Abstract
In the last century, human activities were the primary cause of air, water, and soil contamination. However, in the twenty-first century, while pollutants like sulfur oxides (SOx) and nitrogen oxides (NOx) remain significant, microplastics (MPs) have emerged as a new global environmental concern. Microplastics are plastic fragments that are less than 5 mm in diameter. Their widespread distribution in aquatic and terrestrial ecosystems has adverse impacts on various ecological systems. The presence of MPs has been well documented in diverse matrices, such as table salt, drinking water, indoor and outdoor air, beer, cold drinks, aquatic organisms, plants, and earthworms. The potential adverse effects of MPs consumption have been reported in various organisms, including earthworms and aquatic fishes; however, their potential effects on human health through respiratory, dietary, and other exposures are still being elucidated. This review provides a comprehensive overview of the current knowledge on potential sources, quantities present in water, table salt, air, and possible routes in the human body through different trophic levels. Furthermore, this paper reviews insights into the movement and accumulation of MPs at different trophic levels (i.e., aquatic, and terrestrial organisms) and their impacts on the cycling of soil carbon and nutrients (nitrogen and phosphorus). Additionally, this review paper addresses the current trends in MPs research and proposes strategic management techniques to mitigate MPs pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plastics are a subset of polymers, playing a key role in the daily functioning of modern society worldwide for a long time [1, 2]. However, in recent years its application has exponentially increased from household to industry owing to their versatile properties and low cost of production [3]. This has led to a considerable increase in the production of plastics, which reached 359 million tons in 2018, with maximum production in Asia (ca. 51% of global production). China is a major producer of plastics, contributing almost 30% of the plastic production in Asia, which significantly contributes to the economy of the country. The production, consumption, and disposition of plastics are related to the depletion of natural resources, environmental degradation, and negative effects on human health [4, 5].
Plastics are a cocktail of polymers resistant to microbial degradation. Plastics possess chemical properties that are resistant to microbial decomposition, resulting in high pollutant loads in various environmental components. The intensity of plastic pollution depends on the chemical formulation, raw materials, and plasticizers. Plastics have long residence times in the environment because of their slow decomposability; therefore, the fragments of plastics are divided into micro, meso, or macro debris. Microplastics (MPs) are tiny plastic particles that are usually defined as approximately 5 mm or less in diameter [6]. MPs are categorized as primary when they are produced in small sizes and as secondary when they originate from the breakdown of larger plastic items [7,8,9]. However, the definition of MPs is not straightforward, as various size scales are used to define them [10]. These small plastic particles pose significant environmental issues because of its ingestion by living organisms, which are leading to potentially harm them and disrupting the ecosystem services.
Microplastics (MPs) were first identified in 2004 [11] and have since been found in a wide array of environments, including many marine fish species [8, 9, 12], raw drinking water [13, 14], estuaries, sediments [15], coral reef systems [16], air [17], table salts [18], beer [19], and even human organs. The presence of MPs is increasingly concerning due to their impact on various marine environments, affecting air quality [20], water quality [21], food sources [22], and other ecosystem functions. MPs are an assemblage of heterogeneous particles that vary in size, shape, colour, chemical composition, density, and characteristics. Due to its abundance in different environmental components and notorious properties, studies are failed to clearly demonstrate the potential scientific prove for its effect on health of human being and other organisms. In recent years, the research on MPs has experienced exponential growth (Fig. 1). Despite significantly large number of papers published in the last one decade, there are still considerable gaps in our understanding of the impact of plastics on living organisms and ecosystem services and their possible effective mitigation measures. The key areas of MPs research having knowledge gap include (i) the pathways through which MPs accumulate in the human body, (ii) the long-term effects of microplastic exposure on aquatic plants and animals, soil structure and microbes, soil nutrient cycling, and earthworms, and (iii) the mechanisms by which microplastics affect human physiology, despite their presence being documented in various human and fluids. Additionally, there is limited information available on MPs interaction with other environmental pollutants. MPs can carry harmful chemicals, such as persistent organic pollutants (POPs) and heavy metals, potentially increasing their toxicity. The interactions between MPs and these contaminants, and their combined impacts on ecosystems and human health, require more focus.
Although, extensive research has been done on effects of MPs on numerous ecosystems and organisms, however, significant gaps still remain in our understanding. Notably, there is a paucity of comprehensive analysis that connects findings from several fields to better understand the broader implications of MPs for human health. Although studies have examined the impacts of MPs on aquatic life, plants, soil characteristics, nutrient cycling, earthworms, and trophic transmission, no systematic analysis has yet linked these disparate features to highlight their cumulative significance for human health. The current study aims to fill this vacuum by providing a comprehensive assessment of microplastics’ effects on numerous environmental and biological systems, as well as their consequences for human health. The key questions being addressed in this study are: how can MPs affect various environmental and biological systems? and what are the hazards posed by MPs to human health? This review seeks to connect the effects of MPs across organisms and ecosystems, and highlighting the need for a uniform approach for assessing health hazards associated with microplastic contamination. The review synthesises ideas from aquatic biology, plant science, soil chemistry, and ecosystem to provide a complete understanding of microplastic impacts, interactions, and potential health hazards, such as exposure pathways and bioaccumulation. Through this holistic approach, the study attempts to provide new insights and increase our understanding.
