Abstract
Botanicals from Marigold, Chrysanthemum, Basil, Rosemary and other medicinal plants have been analysed as potential replacements of chemical pesticides as they are effective and non-hazardous to the environment. The bioactive components thiophene is present in large amount in Tagetes sp. which have a wide range of biocidal properties. In this study the crude extracts of different parts of Tagetes erecta and Tagetes patula were obtained by Soxhlet extraction method. The leaf extracts were subjected to GC–MS to identify their components and to confirm the presence of thiophenes. Two major disastrous crop pests namely, Spodoptera litura and Corcyra cephalonica belongs to Order Lepidoptera, Family Noctuidae and Pyralidae were used for the experimental study. The effect of bioactive extract was tested by direct and indirect methods for their larvicidal effect. The S. litura larvae was allowed to feed on castor leaves coated with spraying different concentrations of methanolic sample extracts as indirect method. In the direct spraying method, the S. litura and C. cephalonica larvae were sprayed with low and high concentrations of crude sample extracts of both T. patula and T. erecta. Significant decrease in larval activity and survival rate within 24 h to 48 h were recorded for both the methods. The trials were performed using variousvolumes from 10 to 1000 µl to check larvicidal activity. The roots and stem extracts of T. erecta for 25 µl kill 100% larvae within 24 h whereas 50 µl of T. patula flowers, roots and stem extract effectively kill only 50% of the larvae in 48 h. A mini nursery evaluation were conducted by direct spraying method on larvae growing on jowar saplings, which displayed 100% mortality of larvae within 24 h for 25 µl of T. erecta leaf and flower extracts and T. patula flower extract. From these trials, it can be inferred that the extracts of T. patula and T. erecta provided effective outcomes as larvicides of S. litura and C. cephalonica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Agriculture is the major occupation of more than 60% of the population in India. Most of the cultivated crops go waste due to pest infestations, weeds and plant pathogens [1]. This results in lesser production and becomes a severe loss to the farmers. Use of pesticides has helped farmers curb the pests and increase crop yield. In India, 76% of pesticides used are insecticides, while the remaining 24% are herbicides and fungicides. Cotton and rice crops consume 45% and 22% of pesticides, respectively. Vegetables, plantation crops, pulses, wheat and other crops consume 9%, 7%, 4%, 4% and 9% of pesticides [2]. Initially natural pesticides like manure, vegetable wastes and cow dung were proficiently used. But these natural pesticides were slow in killing pests, and could not maintain the quality and quantity of crop yield. Also, an increased demand for food due to population explosion compelled the farmers to resort to a faster alternative,chemical pesticides, that could kill pests quickly and increase crop yield drastically.
S. litura is called for causing serious damage to polyphagus herbivore around the world. It is widely distributed across South and East Asia and Oceania. Considered as a serious pest in the Asia–Pacific region. It is widely known to cause losses to many economically important cultivated field crops such as eggplants, sweet peppers and tomatoes [3]. Another highly devastating pest C. cephalonica known to be good invaders and package penetrators, very much capable of boring through more than one layer of flexible packaging materials is a concern. Commonly known as Rice-moth leaving dense and tough silken threads after feeding the stored grains are critical as they cause a severe damage to it. C. cephalonica has a short life cycle. It serves as a factitious host for natural enemies, hence used as a suitable host in determining the effect of biocontrol agents [4, 5].
Chemical pesticides persist for a long period of time in the environment by accumulating themselves at various concentrations in different levels of the ecosystem. They percolate through the soil and cause contamination of groundwater and degrade soil quality too [6]. Chemical pesticides cause disastrous effects on humans such as acute poisoning, cancer, neurological effects, reproductive and developmental harm, liver malfunction, immune malfunction, neurologic impairment, birth defects, hormone disruption, diminished intelligence and death. To control and minimize these harmful effects of chemical pesticides, alternate methods of pest control like cultural practices, biological control, use of antifeedants, hormonal insects, plant extracts etc. are being resorted to.
The antibiosis property of biopesticides in plant-pest defense system has been facilitating the use of phytotoxins commercially as a promising biocontrol agent [7].
Biopesticides only kill pests and do not harm the plants also do not cause any adverse health effects on human. They are eco-friendly, biodegradable and more efficient than chemical pesticides, reduce crop losses and are comparatively cheaper [8]. Commonly used botanicals are neem (Azadirachta indica), turmeric (Curcuma longa), garlic (Allium sativum), ginger (Zingiber officinale), eucalyptus (Eucalyptus globulus), and tobacco (Nicotiana tabaccum) [8]. Plants contain primary and secondary metabolites like phenols, phenolic acids, flavones, flavonoids, flavonols, tannins, coumarins, thymol, eugenol, terpenoids, alkaloids, carotenoids, pyrethroids and thiophenes, which provide antibacterial, antifungal, antiviral, nematocidal and insecticidal functions [8] ultimately provide protection to crops against external agents like microorganisms and insects. Development of pesticide resistance is delayed when plant based insecticides are used [6].
The probability of marigold as biopesticide has increased its contribution over the ages as an effective and rapid antifeedant on multiple crops. Tagetes patula flower extract possesses intrinsic activity against L. Hesperus & B. tabaci with high mortality rate. The mortality rate is concentration—dependent [9]. The biocontrol application of marigold foliar extracts from Tagetes patula L tried on tomato crop in US breed has promoted the plant growth. The shoot height, shooting frequency, bud formation, flowering interval and also disease free were recorded, results in increased efficiency in growth and control of diseases [10]. In this study, thiophene was extracted from different parts of Marigold plant and evaluated for its biopesticidal efficacy on crop pests.
2 Materials and methods
2.1 Plant collection
Both plants were collected from the nursery of University of Agriculture, Gandhi Krishi Vignana Kendra (GKVK), Bangalore and Lalbagh Botanical Gardens, Horticulture Centre, Bangalore, during the months of January and February (till the experiment was complete). The two plant species, T. erecta and T. patula, belongs to Order Asterales, Family Asteraceae, were segregated into their respective plant parts and packed into big zip lock bags. They were weighed before storing in the refrigerator, at 4 °C, until further use.
T. erecta flowers, leaves, stem and roots, T. patula flowers, leaves, stem, and roots-all eight samples were washed and air dried before placing them in the hot air oven at 45 °C for complete drying. The dried samples were powdered coarsely using a mixer, packed in small zip lock bags and weighed again.
