These findings suggest that the Thea-mediated inhibition or induction of the expression of genes that promote Cd uptake and translocation may be partially due to the regulation of endogenous Thea biosynthesis. Therefore, the transcript levels of genes involved in Thea synthesis and nitrogen assimilation were investigated under four treatments: 50 μM Thea, 80 μM Cd, combined treatment of 80 μM Cd and 50 μM Thea, and the control (Fig. 4). The expression levels of the nitrate transporters CsNRT1.2 and CsNRT2.5 in the tea roots were significantly reduced under the 80 μM Cd treatment compared with the control. On the other hand, they were significantly upregulated under the combined treatment of 80 μM Cd + 50 μM Thea compared to the 80 μM Cd treatment (P < 0.05) (Fig. 4). Under 80 μM Cd, no significant differences were observed in the expression levels of CsTS1 and CsFd-GOGAT in the leaves and roots when compared to the control. Further, the transcript levels of the genes associated with nitrogen assimilation, Camellia sinensis Alanine Aminotransferase (CsALT), Camellia sinensis Nicotinamide Adenine Dinucleotide Glutamate Synthase (CsNADH-GOGAT), Camellia sinensis Nitrate Reductase (CsNR), and Camellia sinensis Nitrite Reductase (CsNiR), were down-regulated only in roots, while Camellia sinensis Glutamine Synthase 1 (CsGS1) and Camellia sinensis Glutamate Dehydrogenase 1 (CsGDH1) were down-regulated both in shoots and roots. The expression levels of Thea biosynthesis pathway genes, CsTS2, CsGS1, CsNiR, CsNR, CsGDH1, CsALT, and CsNADH-GOGAT, were significantly induced under the combined treatment of 80 μM Cd + 50 μM Thea compared to the 80 μM Cd treatment (Fig. 4).
Exogenous theanine (Thea) induces the expression of genes involved in Thea synthesis and nitrogen assimilation under cadmium (Cd) stress. The expression levels of genes involved in nitrogen assimilation (Camellia sinensis Nitrate Transporters, CsNRT1.2 and CsNRT2.5; Camellia sinensis Alanine Aminotransferase, CsALT; Camellia sinensis Nicotinamide Adenine Dinucleotide-dependent Glutamate Synthase, CsNADH-GOGAT; Camellia sinensis Nitrate Reductase, CsNR; Camellia sinensis Ferredoxin-dependent Glutamate Synthase, CsFd-GOGAT1; and Camellia sinensis Nitrite Reductase, CsNiR; Camellia sinensis Glutamine Synthetase, CsGS1; Camellia sinensis Glutamate Dehydrogenase, CsGDH1 and CsGDH2) and Camellia sinensis Theanine Synthesis (CsTS1, CsTS2) were compared in the leaves and roots of tea plants subjected to 80 µM Cd2+, 50 µM Thea, and a combined 80 μM Cd and 50 μM Thea -treatment. Values are mean ± standard deviation (SD) (n ≥ 3). Different letters indicate significant differences between treatments at P < 0.05. CK, control, with no addition of Cd and Thea
The expression levels of genes upstream of Thea biosynthesis in tea plants were differentially regulated in response to Cd stress. These findings suggest that Thea at physiological levels alleviates the phytotoxic effect of Cd, which confirms the possible physiological function of Thea in Cd stress tolerance in tea plants. Therefore, it was postulated that the identification of the causal genes responsible for the transport of Cd and Thea might explain the mechanisms underlying Thea-mediated alleviation of Cd stress.
Heterologous expression of CsABCG11.2 mediates the transport of Cd and amino acids in yeast
To identify the candidate genes that transport Thea and Cd, we analyzed transcriptomic data (Zhang et al. 2020) for transporter genes that were highly expressed in the tea roots and/or shoots. Due to its expression profile (Fig. S3), CsABCG11.2 could be considered the causal gene encoding for a protein with a putative ability to transport Cd and amino acids simultaneously. To assess the function of CsABCG11.2 in Cd transport, it was expressed, driven by the galactose-inducible promoter GAL1 in the pYES2 vector, in the yeast strain Δycf1 to investigate its Cd transport capacity The strain containing the empty plasmid pYES2 was used as a control. The growth of the strain on media supplemented with a gradient Cd concentration was determined. In the presence of glucose, there was no difference in Cd sensitivity between the yeast strains expressing the vector control or CsABCG11.2 (Fig. 5a). When grown in an induction medium with galactose, the yeast strain expressing CsABCG11.2 was more sensitive to Cd than that expressing the empty vector. The sensitivity increased with the Cd concentration increase (Fig. 5a). Similar results were obtained in a time-series experiment by measuring yeast growth curves in a liquid medium. In the absence of Cd, there was no significant difference in the growth performance between yeast containing the empty vector and those expressing CsABCG11.2 (Fig. 5b). In a series of liquid media supplemented with increasing Cd concentrations, the growth of the yeast strain expressing CsABCG11.2 was significantly more inhibited by 10 μM Cd than 5 μM Cd (Fig. 5b). Further after growth in a medium supplied with 10 μM Cd for 1 h, the yeast strain expressing CsABCG11.2 accumulated significantly more Cd (84.05 μg Cd g−1 dry weight [DW]) than that expressing the empty vector (63.93 μg Cd g−1 DW, Fig. 5c). These findings indicated that CsABCG11.2 could specifically transport Cd.
Heterologous complementation of cadmium (Cd) transport by Camellia sinensis ATP-Binding Cassette subfamily member G 11.2 (CsABCG11.2) expression in yeast. a Yeast Cd transport ∆ycf mutant strains expressing the empty vector pYES2 or CsABCG11.2 were grown in SD-U medium containing different Cd concentrations in the presence of glucose or galactose. b A time-dependent growth curve of different yeast ∆ycf mutant strains was conducted in SD-U liquid medium supplemented with Cd (+ 5/10 μM) for 72 h, and the OD600 was measured at six- or twelve-hour intervals. c The Yeast ∆ycf mutant strain carrying CsABCG11.2 accumulated significantly more Cd in an SD-U liquid medium containing galactose compared to the empty vector pYES2-expressing control. d Assessment of the yeast amino acid transport mutant 22∆10α carrying the empty vector pYES2 (negative control) and CsABCG11.2. The yeast wild-type strain 23344c served as a control. Yeast cells were grown in a liquid medium containing 1 mM theanine (Thea), glutamate (Glu), arginine (Arg), aspartate (Asp), glutamine (Gln), or (NH4)2SO4 as the sole N source. Growth was recorded after 48 h culture at 30°C. e Growth rates of yeast amino acid transporter mutants expressing CsABCG11.2 or the empty vector pYES2 for 72 h. OD600 was measured every 12 h (n = 3) and 50 h in the Thea treatment
Yeast complementation assays were performed to validate the function of CsABCG11.2 as an amino acid transporter. Yeast cells expressing CsABCG11.2 or the empty vector pYES2 were serially diluted and cultured on a solid medium containing 1.0 mM of individual amino acids, which are the primary amino acid constituents in the tea leaves, as the sole nitrogen source. The treatment containing 0.5 mM (NH4)2SO4 was used as a N source control. In the presence of glucose and (NH4)2SO4, no difference in yeast growth was observed among the yeast strains 22∆10α expressing either the empty vector or CsABCG11.2 and the wild type 23344c. Based on the growth performance of yeast, it was confirmed that CsABCG11.2 mediates the transport of various examined amino acids, such as aspartate and glutamate, arginine, glutamine, and theanine (Fig. 5d). The liquid culture assays confirmed these results (Fig. 5e).