Extraction using Methanol (HPLC Grade) as solvent [11] was carried out for all the samples separately using Soxhlet extraction. Each sample of 30 g was run with 250 ml – 400 ml of methanol. The extracted samples were filtered using Whatman filter paper, and stored in brown bottles at room temperature.
Two solvents, hexane and chloroform were taken in the ratio 2:1 (v/v) [11] and mixed well with the help of a magnetic stirrer for 10 min. This mixture, along with the methanolic extract of the sample (T. patula) stem was poured into a separating funnel, and shaken vigorously for 10 min [12]. The separating funnel was covered with aluminium foil since thiophenes are photosensitive [13, 14]. The setup was allowed to stand on a tripod for 48 h, and the same procedure was repeated for all other samples.
The separated samples (50 ml of each sample) obtained from the aqueous two phase method were collected in beakers and petriplates, and kept for drying in the hot air oven at 50 °C for 8 h till the moisture was almost lost. The dried samples were re-dissolved in 10 ml of cyclohexane and stored in centrifuge tubes at 4 °C until further use [11, 15].
2.2 Identification and quantification
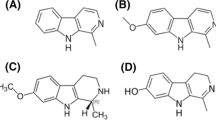
Two crude samples dissolved in cyclohexane (T. erecta and T. patula leaf samples) were given for GC–MS analysis at Vittal Mallya Scientific Research Foundation, Bangalore. The carrier gas used was a mixture of helium, hydrogen and nitrogen. Sample volume of 1 µl was injected into an Rtx-5 capillary column with column oven temperature at 600C and injection temperature at 300 °C. The temperature in the GC–MS instrument was increased at the rate of 10 °C/min from 60 °C (hold time = 2 min) to 320 °C (hold time = 5 min). The results obtained were interpreted, to identify the thiophenes present in the crude samples, and the quantity of each compound was determined as shown in Table 1 and Fig. 2a, b [11, 16].
2.3 Culturing of larvae
Eggs of S. litura (Fig. 1a) were obtained from National Bureau of Agriculturally Important Insects, Indian Council for Agricultural Research (ICAR), Hebbal, Bangalore. Fresh castor leaves were collected and washed thoroughly under tap water and dried using blotting paper. Leaf bouquets were prepared by inserting three to four castor leaf stems into a small glass bottle filled with tap water, and plugged with cotton. This was done to prevent the castor leaves from drying. The bouquet was placed into a big plastic bottle. The S. litura eggs were placed on the castor leaves, and the plastic bottle was sealed using a muslin cloth and tied with rubber bands. This setup was left under humid conditions for 2–3 days for the eggs to hatch. The larvae were then transferred into a new plastic bottle with a fresh castor leaf bouquet, and allowed to grow to the third instar stage (5th to 8th day from hatching). The third instar larvae were used for evaluating the larvicidal effect of thiophene containing secondary metabolites extracted from T. erecta (Fig. 3a–d) and T. patula (Fig. 4a–e) [17].
Eggs of C. cephalonica (Fig. 1b) was also obtained from National Bureau of Agriculturally Important Insects (ICAR), Hebbal, Bangalore. The medium for these larvae was prepared by mixing thoroughly 1 kg of coarsely ground bajra seeds, 50 g of crushed groundnuts, 3.4 g of yeast solution and 0.5 g of Streptomycin sulphate, in a large plastic bottle. The C. cephalonica eggs were spread on this medium evenly. The bottle was covered with muslin cloth and tied with rubber bands. The entire setup was kept under humid conditions for 20 days for the eggs to hatch and grow into the third instar larval stage [18]. These third instar larvae were used for evaluating the larvicidal effect of the plant extracts from T. erecta and T. Patula (Fig. 5).
3 Evaluation methods
3.1 Indirect polishing method
Around 25–30 castor leaf bouquets were made and crude samples (extracts of flowers, leaves, roots and stems of T. erecta and T. patula) of different volumes (10 μl, 100 μl, 500 μl, and 1000 μl) were spread evenly on the leaves of each bouquet using a paint brush. Three similar sized third instar S. litura larvae were introduced into each setup, and the setup was covered using muslin cloth tied with rubber bands. A setup containing three S. litura larvae in a castor leaf bouquet without any crude samples were taken as the positive control. The setup containing leaf bouquet polished with the crude samples were taken as the negative control [19]. The setups were placed under humid conditions for a period of 7 days, and the survival and death rates of the larvae in each setup were observed and recorded every 24 h. The whole experiment was designed by us to evaluate and visualize directly the development and retardation of the larval stages in response to biopesticides effect.
3.2 Direct spraying method
Three S. litura larvae of same size were taken onto a petriplate. Lower volumes (10 μl, 25 μl, 50 μl, 75 μl and 100 μl) of the eight crude extracts (flowers, leaves, roots and stems of T. erecta and T. patula) were sprayed directly onto the larvae, and introduced into setups containing healthy castor leaf bouquets [17]. A setup with larvae and fresh castor leaves were taken as positive control, and a setup containing only the castor leaf bouquet was taken as negative control. To check if cyclohexane harms the larvae, 10 μl, 25 μl, 50 μl, 75 μl and 100 μl of cyclohexane was sprayed onto the larvae and observed for the same duration as the other setups. Eventually, 100 μl, 250 μl, 500 μl, 750 μl, and 1000 μl were also sprayed on the larvae to check its response with higher volume. The survival and death rates of the larvae were observed and recorded every 24 h. The method was repeated for the C. cephalonica larvae using methanolic extracts [19].
3.3 Mini nursery evaluation
Jowar seeds were procured and sown into mud pots containing well mixed soil and manure. The seeds were allowed to germinate into saplings of 5 in. height before transferring them into smaller plastic bottles filled with mixed soil and manure. The S. litura larvae were introduced onto the saplings. The crude samples (crude extracts of flowers, leaves, roots and stems of T. erecta and T. patula) were sprayed directly on the larvae. Survival and death rates of the larvae were observed and recorded every 24 h.
3.4 Sample efficacy interpretation
The starting number of larvae was 3 in all experiments. Following treatment of the plant parts and larvae with bioactive extracts, the larvae were classified into one of the following groups based on their qualitative physical state—active, motile, inactive, partially dead, or dead. The number of larvae in each state was tracked over time of 24 h and is represented in the form of bar plots, and this data was used to infer the biopesticide effects of these bioactive extracts. As the sample size is maintained small and its a preliminary analysis to understand the efficacy of the extract in controlling and inhibiting the pest growth; statistical tool is not applicable to generate data. The study can be further be implicated on a larger sample size and natural environment conditions for longer duration, statistical analysis would be convenient to use and interpret.
4 Results
4.1 Sampling
The T. erecta and T. patula plants were segregated into flowers, leaves, roots and stems. The wet and dry weights were measured and noted. T. patula: Dry weight of flowers (82.63 g), leaves (59.17 g), stem (21.51 g) and roots (30.11 g). T. erecta: Dry weight of flowers (57.80 g), leaves (80.69 g), stem (17.00 g) and roots (88.53 g).
It was observed that the stems and leaves of both the marigold species, T. patula and T. erecta contained higher water content than the flowers and roots, respectively. Roots had the lowest moisture content. Therefore, the stems took the maximum time to dry, followed by the leaves, flowers, and the roots.
4.2 Solvent extraction
The dried ground flowers, leaves, roots and stems of T. erecta and T. patula plants were subjected to solvent extraction using Soxhlet by adding methanol as solvent. T. patula flower samples were run for 26 cycles, leaves for 13 cycles, stem for 12 cycles and roots for 5 cycles. Similarly, T. erecta samples for flowers, leaves, stems and roots were run for 20, 16, 6 and 9 cycles, respectively.
4.3 Aqueous two phase extraction and partial purification
The eight extracted samples of both the plants T. erecta and T. patula were partially purified by aqueous two phase extraction using hexane and chloroform. It was noticed that none of the samples got separated into the two organic solvent fractions.
This might be due to equal solubility of the thiophenes and other secondary metabolites in the flower, leaf, roots and stem samples of T. erecta and T. patula in the organic solvents, hexane and chloroform. The methanolic extracts were probably not completely soluble in both the solvents. Therefore, the components of the methanolic extracts might have dissolved in only one solvent and no separation would have occurred. The ionic strength, pH and temperature also play an important role in phase separation. The methanolic extracts are highly polar and the separating reagents hexane and chloroform are non-polar and polar, respectively. The methanolic extracts would have been soluble in chloroform since methanol and chloroform are polar. Since hexane was completely miscible in chloroform, the entire mixture of the extracts and solvents might have appeared uniform in colour (i.e., colour of the methanolic extract of flowers, leaves, roots and leaves of T. erecta and T. patula). The components of the methanolic extracts might have got separated if the pH had been higher or lower. It is also possible that a higher or lower temperature might have been required for the extracts to solubilize in the separating reagents.
When most of the moisture content was lost, the remaining extract was obtained in a thick and sticky form. The separated liquid extracts of flowers and leaves of T. erecta and T. patula yielded the maximum amount of dry extracts. The roots yielded the minimum. Thiophenes accumulate mostly in the roots of T. erecta and T. patula, and studies have proved that cultured hairy roots produce thiophenes as well [15], [20].
4.4 Identification and quantification
The cyclohexane dissolved crude extracts of the T. erecta and T. patula leaves were analysed for the bioactive substance by GC–MS analysis [12]. The peaks obtained from sample analysis were compared with the standards obtained from libraries and the compounds were identified (Fig. 2a, b).
A total of 49 compounds were identified in this sample by GCMS analysis.
A total of 63 compounds were detected by GC–MS analysis.
The Table 1 shows the major components identified in the hexane-dissolved leaf extracts of T. erecta and T. patula. It was observed that both the extracts contained one thiophene components namely, 2,2′:5′2′′-terthiophene, also called α-terthienyl. 0.33% and 0.23% of α-tertheinyl was present in 1 µl (injection volume into GC–MS column) of each sample of T. erecta leaves and T. patula leaves respectively. The concentration of α-terthienyl in T. erecta and T. patula leaf extracts were found to be 3300 µg/ml and 2300 µg/ml, respectively. This implies that T. erecta has higher thiophene content than T. patula, hence it has more biocidal and larvicidal properties than T. patula.
5 Evaluation of effect of secondary metabolites on the larvae
5.1 Polishing method
Experimental setups containing castor leaf bouquets polished with the crude extracts of T. patula flowers, stem, and leaves were prepared. The leaves were polished with 10 μl, 100 μl, 500 μl and 1000 μl of the crude extracts and larvae were allowed to feed on these leaves. The survival and death rates of the S. litura larvae were observed once every 24 h.
Each methanolic extract was polished on two castor leaves and placed into different setups, into which three larvae were introduced. From the above graph, Fig. 3a–d, the following observations were made.
a Larvicidal effect of methanolic extracts of T. patula stem at different volumes on S. litura. b Larvicidal effect of methanolic extracts of T. patula leaves at different volumes on S. litura. c Larvicidal effect of methanolic extracts of T. patula flowers at different volumes on S. litura. d Control—Effect of methanol at different concentrations on S. litura
It was found that at least one out of the three larvae introduced into each setup was inactive after 24 h for all the volumes (10 μl, 100 μl, 500 μl, 1000 μl) of all the three methanolic extracts (T. patula flowers, leaves, stem extracts). The 10 μl extract volume of the flower and leaf extracts made one larva inactive, while 10 μl of the stem extract could render the two larvae inactive within 24 h. At 100 μl, two larvae were inactive for the flower and stem extracts at 24 h. 500 μl of the leaf and flower extracts gave rise to two inactive larvae, whereas 500 μl of stem extract showed 100% inactivation at 24 h. At 1000 μl capacity the larvae are not affected by the flower extract at all, whereas the leaf and stem extracts inactivate one and two larvae, respectively within 24 h. The minimum concentration required to witness a significant effect on the S. litura larvae begins from 100 μl for all three extracts of T. patula flowers, stem, and leaves.
Death of one larva within 48 h was first observed at 100 μl of T. patula stem. Though the 500 μl and 1000 μl volume of stem extracts also kill one larva at 48 h, the minimum capacity required to kill the larva is 100 μl. At 48 h, 100 μl, 500 μl, and 1000 μl of the leaf and flower extracts each were capable of inactivating 2 larvae. This implies that the T. patula stem extract is more effective than the T. patula flower and T. patula leaf extracts.