Complementation experiments in yeast demonstrated that CsABCG11.2 can transport Cd and Thea. The impact of CsABCG11.2 on Cd uptake, competitive amino acid and Cd uptake, and yeast growth was further analyzed. To this end, mutant yeast strains were used to assess the transport competition between Cd and Thea. Based on the results, the amount of Cd taken up by yeast heterologously expressing CsABCG11.2 decreased with the increase of Thea concentration from 0.1 to 2.0 mM (Fig. 6). When Thea concentration in the medium was greater than 2.0 mM, the Cd concentration in yeast was stabilized and did not decrease more. Therefore, Thea is a substrate that can competitively inhibit the uptake of Cd mediated by CsABCG11.2 (Fig. 6).
Competitive uptake of cadmium (Cd) and theanine (Thea) by Camellia sinensis ATP-Binding Cassette subfamily member G 11.2 (CsABCG11.2). Yeast carrying CsABCG11.2 and an empty vector (pYES2) were cultured in a liquid medium containing 2% Gal in the presence of 10 µM CdCl2 for 1 h. The Cd concentration in yeast was determined with ICP-MS after digestion. Data are means ± SD of three biological replicates, with different letters indicating significant differences at P < 0.05. DW, dry weight
CsABCG11.2 expression is highly induced by Cd
To determine the expression profile of CsABCG11.2 in tea plants, we measured, via RT-qPCR, its expression levels in leaves L1, leaves L2, and Root of tea seedlings (Fig. 7a). CsABCG11.2 was ubiquitously expressed, and it was especially highly expressed in tea leaves (Fig. 7b). The expression levels of CsABCG11.2 in young leaves were 1.2-fold and 2.8-fold higher than those in mature leaves and roots, respectively (Fig. 7b). In addition, to determine the transcriptional responses of CsABCG11.2 in response to Cd stress, the tea plants were grown in solutions supplemented with increasing concentrations of CdCl2 for 12 h. CsABCG11.2 expression was most significantly induced by 10 µM CdCl2 in young leaves, mature leaves, and roots (Fig. 7b). However, when the plants were treated with 40 or 80 µM CdCl2, the expression levels of CsABCG11.2 gradually decreased, indicating potential indirect effects due to cell death after exposure to a high level of Cd rather than direct regulation of gene expression (Fig. 7b). Furthermore, under treatment with 10 µM CdCl2 for 24 h, the transcript levels of CsABCG11.2 increased over time and peaked at 6 h, then gradually decreased (Fig. 7c). These findings further demonstrated that CsABCG11.2 is a Cd-responsive gene and participates in Cd stress adaptation and tolerance.
The expression of Camellia sinensis ATP-Binding Cassette subfamily member G 11.2 (CsABCG11.2) is highly induced by cadmium (Cd) stress in tea plants. a Schematic maps of different tissues of tea plants for gene expression level determination. b Cd-concentration dependent expression levels of CsABCG11.2 in different leaves and roots after treatment for 6 h. c Time-dependent expression levels of CsABCG11.2 after treatment with 10 μM Cd for 0, 6, and 24 h. d Expression analysis of CsABCG11.2 in a ProCsABCG11.2:GUS transgenic Arabidopsis line. Expression patterns of CsABCG11.2 in seedlings, flowers, and roots as revealed by β-glucuronidase (GUS) staining analysis. Images are presented as follows: GUS staining observed in a 3-week-old seedling and flower under the control treatment. Lateral roots and rosette leaf are under control, as well as Cd, theanine (Thea), and Cd + Thea treatments. Scale bar = 1 mm for the root and 5 mm for the leaves. e The subcellular localization of 35S::GFP and 35S::CsABCG11.2::GFP fusion proteins, transiently expressed in tobacco leaves. Scale bar = 20 µm in the images of CsABCG11.2. Scale bar = 50 µm for 35S::GFP. Values are means ± SD of three biological replicates. Different letters indicate significant differences at P < 0.05
To further confirm the tissue-specific expression patterns of CsABCG11.2, proCsABCG11.2::GUS transgenic lines were generated. As shown in Fig. 7d, after histochemical staining in the transgenic lines, in the absence of Cd and Thea, the GUS signals were broadly detected in the vascular system of the cotyledons, rosette leaves, the stamens and stigmata, (Fig. 7d). In roots, proCsABCG11.2:GUS expression was abundantly detected in lateral root primordia (Fig. 7d), in accordance with the expression profile of AtABCG11 (Le Hir et al. 2013). According to the results of the GUS histochemical assay, CsABCG11.2 expression was much higher in the shoot under Cd stress compared with the control, which was consistent with the result of qRT-PCR in tea plants (Fig. 7b). Under 40 µM Cd stress, the GUS staining intensity in root tips and leaves was significantly increased. Higher GUS staining intensity was also observed in the mature region of lateral roots (Fig. 7d). Moreover, the GUS staining in root tips and lateral roots primordia was obviously increased under the 50 µM Thea treatment (Fig. 7d). Under the combined treatment of 40 µM Cd and 50 µM Thea, the GUS staining intensity was also significantly increased.
Subcellular localization of CsABCG11.2
To determine the subcellular localization of CsABCG11.2, a CsABCG11.2::eGFP fusion protein was transiently expressed in N. benthamiana leaves. The green fluorescence of the 35S::eGFP control was observed throughout the cell, especially the plasma membrane and nucleus (Fig. 7e). The CsABCG11.2::eGFP fusion protein fluorescence suggested a localization at the plasma membrane, as it overlapped with the fluorescence localization of CBL1n-OFP, used as a plasma membrane marker (Fig. 7e). These results confirmed that CsABCG11.2 is localized at the plasma membrane.
Overexpression of CsABCG11.2 alleviates Cd stress in the presence of Thea
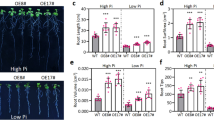
To further confirm the biological function of CsABCG11.2 in mediating Thea absorption and thus alleviating Cd toxicity, Cd sensitivity and tolerance were assessed in CsABCG11.2-overexpressing Arabidopsis lines (OE1-OE3) and WT plants. The root length of plants grown on plates was quantitatively determined after exposure to the different treatments (Fig. 8a, b). The growth of OE lines was similar to the WT in the treatment of Thea and the control treatment (Fig. 8b). The primary root length of OE lines was more severely reduced by Cd treatment relative to WT. In comparison, its growth was maintained in the combined Cd and Theatreatment, and OE lines exhibited greater biomass than the WT plants (Fig. 8b, c).
Growth performance comparison of Arabidopsis thaliana wild-type (WT) and CsABCG11.2 -overexpressing lines (OE1-OE3) treated with theanine (Thea), cadmium (Cd), and their combination. a, b On plate culture experiments, growth phenotypes were recorded in Arabidopsis thaliana WT and Camellia sinensis ATP-Binding Cassette subfamily member G 11.2 (CsABCG11.2)-overexpressing lines treated with 20 μM Thea, 100 μM Cd, and combined Thea and Cd (a), and quantitative comparative analysis was conducted for primary root length (b). c, d In a long-term hydroponic cultivation experiment, growth phenotypes were recorded in Arabidopsis thaliana WT and CsABCG11.2-overexpressing lines treated with 20 μM Thea, 40 μM Cd, and combined Thea and Cd (c), and the accumulation of Cd in the shoot was comparatively analyzed (d). * represents significant difference at P = 0.05 when compared with the control, n = 3
In a long-term hydroponics experiment, 2-week-old Arabidopsis plants were grown under four treatments (Fig. 8c). WT plants showed seriously inhibited growth at 40 μM Cd, which was a lethal concentration for OE lines at the seedling stage (Fig. 8d). At 40 μM Cd, 52.7% of the Cd was transported to the shoots of WT. In contrast, an average of 76.5% Cd was translocated to the shoots of the OE lines. In contrast, OE lines grew better than WT in the combined treatment of 40 μM Cd and 20 μM Thea and had a significantly lower percentage of shoot-Cd (53.5%) accumulation (Fig. 8e). These findings indicated that, at relatively higher Cd levels, overexpression of CsABCG11.2 enhanced the sensitivity to Cd, which might be due to the increased Cd concentration in both shoots and roots or due to markedly increased Cd translocation from the roots to the shoots. Further, both Cd and Thea were further confirmed to be substrates that were taken up in a competitive manner by CsABCG11.2. How Thea affects Cd uptake and transport mediated by CsABCG11.2 in plants remains unknown. However, its application can be highly valuable for producing tea with lower Cd contamination.
4-week-old hydroponically cultured Arabidopsis plants were assessed for Cd response and tolerance. When the plants were exposed to 40 μM Cd, OE lines showed more evident toxicity symptoms of chlorosis in the young leaves and had a smaller rosette size (Fig. S4), consistent with the phenotype of inhibited primary root length on the plate experiment (Fig. 8a, b). In addition, the OE lines exhibited significantly increased Cd concentration in shoots by 1.3-fold compared with WT plants (Fig. S4). As expected, the addition of 10 and 50 µM Thea alleviated the inhibitory effect on growth and chlorosis by Cd in OE lines, and plant biomass was increased by 80% compared to the 40 μM Cd treatment. The Cd concentration of OE lines treated with exogenous Thea decreased by 27.40%, 23.72%, and 33.20%, respectively, compared with the Cd treatment alone (Fig. S4). In WT plants grown under combined Cd and Thea, improved growth performance was observed under the higher Cd concentrations when compared with the Cd treatment alone, which might be attributed to Thea's physiologic role in alleviating Cd-generated stress. Therefore, the underlying mechanisms of CsABCG11.2 mediating the uptake of Thea to alleviate Cd toxicity might be regulated by other genes, such as transporters sequestering Cd into the vacuole.
To assess how CsABCG11.2 and exogenous Thea affect the ROS-caused damage induced by Cd, we determined the MDA contents in WT and OE Arabidopsis lines grown under different Cd and Thea treatments. The MDA contents of Arabidopsis lines were significantly increased under Cd stress, and they were significantly higher in OE lines than in WT. As expected, exogenous Thea application significantly decreased the MDA levels compared to exposure to Cd alone, which was especially apparent in the OE lines, suggesting that the addition of Thea alleviated the oxidative stress induced by Cd (Fig. S5a). Further, we examined the activities of the antioxidant enzymes CAT, SOD, and POD in the Arabidopsis lines. In the OE lines, compared with the control treatment, the CAT, SOD, and POD activities in the leaves consistently increased by 2.2–2.5 fold in plants grown in the medium containing 40 µM Cd (Fig. S5b, c, d). They were slightly increased in the 10 µM or 50 µM Thea treatments, but no significant differences were observed for SOD and POD antioxidant activities between the two treatments (P > 0.05) (Fig. S5b, c, d). However, different responses were observed in WT plants, suggesting that CsABCG11.2 could explain the increased sensitivity to Cd stress. Altogether, the addition of Thea decreased the activity of antioxidant enzymes, promoted the scavenging of Cd-induced ROS, and alleviated Cd-derived growth inhibition, indicating that Thea plays a vital role in tolerance to Cd toxicity via regulating the antioxidant system.
Knockdown of CsABCG11.2 reduces Cd accumulation in tea plants
Three weeks after VIGS inoculation, the plants were treated with Cd. As expected, after one week, the tea plants treated with a high Cd concentration of 200 μM showed severe symptoms of toxicity, root browning, and leaf abscission. Under 80 μM Cd treatment for 20 days, in comparison to theTRV1-TRV2 lines (Fig. S6), the three CsABCG11.2 knockdown lines exhibited significantly reduced Cd concentrations in the young leaves, ranging between 2.20 ~ 2.41 mg Cd kg−1·DW (Fig. 9). However, no remarkable difference was observed in the mature leaves between the knockdown lines and control lines. Furthermore, no significant difference was observed in the combined treatment with Thea and Cd between the CsABCG11.2-knockdown and control lines in both young and mature leaves. VIGS-mediated CsABCG11.2 silencing in tea plants revealed its important role in Cd translocation from the roots to the young shoot. The different results between tea plants and Arabidopsis might be attributed to the increased Thea biosynthesis in tea plants after exposure to Cd stress. Therefore, the results from the OE Arabidopsis and the CsABCG11.2-knockdown tea plants demonstrated that Thea is a significant competitive substrate for plant transporters that can contribute to tea production with lower Cd and other metal contamination. Overall, the physiological roles of Thea in the increased tolerance to Cd stress and the competition in the absorption of Thea and Cd by CsABCG11.2 significantly reduced Cd absorption, resulting in improved tea growth.