There was not much difference in the activity of larvae in all setups for the next 72 h. After 168 h, a slow increase in the death rate is observed for the flower, leaf and stem extracts. All three larvae were found dead on treatment with 500 μl of T. patula flower extract at the end of 192 h. Moreover, larvae were found to be missing during the observation period (500 μl of T. patula flower extract). This is because the S. litura larvae feed on themselves. This experiment proves that all the extract volumes of methanolic extracts from this species are capable of causing inactive the larvae, but it takes time to kill the larvae when indirect polishing method is used. Maximum death was seen at the end of 192 h for T. patula stem and leaves extracts.
The control shows us that these volumes of methanol are toxic to the S. litura larvae Fig. 3d. Therefore, we cannot infer whether the death of the larvae was caused solely by the components present in the extracts, or due to the toxic effect of methanol as well during the first 24 h. Hence, cyclohexane-dissolved extracts were used in the further experiments to determine the larvicidal activity of the T. erecta and T. patula extracts.
6 Spraying method
6.1 Effect of lower volume of T. patula crude extracts on S. litura
Cyclohexane dissolved crude extracts of flowers, leaves, roots and stems of T. patula were sprayed directly onto S. litura larvae (third instar) in different volumes (10 μl, 25 μl, 50 μl, 75 μl and 100 μl). The larvae were left for observation for a period of 192 h, and their activity, survival and death rates were noted once every 24 h.
Cyclohexane-dissolved extracts of T. patula flowers, leaves, roots and stem were used in this experiment. These cyclohexane-dissolved extracts of different volumes (10 μl, 25 μl, 50 μl, 75 μl, 100 μl) were sprayed onto three S. litura larvae each, and subsequently, introduced into different setups having castor leaf bouquets. From the above graph, the following observations were made (Fig. 4a–e).
a Larvicidal effect of cyclohexane-dissolved extracts of T. patula stem at different volumes on S. litura. b Larvicidal effect of cyclohexane-dissolved extracts of T. patula leaves at different volumes on S. litura. c Larvicidal effect of cyclohexane-dissolved extracts of T. patula flowers at different volumes on S. litura. d Larvicidal effect of cyclohexane-dissolved extracts of T. patula roots at different volumes on S. litura. e Control Setup for effect of different volumes of cyclohexane on S. litura
The cyclohexane-dissolved crude extract of T. patula stem of 100 μl killed all the S. litura larvae within 24 h of incubation. The 10 μl, 25 μl, 50 μl and 75 μl volumes each killed one larva in their respective setups within 24 h, but no change was seen after 48 h. After 72 h, there was a gradual death rate seen in all the stem extract. 100% death was observed at 50 μl volumes of T. patula stem extract amongst all the concentrations at 72 h (Fig. 4a).
In the setups containing larvae sprayed with the crude extract of T. patula leaves, one S. litura larva was dead for each 10 μl, 25 μl, 50 μl, 75 μl treatment and two larvae were dead for 100 μl exposure within 24 h of incubation. The survival and death count remained the same for all treatment volumes till 48 h of incubation (Fig. 4b). At 72–96 h, one larva became inactive at the 50 μl, and 100% death was observed at 75 μl and 100 μl volumes. Further, incubation periods showed a constant decline in the larval activity and eventually the larvae died at the end of 192 h. This implies that 75 μl is the minimum treatment volume required to kill all larvae within the minimum time of 72 h effectively.
The T. patula flower extract was capable of killing three S. litura larvae at volumes of 50 μl, 75 μl and 100 μl within 24 h. Death of the larvae at 25 μl began only after 72 h, and at 10 μl after 96 h. The 25 μl could make the larvae inactive within 24 h, and 50 μl could kill all the larvae within 24 h. The 50 μl extract is found to be most efficient comparatively in killing all three larvae within 48 h (Fig. 4c).
The T. patula root extract started killing the larvae at 25 μl within 48 h. Simultaneously, it was observed that all three larvae were dead for 75 μl and 100 μl.10 μl and 25 μl take a longer time to inactivate and kill at least one larva. Beyond 96 h, there was a constant death rate of larvae in setups of 10 μl and 25 μl volumes. Hence, 50 μl is considered to be effective in killing and larvae were inactive more quickly (within 48 h) (Fig. 4d).
From the above analysis, it can be concluded that T. patula flower, stem and roots extracts are the most effective, as it can kill the maximum number of S. litura larvae within a short duration. Control was recorded at higher concentration above 500–1000 μl by 48 h the 2 of 3 larvae were found dead.
6.2 Effect of lower volumes of T. erecta crude samples on S. litura
Similar procedure was followed for lower volumes. Cyclohexane dissolved crude extracts of flowers, leaves, roots and stems of T. erecta were sprayed directly onto third instar larvae of S. litura in different volumes (10 μl, 25 μl, 50 μl, 75 μl and 100 μl), and subsequently introduced into different setups having castor leaf bouquets. The larvae were left for observation for a period of 192 h, and their activity, survival and death rates were noted once every 24 h.
The T. erecta stem extract could kill all the three S. litura larvae in the 25 μl setup within 24 h of incubation, whereas the other volumes (10 μl, 50 μl, 75 μl, 100 μl) could kill only two larvae within the same time. After 48 h, significant death of the larvae in all setups was observed. This suggests that T. erecta stem extract is very effective in killing these worms.
T. erecta leaf extract samples, within 24 h of spraying, could kill only one larva as observed in all volumes (10 μl, 25 μl, 50 μl, 75 μl, 100 μl). But by 48 h, there was sudden death of all three larvae in all the (10 μl, 25 μl, 50 μl and 100 μl setups containing the leaf extract, whereas two larvae were dead at 75 μl. By the end of 96 h, all the larvae were found dead. This infers that the T. erecta leaf extract is highly effective at the lowest volume (10 μl), but some larvae might have survived at higher concentrations because of their resistance to the constituents of the extract.
In the case of T. erecta flower extract, all three larvae were dead at 25 μl and 50 μl within 24 h. Two out of the three larvae that were sprayed with 10 μl and 75 μl of the extract, died, whereas one larva was dead out of the three larvae sprayed with 100 μl of the extract. The observations remained the same till 48 h, with 10 μl, 75 μl and 100 μl volumes. Decline in the active forms of the larvae was observed post 48 h. At the end of 192 h, all the larvae were found dead. Hence, 25 μl of this extract can be identified as an ideal and effective for biocidal activity for the maximum number of larvae within 24 h.
The crude extract of T. erecta roots could kill all the larvae that were sprayed with 25 μl and 50 μl of the extract within 24 h. 10 μl and 75 μl of the extract were efficient in killing two larvae, whereas only one larva died when sprayed with 100 μl of the extract. Observations remained constant till 72 h, after which all the larvae started dying. By the end of 192 h, all the larvae were dead. This shows that 10 μl extract of the T. erecta root extract was effective in affecting the S. litura larvae within a day.
From the above experiment, it refers to, that the T. erecta flower, stem and root extracts are more efficient than the T. erecta leaf extract, as they can kill a maximum number of larvae within 24 h at 25 μl concentration.
The control setup showed that hexane did not affect the S. litura larvae at lower volumes of 10–75 μl. Inactivity of larvae is shown at 75 μl and 100 μl towards the end of 48 h.
6.3 Effect of higher volumes of T. patula crude extracts on S. litura
Cyclohexane-dissolved crude extracts of flowers, leaves, roots and stems of T. patula were sprayed directly onto S. litura larvae with different higher volumes (100 μl, 250 μl, 500 μl, 750 μl and 1000 μl). The larvae were left under observation for a period of 192 h, and their activity, survival and death rates were noted once every 24 h.
Exposure to all the volumes of T. patula stem extract were efficient in killing all the larvae within 24 h of spraying. The T. patula leaf extract could kill all the three larvae at 100 μl and 250 μl, and 500 μl, 750 μl and 1000 μl volumes each could kill only one out of three larvae in 24 h. At 48 h, it was observed that all three larvae were dead at 500 μl and 750 μl treatment, whereas two larvae were still alive at 1000 μl. Subsequent hours show the death of another larva at 1000 μl, but at the end of 192 h, all three larvae in the 1000 μl setup were not dead. This could be because the larvae were resistant to the extract. The higher volume repelled the larvae to feed on the leaves but due to the starvation condition on feeding all the larvae were found dead.
The T. patula flower extract could kill one larva for all volumes (100 μl, 250 μl, 500 μl, 750 μl, 1000 μl) within 24 h. The observations remained the same for 100 μl and 250 μl till 72 h. 100% mortality of larvae was seen for extract at 750 μl volume at 72 h. After 96 h, a significant reduction in the activity of the larvae was observed for all volumes (100 μl, 250 μl, 500 μl, 750 μl, 1000 μl). Thus, it can be concluded that 750 μl is effective in killing the S. litura larvae sooner than the other volumes (72 h).
In the setups sprayed with T. patula roots, all the larvae were found dead with treatment of 1000 μl volume.. The 100 μl, 250 μl, 500 μl and 750 μl volumes could kill one larva at the end of 24 h. At 48 h, the survival and death rates remained the same for all the volumes (100 μl, 250 μl, 750 μl, 1000 μl), except for the 500 μl, when two larvae were found dead. Significant change in larvae activity and death was observed only after 96 h for the reamaining100 μl, 250 μl, 750 μl, 1000 μl extracts. Hence it was evident that the S. litura larvae react most within 48 h when sprayed with 500μlof T. patula root extract.
The control shows that when lower volumes of cyclohexane were sprayed, the larvae were able to survive. But exposure to above 500 μl, the larvae died. This could be possible due to excessive cyclohexane being sprayed, which could have led to drowning of the larvae. Therefore it is better to use a lesser volume of the extracts, to evaluate their larvicidal activity.
6.4 Effect of higher volumes of methanolic extracts of T. patula on C. cephalonica
Methanolic extracts of flowers, leaves, roots and stems of T. patula were sprayed directly onto third instar larvae of C. cephalonica in different volumes (100 μl, 250 μl, 500 μl, 750 μl and 1000 μl). The larvae were left for observation for a period of 48 h, and their activity, survival and death rates were noted once every 24 h.
It was observed that the C. Cephalonica larvae were all killed at concentrations of 250 μl, 500 μl, 750 μl, and 1000 μl within 24 h. Though 100 μl of methanol rendered the larvae inactive only at 24 h, death was observed at the end of 48 h. This indicates that methanol is toxic to the C. cephalonica larvae.
All volumes of the methanolic extract of T. patula flowers sprayed onto the C. cephalonica larvae caused their death. The same was observed for all volumes of T. patula stem extract also. This implies that C. cephalonica larvae are extremely sensitive to methanol, and get killed very quickly by the methanolic extracts as well. Hence it cannot be concluded that only the components in the methanolic extracts were affecting the larvae.
Testing the larvicidal effect of the T. patula leaf extract, T. patula root extract and the extracts of T. erecta (flowers, leaves, roots, stem) was not possible due to the shortage of C. cephalonica larvae. The turnover ratio of the eggs kept for hatching to the larvae developed was 1:5. This might be due to unfavourable conditions of growth (Fig. 5).
7 Mini nursery evaluation
From the above experiments, it was concluded that the most ideal treatment volume of hexane- dissolved extracts (extracts of flowers, leaves, roots, and stems of T. erecta and T. patula) to be sprayed on the larvae were 25 μl, 50 μl, and 75 μl. At these extract size, the larvicidal effect of the extracts can be monitored as the cyclohexane does not affect the larvae. The mini nursery was set up with minimum natural environment conditions provided for the larvae development and to evaluate the effect of biopesticide on its survival. The results are represented in the graph (Fig. 6).
All volumes of the cyclohexane-dissolved T. erecta extracts were effective in killing the S. litura larvae within 24 h of spraying. The order was:flower and leaf extracts were better than stem and root extracts, as they could give a 100% death rate at 25 μl within 24 h. Higher volumes showed complete mortality of the larvae Fig. 7a, b.
The cyclohexane-dissolved T. patula extracts were also capable of killing the S. litura larvae within 24 h. Only T. patula flower extract gave 100% mortality of larvae at 25 μl (Fig. 8) within 24 h. The other extracts took more time to kill all the larvae in their respective setups. Therefore, T. patula flower extract is the most efficient out of the T. patula stem, leaf and root extracts at lower volumes.