Comparative analysis of cadmium (Cd) accumulation in the shoot of tea plants, wild type (WT) and Camellia sinensis ATP-Binding Cassette subfamily member G 11.2 (CsABCG11.2)-knockdown tea lines, under different treatments: 40 µM Cd, 50 µM Thea with 40 µM Cd, and the control. After three weeks of culture, the Cd concentrations in the young leaves (YL) and mature leaves (ML) were determined. * represents a significant difference at P = 0.05 when compared with the control, n = 3. NS, non-significant; TRV, tobacco rattle virus
Discussion
Thea is an important metabolite in tea plants' resistance to Cd stress
In this study, it was demonstrated that Thea at physiological levels could effectively alleviate the reduction of photosynthetic efficiency of tea plants caused by Cd toxicity. As expected, it enhanced the resistance of tea plants to Cd toxicity by increasing the activity of SOD, POD, and CAT enzymes resulting in significantly reduced MDA levels in the leaves of Cd-treated tea plants (Fig. 2). Hence, the presence of Thea plays an essential role in tea plant-environment interactions to adapt and resist to Cd stress. The expression of genes e involved in nitrogen assimilation and Thea metabolism were affected by Cd, which might suggest the necessity of amino acid metabolism as a key intercellular signal for tea plant adaptation to Cd stress. Further investigations should be conducted to explore the mechanisms underlying this proposed function. The Cd content in the upper young leaves decreased significantly under the combined treatment with Thea and Cd in comparison to the Cd treatment alone. Exogenous Thea significantly reversed the Cd-induced decrease in the contents of FAA and CAF in the young tea leaves (Fig. 3). The addition of Thea reversed the Cd-mediated downregulation in the expression levels of nitrate transporters CsNRT1.2 and CsNRT2.5 and nitrogen assimilation pathway-related genes CsGS1, CsNiR, CsNR, CsGDH1, CsALT and CsNADH-GOGAT in tea roots. This suggests that Thea can alleviate Cd toxicity by regulating nitrogen assimilation and Thea synthesis in the tea roots. These findings are consistent with previous reports that plants actively regulate the biosynthesis of certain metabolites under heavy metal stress (Zhu et al. 2018), inhibiting the Cd uptake by roots and the translocation of Cd from the roots to the shoot (Köhl 1996; Takahashi et al. 2003; Kobayashi et al. 2005). It is suggested that they be regulated towards increased biosynthesis of Thea to adapt to environmental stresses. It has been reported that upregulated Thea biosynthesis enhanced salt stress tolerance through a redox homeostasis pathway in tea plants (Chen et al. 2021). Therefore, these findings indicate the importance of Thea in plant tolerance to abiotic stress conditions. We hypothesize that Thea plays a crucial role as a secondary metabolic regulatory compound in detoxifying Cd in tea plants, as evidenced by its increased biosynthesis and its ability to alleviate Cd stress in WT tea seedlings. Overall, Thea in the growth medium or the soil might contribute to plant tolerance and adaptation to different environmental stresses. This hypothesis deserves further investigation for the efficient utilization of tea plant pruning materials and to reveal the underlying mechanisms.
Competitive absorption of Cd and Thea by CsABCG11.2
Here, we have demonstrated that, in tea plants and Arabidopsis, the Thea-mediated reduction in Cd uptake and translocation was partially attributed to the fundamental competition for their absorption by CsABCG11.2, resulting in the direct Thea-mediated Cd toxicity alleviation. CsABCG11.2 is not a Cd-specific transporter for Cd uptake and translocation (Fig. 5, Fig. S7), as it has been reported in a review study on ABC transporters in Arabidopsis (Gräfe and Schmitt 2021). This implies the multifunctionality of these transporters, which involves the transport of a broad range of substrates. The numerous ABCG subfamily members are involved in diverse processes such as pathogen responses, tissue development, phytohormone transport, plant growth, etc. (Le Hir et al. 2013; Gräfe and Schmitt 2021). Until now, it has been unclear if Thea affects plants through an unidentified signaling pathway. Here, we proposed that Thea exerts its multiple physiological effects in plants via the transporter CsABCG11.2. Studies have shown that Thea has beneficial effects on human health, including antioxidant and immunomodulatory properties (Deng et al. 2016; Gong et al. 2019). Thea likely acts as a signaling molecule in both plants and animals. In addition to Thea, the non-protein amino acid γ-aminobutyric acid (GABA) has been well studied, possessing various physiological functions and improving plant stress resilience to environmental stresses (Ramesh et al. 2015; Xu et al. 2021).
Thea synthesis/N assimilation in tea plants is inhibited by Cd stress. Therefore, the roles of Thea as a metabolite in tea plants are associated with redox processes. It is currently unknown whether Thea is directly involved in physiologic and metabolic activities as a signal molecule or whether Thea or nitrogen metabolism is feedback-regulated, and the key genes involved in these biological processes have still not been identified. It has been reported that ABC family transporters can absorb and transport heavy metals, but these studies are mainly concentrated in model plants, Arabidopsis, and rice (Oda et al. 2011; Park et al. 2012; Brunetti et al. 2015). However, how Thea and Cd regulate the expression of CsABCG11.2 under Cd stress conditions is unknown. Based on heterologous yeast expression experiments, this study demonstrated that CsABCG11.2 transports Cd and the major amino acids in tea plants. Although CsABCG11.2 is a transporter responsible for Cd and Thea transport, the extent of competitive transport of Thea and Cd via CsABCG11.2 in tea plants remains unknown but deserves further investigation. In CsABCG11.2 overexpressing Arabidopsis lines, the combined treatment with Cd and Thea could significantly reduce Cd toxicity. At the same time, CsABCG11.2 can mediate Thea transport, resulting in enhanced oxidative stress tolerance by significantly reducing MDA content (Fig. S5). It is worth noting that the Cd content of OE Arabidopsis strains was significantly decreased under the combined Cd and Thea treatment compared with the Cd treatment alone. Moreover, there was no significant difference in Cd content between OE lines under the combined Cd and Thea-treatment and wild-type plants under the Cd treatment (Fig. S5). These results suggested that the reduction of Cd accumulation in the shoot of plants under the combined treatment was associated with the competitive absorption of Thea and Cd mediated by CsABCG11.2. The ABC family members have been documented to be involved in Arsenic (As) or Cd resistance due to their Cd transport into the vacuole (Sasaki et al. 2014; Song et al. 2010).
The heavy metal Cd is a foreign toxic compound to plant cells, while the endogenous synthesis of Thea is beneficial to tea plants. It was confirmed that the competitive absorption of Thea by CsABCG11.2 inhibited the internal transport of Cd. However, the underlying mechanism remains unclear. On the other hand, the uptake of Cd in yeast heterologously expressing CsABCG11.2 did not decrease linearly when Thea concentration exceeded 2 mM (Fig. 6). Camellia sinensis Heavy-Metal ATPases 3 (CsHMA3), Camellia sinensis Natural Resistance-Associated Macrophage Protein 5 (CsNRAMP5), and Camellia sinensis Iron-Regulated Transporter 1 (CsIRT1) were significantly upregulated by the organic nitrogen treatment in the medium containing Thea (Fig. S3), and their homologous genes were demonstrated to transport Cd in rice or Arabidopsis (Sasaki et al. 2012, 2014; Uraguchi and Fujiwara 2012; Yan et al. 2016). How Thea regulates the expression of other genes involved in Cd uptake and accumulation cannot be concluded from the current study. Therefore, the findings underscore a complex system for the adaptation to environmental stresses through the fine regulation of key genes involved in nitrogen assimilation, Thea synthesis, Cd transport, or other transporters.