8 Discussion
Several studies have been conducted earlier using thiophene extracts of flowers, leaves, roots and stems of T. erecta, T. patula and other species of Tagetes, to test their biocidal activity on various pests. The extracts have been subjected to different types of characterization methods to identify the thiophene components they possess [21]. These studies have also been carried out using biological controls and botanicals to assess their larvicidal activity on S. litura and C. cephalonica. T. erecta leaf extracts were tested against different types of gram positive and gram negative bacteria to understand its antibacterial activity. Maximum antibacterial activity of T. erecta leaf extract was observed in Acinetobacter baumannii and Propoinobacterium acne. Minimum antibacterial activity was obtained for Streptococcus pneumoniae, due to the presence of a capsule around its cell membrane. This study proved that T. erecta has antibacterial properties and can be used in developing antiseptics and drugs for skin infections [22]. Weaver et al., [23] reported that the leaf and root extracts of T. minuta had insecticidal activities as they could effectively kill the male and female bean weevils, Zabrotes subfasciatus. Male adults were found to be more susceptible to the extracts than the female insects. In addition to biocidal activity the extracts also has been reported to have antifungal activity [11].
Ethanol extract, chloroform and petroleum ether fractions of flowers of T. erecta L. were tested against larval and adult stages of Tribolium castanuem. The chloroform fraction was more toxic to the larvae and adult forms of T. castaneum, than the ethanol extract and petroleum ether fraction. Significant mortality was seen in the lower instar stages of T. castaneum [24]. Many other findings, thiophene extracts derived from the callus of T. patula leaves and hairy roots were found to have high larvicidal activity against mosquito larvae of genera Aedes, Anopheles and Culex [13, 15].
Hexane-dissolved extracts of T. erecta, T. patula, T. tenuifolia and T. lucida flowers were subjected to TLC and HPLC methods. Thiophenes were detected by TLC and were visible as blue fluorescent spots when the TLC plate was viewed under 366 nm UV light. HPLC analysis showed that there was a complex mixture of thiophenes present in different compositions in the different species, though α-terthienyl and C13bithienyl were found to be major phototoxic thiophenes in all the species under study [25]. Thiophene rich extracts of T. minuta leaves and roots were subjected to GC–MS analysis, and three major thiophene compounds were identified, namely BBT, α-terthienyl and BBTOAc [11]. GC–MS analysis was conducted for thiophene extracts from T. patula roots using several injection modes. A new method of GC–MS was devised for analyzing thiophenes from the solvent extracts of T. patula roots. Major thiophenes like BBT, α-terthienyl, BBT(OAC)2, BBTOAc and other minor components were identified [12].
Sun Ho Park et al., [26] had studied the control of S. litura Fabricus using entomopathic nematodes of Steinernema and Heterorhabditis species. 100% mortality was observed within 20 h. Highest number of nematodes was reported in 2nd to 3rd instar of S. litura by Heterorhabditis bacteriophora HY. In vitro cultured Steinernema carpocapsae PC produced 100% mortality in 4th to 6th instar larvae within three days against S. litura. This study showed that nematodes produced in vitro with S. litura were capable of controlling the population of S. litura. A study by Ashokaraj et al. [27] reported the larvicidal activity of various concentrations of crude aqueous leaf extracts of Eupatorium triplinerve against the fourth instar larvae of S. litura. It was proved that percentage mortality of S. litura increased with increasing extract concentrations. High pupal mortality, morphological deformations and inhibition of adult emergence was reported in the S. litura larvae and pupae.
Crude ethyl acetate, diethyl ether and hexane extracts of Solanum pseudocapsicumwere tested at different concentrations on fourth instar larvae of S. litura and Helicoverpa armigera. Maximum antifeedant activity and maximum insecticidal activity was recorded in the ethyl acetate extract, which could extensively kill the two types of larvae after treatment. It was reported that the ethyl acetate extract produced maximum larval, pupal and adult deformities, and also effectively inhibited adult emergence of S. litura and H. armigera Order Lepidoptera, Family Noctuidae [17]. Azadhirictin was used to inhibit the growth of S. litura. The study proved that increase of caspase mRNA expression cleaved caspase 3 protein expression. Due to the upregulation mechanism of caspase 3-protein released mitochondrial Cyt C in an invivo study. The regulatory mechanism of Azadhirictin activated apoptosis via the caspase dependent mitochondrial apoptotic pathway by regulating both MAPK and calcium signalling pathways hence restricting the larvae development of S. litura [28].
The effect of Nigella sativa, Acorus calamus, Azadiracta indica and Lantana camara exracts were applied to control the growth rate of pest S. litura and its infestation in tomato plant [29]. On application of these plant extracts after 7 days of interval, checked the activity against the pest population. Azadiracta indica resulted in 74.85% mortality rate, Nigella sativa treated were 72.96%. Comparing Acorus calamus and Lantana camara were insignificant in the infestation process.
Entomogenous fungi has been studied as an alternative biocontrol agent in integrated management strategies against S. litura [30]. Beauveria brongniartii SB10 isolate proved high pathogenic activity against different insects [31, 32]. Matrine, heterocyclic compound and B. brongniartii cause significant combination in the treatment of S. litura. The reduction in pupation percentage and adult emergence was high with the synergistic application matrine and B. brongniartii as an antifeedant [3]. The effect of a commercially available terpenoid lactone, andrographolide, was tested on pupa to adult transformation in C. cephalonica. Meta-morphogenic deformities were observed, wherein the most of the pupae could not survive on exposure to treatment with andrographolide. The emerged adults also showed morphogenic abnormalities and could not survive beyond 24 h of emergence due to their shriveled wings [33].
Commercially available insecticide Malathion was used to estimate its effect on biochemical reactions in C. cephalonica. It was reported that the glucose and glycogen levels in C. cephalonica had considerably decreased, whereas there was an increase in the protein levels. This proved that there were significant changes in the biochemical pathways of C. cephalonica when treated with Malathion [34].
Pathak et al. [18] showed that increasing concentration of neem. (Azadirachtin indica A. Juss) extracts could induce significant larval, pupal and adult mortality of C. cephalonica, and hence proved the insecticidal, antifeedant and growth inhibiting activity of neem against this storage pest. To control the pest population of C. cephalonica. Azadirachtin indica and Eucalyptus globules powdered leaves were tested with different concentrations (0.5–2.0 g) on all stages of larvae. Highest mortality rate was achieved with 2.0 g with both the plant samples in 1st and 2nd instar of C. cephalonica [5]. Radwa et al. [35] in 2020 encapsulated Cinnamon oil with silica gel nanoparticles (CESN), cinnamon oil and mesoporous silica gel nanoparticles on pupation %age, pupal duration, %of adult emergence and adult longevity. The treatment with CESN and its sub-lethal concentration (LC50) was recorded to be 47.5% of pupation, 45.0% with Mesoporous silica nanoparticles and 37.5% with cinnamon oil. Randomized trial on C. cephalonica on stored grains were performed. They used neem leaf powder, Tulsi leaf powder, Karanj leaf powder, Marigold flower powder, Eucalyptus leaf powder and Coragen to investigate on the larval mortality and grain damage. Neem reported as highest upto 81.67%. Marigold treated were 5.8% larvae showed larval orientation after 72 h of application [36, 37]. To carry forward the studies climate optimization and cost-effective extraction methods can be opted using green technology.