CsABCG11.2 is a key contributor to the alleviation of Cd toxicity by promoting Thea uptake
Our results suggest that Thea plays a positive role in the growth performance of WT Arabidopsis (Fig. 8c) through increased Cd concentration (Fig. S4b) and increased Cd accumulation in the shoots (Fig. 8d). On the other hand, it contributed to better growth and decreased root and shoot Cd concentration in the CsABCG11.2-overexpression lines (Fig. 8; Fig. S4). The plasma membrane-localized CsABCG11.2 was significantly upregulated by Cd (Fig. 7b, c). It has been suggested that different conformations of ABC transporters regulate the substrate specificity for transmembrane transport (Jones and George 2004). The choice of transport substrates is determined by the alternation of the two conformations (Yu et al. 2021). Previous studies have shown that both AtABCG12 and AtABCG11 are multimeric transporters. They form heteromorphic dimers, and two AtABCG11 units can either form homodimers or heterodimers with AtABCG9 and AtABCG14, and the diversity of their dimerization enables them to transport different substrates (Le Hir et al. 2013). Therefore, we also speculated that CsABCG11.2 can mediate Thea transport to reduce Cd uptake in tea roots, which might be a result of the conformation or dimer formation by CsABCG11.2. Its conformation is related to the type of substrate, and Cd can be pushed out of the substrate binding site through a conformation change, thus reducing its transmembrane transport and influx. Thus, Cd homeostasis in plants is strictly controlled at the cellular level.
Furthermore, Thea in the tea plantation soil originates from the residues of tea plants or root exudates and ranges from 4.89 μM to 56.98 μM throughout the year (Fig. S1). In this study, the Thea concentration contributing to Cd stress fits well with the natural environmental concentration levels. However, in a previous study, Shan et al. (2015) found that exogenous Thea at relatively high levels (0.5, 1, 5 mM) resulted in antioxidant enzyme system imbalances and caused oxidation reactions in tobacco seedlings. In contrast, a high level of 10 mM Thea was demonstrated to increase the tolerance to salt stress in tea plants (Chen et al. 2021). It is well known that Thea is a unique metabolite in tea plants. It can be hydrolyzed into glutamate and ethylamine due to environmental factors or through metabolic reactions. The ethylamine is physiologically toxic to most plant species. Still, ethylamine is not toxic, or its toxic threshold is relatively higher in tea plants, suggesting that a threshold concentration of Thea could be beneficial in agronomic practices in tea plantations.
Studies have shown that a large number of differentially phosphorylated proteins are involved in signal transduction, stress tolerance, reactive oxygen species neutralization, and other processes, and n pathways mediating phosphorylation changes quickly respond to and enhance resistance to Cd stress (Zhong et al. 2017; Fang et al. 2019). Raichaudhur (2016) analyzed the phosphorylation proteomics of Arabidopsis thaliana under arsenic stress and found that phosphorylation of the C-terminal nucleotide binding domain (NBD) serine triplet of AtMRP1 plays a key role in Arabidopsis resistance to arsenic stress. Six phosphorylation sites were predicted in the intracellular domains of CsABCG11.2, including five serine phosphorylation sites and one tyrosine phosphorylation site (Fig. S8). Regarding the competition between Thea and Cd, whether substrate recognition and affinity by CsABCG11.2 are regulated by serine phosphorylation requires further studies at the biochemical level. The hypothesis of CsABCG11.2 protein modification is intriguing and warrants additional experiments to further optimize it as an ideal transporter, targeted with molecular breeding, for the production of tea with a lower concentration of contamination. Therefore, the characterization of the conformation change mechanisms of CsABCG11.2 involved in substrate affinity or transport will be further investigated.
As CsABCG11.2 was expressed in the petals in proCsABCG11.2::GUS Arabidopsis, it was speculated that CsABCG11.2 may be involved in the development of reproductive organs. In addition, except for the treatments of control and Thea, it was observed an increase in Mn and Cu concentration in OE lines in the two other treatments (Fig. S9) despite the inability of Cu transport observed in yeast expressing CsABCG11.2 (Fig. S7). Mn and Cu are required as cofactors for enzymes with multiple functions. Therefore, the exact mechanism of how the CsABCG11.2 affects Mn and Cu transport and their involvement in Cd stress tolerance needs further investigation. Additionally, significantly decreased Cd concentration was observed in the CsABCG11.2 knockdown tea plants in the presence of Cd. Homologous genes responsible for heavy metal transport, including Cd, have been identified, which are associated with more Cd accumulation in the roots and reduced root-to-shoot Cd translocation and Cd concentration in grain or other edible parts (Yan et al. 2016; Zhang et al. 2019). Considering the fact that both Cd and Thea are the substrates for CsABCG11.2, the selection of superior CsABCG11.2 alleles showing a greater affinity for Thea or low transport ability for Cd is a more effective strategy for germplasm breeding for contaminant-free tea production rather than the knockout/knocking-down of CsABCG11.2.
Conclusions
The Cd transport and distribution in tea leaves result from synergies and competition between Cd and other potential substrates. It is, therefore, worthwhile to explore substrates and the causal genes associated with Cd accumulation variation in tea plants' leaves. Our findings confirmed that Thea alleviates Cd toxicity by regulating photosynthetic efficiency, MDA content, antioxidant enzyme activity, and expression of genes related to the nitrogen assimilation pathway. This study preliminarily elucidated the physiological regulatory mechanism of CsABCG11.2-mediated Thea alleviating Cd toxicity. As a result, our observations suggest that Thea application at the environmental and biologically normal level provides an agronomic practice to alleviate the damaging effects of Cd toxicity in agricultural plants. Therefore, the feasibility of designing a tea plant cultivar with elevated Thea and decreased Cd, as well as a novel approach of reducing Cd and enhancing Thea levels in young shoots, should be evaluated and implemented in future studies.
Availability of data and materials
The data in the current manuscript is available from the corresponding author upon reasonable request.
References
Åkesson A, Barregard L, Bergdahl IA, Nordberg GF, Nordberg M, Skerfving S. Non-renal effects and the risk assessment of environmental cadmium exposure. Environ Health Perspect. 2014;122:431–8. https://doi.org/10.1289/ehp.1307110.
Besnard J, Pratelli R, Zhao C, Sonawala U, Collakova E, Pilot G, et al. UMAMIT14 is an amino acid exporter involved in phloem unloading in Arabidopsis roots. J Exp Bot. 2016;67:6385–97. https://doi.org/10.1093/jxb/erw412.
Brunetti P, Zanella L, DePaolis A, DiLitta D, Cecchetti V, Falasca G, et al. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J Exp Bot. 2015;66:3815–29. https://doi.org/10.1093/jxb/erv185.
Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhanceactivities of superoxide dismutase, ascorbate peroxidase, and glutathione reductasein bean leaves. Plant Physiol. 1992;98:1222–7. https://doi.org/10.1104/pp.98.4.1222.
Chen Z, Lin S, Li J, Chen T, Gu Q, Yang T, et al. Thea improves salt stress tolerance via modulating redox homeostasis in tea plants (Camellia sinensis L.). Front Plant Sci. 2021;12:770398. https://doi.org/10.3389/fpls.2021.770398.
Deng Y, Xiao W, Chen L, Liu Q, Liu Z, Gong Z. In vivo antioxidative effects of L-Thea in the presence or absence of Escherichia coli-induced oxidative stress. J Funct Foods. 2016;24:527–36. https://doi.org/10.1016/j.jff.2016.04.029.
Dong C, Li F, Yang T, Feng L, Zhang S, Li F, et al. Thea transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020;101:57–70. https://doi.org/10.1111/tpj.14517.
Du Y, Hu X, Wu X, Shu Y, Jiang Y, Yan X. Affects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China. Environ Monit Assess. 2013;185:9843–56. https://doi.org/10.1007/s10661-013-3296-y.
Fang Y, Deng X, Lu X, Zheng J, Jiang H, Rao Y, et al. Differential phosphoproteome study of the response to cadmium stress in rice. Ecotoxicol Environ Saf. 2019;180:780–8. https://doi.org/10.1016/j.ecoenv.2019.05.068.
Feng L, Yang T, Zhang Z, Li F, Chen Q, Sun J, et al. Identification and characterization of cationic amino acid transporters (CATs) in tea plant (Camellia sinensis). Plant Growth Regul. 2018;84:57–69. https://doi.org/10.1007/s10725-017-0321-0.
Fu S, Lu Y, Zhang X, Yang G, Chao D, Wang Z, et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J Exp Bot. 2019;70:5909–18. https://doi.org/10.1093/jxb/erz335.
Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in Higher. Plants Plant Physiol. 1977;59:309–14. https://doi.org/10.1104/pp.59.2.309.
Gong Z, Lin L, Liu Z, Zhang S, Liu A, Chen L. Immunemodulatory effects and mechanism of action of L-Thea on ETEC-inducedimmune-stressed mice via nucleotide-binding oligomerization domain-likereceptor signaling pathway. J Funct Foods. 2019;54:32–40. https://doi.org/10.1016/j.jff.2019.01.011.
Gräfe K, Schmitt L. The ABC transporter G subfamily in Arabidopsis thaliana. J Exp Bot. 2021;72:92–106. https://doi.org/10.1093/jxb/eraa260.
Guan M, Zhu Y, Liu X, Jin C. Induction of S-nitrosoglutathione reductase reduces root cadmium uptake by inhibiting Iron-regulatedtransporter 1. Plant Soil. 2019;438:251–62. https://doi.org/10.1007/s11104-019-04014-z.
Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–11. https://doi.org/10.1007/s004250050524.
Hua Y, Chen J, Zhou T, Zhang T, Shen D, Feng Y, et al. Multiomics reveals an essential role of long-distance translocation in regulating plant cadmium resistance and grain accumulation in allohexaploid wheat (Triticum aestivum). J Exp Bot. 2022;73:7516–37. https://doi.org/10.1093/jxb/erac364.
Huang W, Ma D, Liu H, Luo J, Wang P, Wang M, et al. Genome-wide identification of CsATGs in tea plant and the involvement of CsATG8e in nitrogen utilization. Int J Mol Sci. 2020;21:7043. https://doi.org/10.3390/ijms21197043.
Hwang J, Song W, Hong D, Ko D, Yamaoka Y, Jang S, et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol Plant. 2016;9:338–55. https://doi.org/10.1016/j.molp.2016.02.003.
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C. Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics. 2019;11:225. https://doi.org/10.1039/c8mt00247a.
Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682–99. https://doi.org/10.1007/s00018-003-3336-9.
Kang J, Hwang J, Lee M, Kim Y, Assmann SM, Martinoia E, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc Natl Acad Sci. 2010;107:2355–60. https://doi.org/10.1073/pnas.0909222107.
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007;50:207–18. https://doi.org/10.1111/j.1365-313x.2007.03044.x.
Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, et al. Expression of iron-acquisition-related genes in iron-deficientrice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot. 2005;56:1305–16. https://doi.org/10.1093/jxb/eri131.
Köhl K. Population-specific traits and their implication for the evolution of a drought-adapted ecotype in armeria maritima. Bot Acta. 1996;109:206–15. https://doi.org/10.1111/j.1438-8677.1996.tb00565.x.
Le Hir R, Sorin C, Chakraborti D, Moritz T, Schaller H, Tellier F, et al. ABCG9, ABCG11 and ABCG14 ABC transporters are required for vascular development in Arabidopsis. Plant J. 2013;76:811–24. https://doi.org/10.1111/tpj.12334.
Lee M, Lee K, Lee J, Noh EW, Lee Y. AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol. 2005;138:827–36. https://doi.org/10.1104/pp.104.058107.
Lei M, Tie B, Song Z, Liao B, Lepo JE, Huang Y. Heavy metalpollution and potential health risk assessment of white rice around mine areas in Hunan Province, China. Food Secur. 2015;7:45–54. https://doi.org/10.1007/s12571-014-0414-9.
Li Z, Li L, Pan G, Chen GPJ. Bioavailability of Cd in a soil-rice system in China: soil type versus genotype effects. Plant Soil. 2005;271:165–73. https://doi.org/10.1007/s11104-004-2296-7.
Li X, Ahammed GJ, Zhang Y, Zhang G, Sun Z, Zhou J, et al. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015;17:81–9. https://doi.org/10.1111/plb.12211.
Li X, Wei J, Ahammed GJ, Zhang L, Li Y, Yan P, et al. Brassinosteroids attenuate moderate high temperature-caused decline in tea quality by enhancing Thea biosynthesis in Camellia sinensis L. Front Plant Sci. 2018;9:1016. https://doi.org/10.3389/fpls.2018.01016.
Li F, Dong C, Yang T, Bao S, Fang W, Lucas WJ, et al. The tea plant CsLHT1 and CsLHT6 transporters take up amino acids, as a nitrogen source, from the soil of organic tea plantations. Hort Res. 2021;8:178. https://doi.org/10.1038/s41438-021-00615-x.
Liu J, Zhang Q, Liu M, Ma M, Shi Y, Ruan J. Metabolomic analyses reveal distinct change of metabolites and quality of green tea during the short duration of a single spring season. J Agric Food Chem. 2016;64:3302–9. https://doi.org/10.1021/acs.jafc.6b00404.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.
Meng X, Li W, Shen R, Lan P. Ectopic expression of IMA small peptide genes confers tolerance to cadmium stress in Arabidopsis through activating the iron deficiency response. J Hazard Mater. 2022;422:126913. https://doi.org/10.1016/j.jhazmat.2021.126913.
Nordberg GF. Historical perspectives on cadmium toxicology. Toxicol Appl Pharmacol. 2009;238:192–200. https://doi.org/10.1016/j.taap.2009.03.015.
Oda K, Otani M, Uraguchi S, Akihiro T, Fujiwara T. Rice ABCG43 is Cd inducible and confers Cd tolerance on yeast. Biosci Biotech Bioch. 2011;75:1211–3. https://doi.org/10.1271/bbb.110193.
Park J, Song W, Ko D, Eom Y, Hansen TH, Schiller M, et al. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012;69:278–88. https://doi.org/10.1111/j.1365-313x.2011.04789.x.
Raichaudhuri A. Arabidopsis thaliana MRP1 (AtABCC1) nucleotide binding domain contributes to arsenic stress tolerance with serine triad phosphorylation. Plant Physiol Biochem. 2016;108:109–20. https://doi.org/10.1016/j.plaphy.
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, et al. GABA signalling modulates plant growth bydirectly regulating the activity of plant-specific anion transporters. Nat Commun. 2015;6:7879. https://doi.org/10.1038/ncomms8879.
Rea PA. Plant ATP-binding cassette transporters. Annu Rev Plant Biol. 2007;58:347–75. https://doi.org/10.1146/annurev.arplant.57.032905.105406.
Sarwar S, Ahmad F, Waheed A, Zaman QU. Study on the determination of nutrient status of NTRI tea gardens soils. Sci Technol Dev. 2011;30:39–43.
Sasaki A, Yamaji N, Yokosho K, Ma J. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell. 2012;24:2155–67. https://doi.org/10.1105/tpc.112.096925.
Sasaki A, Yamaji N, Ma J. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot. 2014;65:6013–21. https://doi.org/10.1093/jxb/eru340.
Shan D, Zhang Q, Guo J, Liu S, Chen Z, Zhou T, et al. Influence of Thea on the growth and physiological indexes of tobacco seedlings. J Anhui Agric University. 2015;42:283–9.
Shi M, Wang S, Zhang Y, Wang S, Zhao J, Feng H, et al. Genome-wide characterization and expression analysis of ATP-binding cassette (ABC) transporters in strawberry reveal the role of FvABCC11 in cadmium tolerance. Sci Hortic. 2020;271:109464. https://doi.org/10.1016/j.scienta.2020.109464.
Song WY, Park J, Mendoza-Cozatl DG, Suter-Grotemeyer M, Shim D, Hortensteiner S, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107:21187–92. https://doi.org/10.1073/pnas.1013964107.
Su Y, Frommer W, Ludewig U. Molecular and functional characterization of a family of amino acid transporters from Arabidopsis. Plant Physiol. 2004;136:3104–13. https://doi.org/10.1104/pp.104.045278.
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, et al. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell. 2003;15:1263–80. https://doi.org/10.1105/tpc.010256.
Terasaka K, Blakeslee JJ, Titapiwatanakun B, Bandyopadhyay A, Makam SN, Lee OR, et al. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;17:2922–39. https://doi.org/10.1105/tpc.105.035816.
Uraguchi S, Fujiwara T. Cadmium transport and tolerance in rice: persperctives for reducing grain cadmium accumulation. Rice. 2012;5:5. https://doi.org/10.1186/1939-8433-5-5.
Wang W, Xin H, Wang M, Ma Q, Wang L, Kaleri NA, et al. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front Plant Sci. 2016;7:385. https://doi.org/10.3389/fpls.2016.00385.
Wang H, Liu Y, Peng Z, Li J, Huang W, Liu Y, et al. Ectopic expression of poplar ABC transporter PtoABCG36 confers Cd tolerance in Arabidopsis thaliana. Int J Mol Sci. 2019a;20:3293. https://doi.org/10.3390/ijms20133293.
Wang P, Chen H, Kopittke PM, Zhao FJ. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ Pollut. 2019b;249:1038–48. https://doi.org/10.1016/j.envpol.2019.03.063.
Wilkens S. Structure and mechanism of ABC transporters. F1000prime Rep. 2015;7:14. https://doi.org/10.12703/p7-14.
Xu B, Long Y, Feng X, Zhu X, Sai N, Chirkova L, et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat Commun. 2021;12:1952. https://doi.org/10.1038/s41467-021-21694-3.
Yan J, Wang P, Wang P, Yang M, Lian X, Tang Z, et al. A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant Cell Environ. 2016;39:1941–54. https://doi.org/10.1111/pce.12747.
Yan P, Wu L, Wang D, Fu J, Shen C, Li X, et al. Soil acidification in Chinese tea plantations. Sci Total Environ. 2020;715:136963. https://doi.org/10.1016/j.scitotenv.2020.136963.
Yang T, Li H, Tai Y, Dong C, Cheng X, Xia E. Transcriptional regulation of amino acid metabolism in response to nitrogen deficiency and nitrogen forms in tea plant root (Camellia sinensis L.). Sci Rep. 2020;10:6868. https://doi.org/10.1038/s41598-020-63835-6.
Yang G, Fu S, Huang J, Li L, Long Y, Wei Q, et al. The tonoplast-localized transporter OsABCC9 is involved in cadmium tolerance and accumulation in rice. Plant Sci. 2021;307:110894. https://doi.org/10.1016/j.plantsci.2021.110894.
Yang T, Xie Y, Lu X, Yan X, Wang Y, Ma J, et al. Shading promoted Thea biosynthesis in the roots and allocation in the shoots of the tea plant (Camellia sinensis L.) cultivar Shuchazao. J Agric Food Chem. 2021;69:4795–803. https://doi.org/10.1021/acs.jafc.1c00641.
Yu Q, Ni D, Kowal J, Manolaridis I, Jackson SM, Stahlberg H, et al. Structures of ABCG2 under turnover conditions reveal a key step in the drug transport mechanism. Nat Commun. 2021;12:4376. https://doi.org/10.1038/s41467-021-24651-2.
Zhang L, Wu J, Tang Z, Huang X, Wang X, Salt DE, et al. Variation in the BrHMA3 coding region controls natural variation in cadmium accumulation in Brassica rapa vegetables. J Exp Bot. 2019;70:5865–78. https://doi.org/10.1093/jxb/erz310.
Zhang L, Gao C, Chen C, Zhang W, Huang X, Zhao F. Overexpression of rice OsHMA3 in wheat greatly decreases cadmium accumulation in wheat grains. Environ Sci Technol. 2020;54:10100–8. https://doi.org/10.1021/acs.est.0c02877.
Zhao F, Ma Y, Zhu Y, Tang Z, McGrath SP. Soil contamination in China: current status and mitigation strategies. Environ Sci Technol. 2015;49:750–9. https://doi.org/10.1021/es5047099.
Zhao H, Guan J, Liang Q, Zhang X, Hu H, Zhang J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci Rep. 2021;11:9913. https://doi.org/10.1038/s41598-021-89322-0.