9 Conclusions
Crude thiophene extracts obtained from T. erecta and T. patula leaves were subjected to GC–MS analysis contained thiophenes (α-terthienyl) present in T. erecta was higher than that in T. patula. This infers that T. erecta plant extracts would be a better source as larvicidal than T. patula. This study offers 100% mortality rate for higher and lower concentrations. Comparatively lower concentrations of T. erecta can be appropriately sprayed on the larvae of S. litura that can lead to the death of larvae in 24 h effectively. On the other hand, two days were required for a higher concentration of T. patula flower, stem and root extracts to kill the same number of larvae. These observations infer that T. erecta plant extracts were more efficient in killing S. litura larvae within a short duration of time with lesser concentration, than the T. patula plant extracts.
A mini nursery evaluation was conducted to test the efficacy of cyclohexane-dissolved T. erecta and T. patula plant extracts (flowers, leaves, roots and stem extracts) on third instar S. litura larvae. It was observed that the T. patula flower extract, as well as the T. erecta flower and leaf extracts could efficiently kill all the larvae at the lowest concentration within 24 h. Hence it was concluded that cyclohexane-dissolved T. erecta plant extracts (flower and leaf extracts) have better larvicidal activity than the cyclohexane-dissolved T. patula plant extracts. The application of green technology if implied for extraction can have safer and cost-effective bioactive agents isolated from these plants. Another advantage can be considered for Tagetes plants, they are short height and have short life, if planted with the crops can cause no damage either to the crops, or environment or even human, but can protect the crops from disastrous pests. Safety limits for using thiophene rich extracts can be determined to process them into botanical pesticides, and large field trials can be done to bring this into reality.
Data availability
The charts, plots and tables summarise all amounts of data generated from the experiments. Methods subsections describe the steps of investigation and data processed obtaining the results. (Experimental design and results). The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The experimental research and lab studies on plants including collection of plant material, identification and authentication has been made by the national relevant institution and no other such plant material has been used without any proper authentication (Mentioned in the manuscript and Acknowledgement).
References
Bhardwaj T, Sharma JP. Impact of pesticides application in agricultural industry: an Indian scenario. Int J Agric Food Sci Technol. 2013;4(8):817–22.
Radcliffe EB, Hutchison WD, Cancelado RE, editors. Integrated pest management, concepts, tactics, strategies and case studies. USA: Cambridge University Press; 2009.
Wu J, Li J, Zhang C, Yu X, Cuthbertson AGS, Ali S. Biological impact and enzyme activities of Spodoptera litura (Lepidoptera: Noctuidae) in response to synergistic action of matrine and Beauveria brongniartii. Front Physiol. 2020;11:584405. https://doi.org/10.3389/fphys.2020.584405.
Vincent A, Singh D, Mathew IL. Corcyra cephalonica: a serious pest of stored products or a factitious host of biocontrol agents? J Stored Prod Res. 2021;94(2): 101876. https://doi.org/10.1016/j.jspr.2021.101876.
Singh H, Singh A, Brar JS. Efficacy of Azadirachta indica and Eucalyptus globulus against Corcyra cephalonica. Int J Adv Res Biol Sci. 2019;6(7):119–23. https://doi.org/10.22192/ijarbs.2019.06.07.015.
Khater HF. Prospects of botanical biopesticides in insect pest management. Pharmacologia. 2012;3(12):641–56. https://doi.org/10.17311/pharmacologia.2012.641.656.
Campos EVR, Proença PLF, Oliveira JL, Bakshi M, Abhilash PC, Fraceto LF. Use of botanical insecticides for sustainable agriculture: future perspectives. Ecol Indic. 2019;105:483–95. https://doi.org/10.1016/j.ecolind.2018.04.038.
Singh GM, Ali S, Akhtar M, Singh KS. Efficacy of plant extracts in plant disease management. Agric Sci. 2012;3(3):425–33.
Fabrick JA, Yool AJ. Spurgeon Insecticidal activity of marigold Tagetes patula plants and foliar extracts against the hemipteran pests, Lygus hesperus and Bemisiatabaci. PLoS ONE. 2020;5(5):e0233511. https://doi.org/10.1371/journal.pone.0233511.
Nahak G, Sahu RK. Bio-controlling effect of leaf extract of Tagetes patula L. (Marigold) on growth parameters and diseases of tomato. Pak J Biol Sci. 2017;20:12–9. https://doi.org/10.3923/pjbs.2017.12.19.
Supradip S, Walia S, Kundu A, Kumar B, Joshi D. Antifungal acetylinic thiophenes from Tagetes minuta: potential biopesticide. J Appl Botany Food Qual. 2012;85:207–11.
Scabolcs S, Hethelyi E, Kuzovkina IN, Lemberkovics E, Szoke E. GC–MS method development for the analyses of thiophenes from solvent extracts of Tagetes patulaL. Chromatogr Suppl. 2008;68:S63–9.
Brigit SA, Anooja PA, Mitha VM, Paulson N, Sushitha SK, Nair T, Praveena P. Production of Thiophene from Tagetes patula. J Plant Pathol Microbiol. 2013;4(1):1–4.
Amor TB, Jori G. Sunlight-activated insecticides: historical background and mechanisms of phototoxic activity. Insect Chem Mol Biol. 2000;30:915–25.
Rajasekaran T, Ravishankar GA, Obul Reddy B. In vitro growth of Tagetes patula L. hairy roots, production of thiophenes and its mosquito larvicidal activity. Indian J Biotechnol. 2004;3:92–6.
Margl L, Tei A, Gyurjan I, Wink M. GLC and GLC-MS analyis of thiophene derivatives in plants and in in vitro cultures of Tagetes patula L. (Asteraceae). Zeitschrift fur Naturforschung. 2002;57:63–71.
Jeyasankar A, Premalatha S, Elumalai K. Biological Activities of Solanum pseudocapsicum (Solanaceae) against cotton bollworm, Helicoverpa armigera Hubner and armyworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae), Asian Pacific. J Trop Biomed. 2012;2(1):981–6.
Pathak CS, Tiwari SK. Insecticidal action of neem seed (Azadirachta indica A. Juss) acetone extract against the life cycle stages of Rice Moth Corcyra cephalonica Staint. (Lepidoptera: Pyralidae). World J Agric Sci. 2012;8(5):529–36.
Deshmukhe Pratibha V, Hooli AA, Holihosur SN. Effect of Lantana camara (L.) on growth, development and survival of tobacco caterpillar (Spodoptera litura Fabricius). Karnataka J Agric Sci. 2011;24(2):137–9.
Szarka. Szabolcs Studies on the Thiophene Metabolism in Genetically Transformed Hairy Root Cultures of Tagetes patula L. 2010, 1–14.
Priyanka D, Shalini T, Navneet VK. A brief study on Marigold (TagetesSpecies): a review. Int Res J Pharm. 2013;4(1):43–8.
Nandita D, Ranjan S, Saha P, Jain R, Malhotra S, ArabiMohamedSaleh MA. Antibacterial activity of leaf extract of Mexican marigold (Tagetes erecta) against different gram positive and gram negative bacterial strains. J Pharm Res. 2012;5(8):4201–3.
Weaver DK, Wells CD, Dunkel FV, Bertsch W, Sing SE, Sriharan S. insecticidal activity of floral, foliar, and root extracts of Tagetes minuta (Asterales: Asteraceae) against adult Mexican Bean Weevils (Coleoptera: Bruchidae). J Econ Entomol. 1994;87(6):1718–25.
Nikkon F, RowshanulHabib M, RezaulKarim M, Ferdousi Z, MotiurRahman M, EkramulHaque M. Insecticidal activity of flowers of Tagetes erecta L. against Tribolium castaneum (Herbst). Res J Agric Biol Sci. 2009;5(5):748–53.
Dieneka VI, Tretyakov MY, Lapshova MS, Deineka LA. Thiophenes of Tagetes flowers and partial purification of xanthophyll esters. Univ J Agric Res. 2014;2(3):101–6. https://doi.org/10.13189/ujar.2014.020304.
Park SH, Yu YS, Park JS, Choo HY, Bae SD, Nam MH. Biological control of Tobacco Cutworm, Spodoptera litura Fabricius with Entomopathis Nematodes, biotechnology. Bioprocess Eng. 2001;6(2):139–43.
Ashokaraj KS, Mahadev C. Khetagoudar, study on larvicidal activity of weed extracts against Spodoptera litura. J Environ Biol. 2013;34:253–7.
Shu B, Zhang J, Cui G, Sun R, Yi X, Zhong G. Azadirachtin affects the growth of Spodoptera litura Fabricius by inducing apoptosis in larval midgut. Front Physiol. 2018;9:137. https://doi.org/10.3389/fphys.2018.00137.
Awasthi A, Avasthi S. National Conference Ecological Imbalance: a threat to flora, fauna, economy and human survival. 2018.
Yang H, Qin C-S, Chen Y-M, Zhang G-Y, Dong L-H, Wan S-Q. Persistence of Metarhizium (Hypocreales: Clavicipitaceae) and Beauveria bassiana (Hypocreales: Clavicipitaceae) in tobacco soils and potential as biocontrol agents of Spodoptera litura (Lepidoptera: Noctuidae). Environ Entomol. 2019;48:147–55. https://doi.org/10.1093/ee/nvy161.
Huang LP. Identification of 29 Beauveria isolates and their virulence against Bemisiatabaci. M.Sc thesis. Guangzhou. 2019. China: South China Agricultural University (in Chinese).
Ou D. Toxicity evaluation of entomopathogenic fungi against Asian citrus psyllid. M.Sc thesis. Guangzhou. 2019. China: South China Agricultural University (in Chinese).
Jagajothi A, Martin P. Efficacy of Andrographolide on pupa—adult transformation of Corcyra cephalonica Stainton. J Biopestic. 2010;3(2):508–10.
Ismail MA, Patel NG, Wankhedkar PT. Effect of malathion on biochemical alterations in Corcyra cephalonica. J Nat Product Plant Resour. 2013;3(1):74–7.
Attia RG, Rizk SA, Hussein MA, AbdelFattah HM, Khalil MMH, Ma’Moun SAM. Effect of cinnamon oil encapsulated with silica nanoparticles on some biological and biochemical aspects of the rice moth, Corcyra cephalonica (Staint) (Lepidoptera: Pyralidae). Ann Agric Sci. 2020;65(1):1–5. https://doi.org/10.1016/j.aoas.2020.05.003.
Sanjoy S, Usha Y. Efficacy of indigenous plant products on Corcyra cephalonica (Stainton) in stored rice grains. Pharma Innov J. 2021;10(7):97–103.
Allotey J, Azalekor W. Some aspects of the biology and control using botanicals of the rice moth, Corcyra cephalonica (Stainton), on some pulses. J Stored Prod Res. 2000;36(3):235–43.
Acknowledgements
We express our sincere gratitude to Dr. R. Jayanthi, Professor of Department of Horticulture, GKVK, Bangalore, Mr. Karunakara, Analytical Lab In-charge, VittalMallya Scientific Research Foundation, Bangalore, Dr. ChandishBalal, Principal Scientist and Head of National Bureau of Agriculturally Important Insects (ICAR), Hebbal, Bangalore for their patience and constant support.
Funding
This project did not receive any funding from any agency either internal or external.
Author information
Authors and Affiliations
Contributions
SS, KK, PR & KBN & AR: Experimental Layout, Result Analysis, Interpretation of the data, Development of the graphs, Preparation of the manuscript, Final Editing. SS: Conceptualization, Design, Experimental Layout, Result Analysis, Interpretation of the data, Preparation of the manuscript, Final Editing and Communication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kannan, K., Raju, P., Keerthy, B.N. et al. Biopesticide effect on crops for the bioactive components extracted from Tagetes erecta and Tagetes patula. Discov Agric 2, 37 (2024). https://doi.org/10.1007/s44279-024-00045-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00045-y