Zhong M, Li S, Huang F, Qiu J, Zhang J, Sheng Z, et al. The phosphoproteomic response of rice seedlings to cadmium stress. Int J Mol Sci. 2017;18:2055. https://doi.org/10.3390/ijms18102055.
Zhou Y, Deng Y, Liu D, Wang H, Zhang X, Liu T, et al. Promoting virus-induced gene silencing of pepper genes by a heterologous viral silencing suppressor. Plant Biotechnol J. 2021;19:2398–400. https://doi.org/10.1111/pbi.13724.
Zhu G, Xiao H, Guo Q, Zhang Z, Zhao J, Yang D. Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol Environ Saf. 2018;158:300–8. https://doi.org/10.1016/j.ecoenv.2018.04.045.
Acknowledgements
The authors appreciate Dr. Pilot Guillaume, who is from School of Plant and Environmental Sciences, Virginia Tech, for providing the yeast strains, 22Δ10α and 23344c. Also, the authors appreciate Prof. Guohua Xu, who is from Nanjing Agricultural University, for helping to get the strains easily.
Funding
This study was jointly supported by the National Natural Science Foundation of China (Grant No. 32070376 and Grant No. 32370390).
Author information
Authors and Affiliations
Contributions
Xulei Hao: conceptualization, validation, methodology, investigation, data curation, writing original draft; Long Xiahou: investigation, data curation; Hanyang Zhao: investigation; Jiatong Liu: investigation, data curation; Hua Zhao: supervision, funding acquisition, conceptualization, writing and editing. The manuscript was finished with contributions from Fei Guo, Pu Wang, Mingle Wang, Yu Wang and Dejiang Ni. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
44281_2024_36_MOESM1_ESM.tif
Supplementary Material 1. The dynamic variation of five amino acids in the tea garden soil of arable layer. The five amino acids are theanine (Thea), glutamate (Glu), arginine (Arg), aspartate (Asp), glutamine (Gln).
44281_2024_36_MOESM2_ESM.tif
Supplementary Material 2. TRV-mediated VIGS of the CsPDS1 in tea seedling leaves. a WT, ten-month-old wild-type tea seedlings (WNZ). b pTRV2, infected seedlings with pTRV1 + pTRV2 Agrobacterium. c, d pTRV2-CsPDS1 (462 bp) tea seedlings, 70 days (c), 88 days (d) after agroinfiltration with pTRV1 + pTRV2-CsPDS1 Agrobacterium. e, f The expression of CsPDS1 was down-regulated in the infected (e) and non-infected (f) leaves of tea seedlings. The values and error bars represent the mean with standard deviations (n = 3). Different lowercase letters indicate a significant difference at P < 0.05 by Duncan’s multiple range test. SD, standard variation; TRV, tobacco rattle virus; WT, wild type tea plants; VIGS, virus-induced gene silencing; WNZ, tea plant cultivar Wuniuzao; CsPDS1, Camellia sinensis phytoene desaturase gene1.
44281_2024_36_MOESM3_ESM.tif
Supplementary Material 3. The expression profile of the four genes, Camellia sinensis Heavy-Metal ATPases 3 (CsHMA3), Camellia sinensis Natural Resistance-Associated Macrophage Protein 5 (CsNRAMP5), Camellia sinensis Iron-Regulated Transporter 1 (CsIRT1) and Camellia sinensis ATP-Binding Cassette G 11.2 (CsABCG11.2), in the young shoot and root of tea plants when exposure to ON and CK. ON: organic nitrogen treatment, containing amino acids of glutamine (Gln), glutamate (Glu), aspartate (Asp), arginine (Arg), and theanine (Thea), each at 30 μM; CK: normal condition.
44281_2024_36_MOESM4_ESM.tif
Supplementary Material 4. Comparison of growth performance and Cadmium (Cd) content in the shoot between wild-type (WT)and CsABCG11.2-overexpressing Arabidopsis thaliana lines(OE)treated with different treatments of theanine (Thea) and Cd. In a short-term hydroponic experiment, the growth phenotypes of OE lines and WT was recorded when the plants supplied with Cd showing phytotoxic signs of chlorosis (a), and the Cd concentration in the shoot (b) were measured. Each data is the mean of three replicates (± standard deviation, SD). Different letters indicate that groups are significantly different at P < 0.05. ns, no siginificance.
44281_2024_36_MOESM5_ESM.tif
Supplementary Material 5. Comparison of malondialdehyde (MDA) concentration and activity of antioxidant enzymes in the shoot between wild-type (WT) and CsABCG11.2-overexpressing Arabidopsis thaliana lines (OE)treated with different treatments of theanine (Thea)and cadmium (Cd). In a short-term hydroponic experiment, the samples were collected for the determination of activities of antioxidant enzymes of OE lines and WT when the plants supplied with Cd showing phytotoxic signs of chlorosis. Each data is the mean of three replicates (± standard deviation, SD). Different letters indicate that groups are significantly different at P < 0.05.
44281_2024_36_MOESM6_ESM.tif
Supplementary Material 6. The relative expression level of Camellia sinensis ATP-Binding Cassette G 11.2 (CsABCG11.2) in the knockdown silencing tea plants (TRV2-CsABCG11.2)and control lines with empty vector (TRV2). Six days after agroinfiltration, we detected the expression level of CsABCG11.2, two or three knockdown tea plants were conducted as a biological repeat, and three biological repeats’ plants were employed to the treatments—(1) CK, normal condition, (2) 40 µM cadmium (Cd), (3) 50 µM theanine (Thea) and 40 µM Cd. Each data is the mean of three replicates (± standard deviation, SD). * indicates that groups are significantly different at P = 0.05.
44281_2024_36_MOESM7_ESM.tif
Supplementary Material 7. Heterologous complementation of CsABCG11.2 function in yeast. Yeast strains expressing the empty vector pYES2 or CsABCG11.2 were grown in SD-U medium containing Cr (a), Fe (b), Zn (c), Mn (d), and Mg (e) in the presence of galactose. No visiable difference was observed.
44281_2024_36_MOESM8_ESM.tif
Supplementary Material 8. Predication of CsABCG11.2 phosphorylation modification sites. It was performed against the website, https://services.healthtech.dtu.dk/service.php?NetPhos-3.1.
44281_2024_36_MOESM9_ESM.tif
Supplementary Material 9. Comparison of Cu and Mn content in the shoot between wild-type (WT) and CsABCG11.2-overexpressing Arabidopsis thaliana lines (OE)treated with different treatments of theanine (Thea) and cadmium (Cd). Each data is the mean of three replicates (± standard deviation, SD). * and ** indicate that groups are significantly different at P = 0.05 and P = 0.01, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hao, X., Xiahou, L., Zhao, H. et al. CsABCG11.2 mediates theanine uptake to alleviate cadmium toxicity in tea plants (Camellia sinensis). HORTIC. ADV. 2, 19 (2024). https://doi.org/10.1007/s44281-024-00036-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44281-024-00036-5