2 Methodology
In this review, we systematically collected and compiled information from various credible sources, including annual reports from reputed organizations and articles published in peer-reviewed journals. To access relevant literature, we utilized multiple scientific databases such as Google Scholar, Web of Science, PubMed, and ResearchGate. The data retrieval process involved the use of specific keywords, including “microplastics,” “nanoplastics,” “plastic fragments,” “plastisphere,” “microplastic pollution,” “microplastics and aquatic animals,” “microplastics and earthworms,” “microplastics and nutrient cycling,” “microplastics and soil physicochemical properties,” and “microplastics and aquatic plants.” The majority of the scientific articles reviewed were published between 2000 and 2021. However, we also included seminal articles predating this period, which were selected for their significant contributions to the field. The amalgamation of data from these diverse sources enhances the comprehensiveness and depth of the review, proving a nuance perspective on the subject matter.
3 Factors affecting the distribution of microplastics
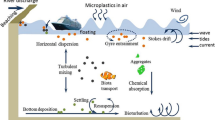
The transport and dispersion of MPs are strongly influenced by weather phenomena owing to their low density, small size, and ease of entertainment by atmospheric processes [5]. Weather phenomena, such as rainfall, wind, cyclones, and eddies, play a pivotal role in the dispersal of MPs over considerable distances from the source area. For instance, weather phenomena, such as snowfall and rainfall, exhibit a robust correlation with the deposition of MPs rates and are marked with an increased deposition rate five times more during the rainy season [23]. Population density, local environment, and human activities significantly influence the distribution of plastics. The higher the population density, the higher the MPs concentration, and the deposition of MPs tends to be more pronounced in rural areas than in urban areas owing to various weather phenomena in rural areas. Additionally, local topography and altitude influence the dispersal of MPs, and a higher altitude is generally associated with a lower concentration of MPs [24].
In indoor environments, the concentration of MPs tends to be higher compared to outdoor settings due to less air circulation [25]. Moreover, indoor samples typically show elevated levels of MPs due to the presence of furniture, textiles, building materials, lifestyles, and activities [26]. For example, cleaning floors, mopping, and washing clothes generate thousands of MPs indoors, whereas vacuuming reduces MP emissions [27].
4 Microplastics in air
Air, an essential component of all living organisms, including humans, requires cleanliness to sustain life. Inhalation of contaminated air poses significant health risks and can lead to mortality in both humans and animals [28]. Unwanted substances that alter the physical and chemical properties of air are considered pollutants. Air pollution is a pressing global issue exacerbated by increasing industrialization, urbanization, and population growth. Polluted air worldwide poses a significant threat to various ecosystems. Among the various types of pollutants, recently identified airborne MPs have garnered considerable attention from scientists, NGOs, and public media [29].
Many scientific studies on MPs pollution in the marine environment and their effects on marine organisms and sea birds have been conducted to date. Currently, there is a paucity of data regarding airborne MPs pollution. Dris et al. [16], reported the presence of MPs pollutants in the atmosphere in recent studies published in the Marine Pollution Bulletin (2016) and Environment Pollution (2017). Various types of MPs are present in the atmosphere, including polyethylene terephthalate (PET), polyethylene (PE), polyester (PES), polyacrylonitrile (PAN), poly (N-methyl acrylamide) (PAA), rayon (RY), ethylene vinyl acetate (EVA), epoxy resin (EP), and alkyd resin (ALK). Atmospheric MPs originate from various sources; however, the synthetic textile industry is the primary source. Minutes of fiber can get out quickly from wearing, drying, tailoring, producing clothes, and other fiber products. Analyzing and characterizing atmospheric MPs helps determine the origin of airborne MPs [17, 30]
Nevertheless, myriad sources contribute significantly to the presence of MPs in the atmosphere, including tire erosion from automobiles, trucks, household objects, waste incineration, building materials, sewage sludge, landfills, abrasive powder, and 3D printing processes. For instance, the washing and drying of a single garment may release approximately 1900 fibers in the air. Studies on particulate matter have reported that hydrogen-based polymer fibers from automobile tires are removed by mechanical abrasion. Countries like Germany and the Netherlands have reported significant emissions of microplastic threads from automobile tires, estimated at approximately 92,594 tons and 17,000 tons per year, respectively. Germany alone contributes an average tire wear emission of 0.81 kg per capita annually on a global scale. Another potential source of airborne microplastics is the use of 3D printers, particularly Fused Deposition Modeling (FDM) printers that utilize filament materials such as PET, PPT, and PLA. The printing process generates numerous ultrafine particles or nanoparticles [31].
Additionally, the accumulation of plastic waste at landfill sites can result in the production of atmospheric microplastics through degradation induced by UV radiation and physical abrasion [8]. The incomplete incineration of garbage also releases enormous amounts of MPs into the atmosphere, and atmospheric transporting agents such as wind and others facilitate the dispersion of MPs in remote and sparsely inhabited areas. Recently, Allen et al. [24] documented the presence of MPs in the Pyrenees Mountains, near Paris.
4.1 Microplastics in table salt
Salt, also known as table salt, provides essential nutrition to most living organisms, including plants and animals. Since ancient times, salt has been used as a food preservative agent after lowering the quantity of water, thereby inhibiting bacterial growth. Salt is used in various sectors, such as cosmetics, personal care products, drinks, and pharmaceutical products [20, 33].
Salt naturally occurs in the sea, lakes, rocks, and wells [34]. Seawater deposited in an enclosed area produces salinas (solar pond work) after crystallization due to evaporation and the heating effect of sunlight. Globally, salt mines often coincide with anthropogenically impacted regions and serve as hotspots for MPs contamination, making them highly susceptible to MP exposure [32]. In rock and well salt, the change in intruding MPs into the salt occurs during the collection, transport, and packing processes. In the solar saltwork pond, fresh seawater circulates in a series of successive ponds with different salinity concentration gradients before the crystallization process; some of this fresh seawater may contain MPs that act as a source of MPs solar pond salt.
Zhang et al. [11], studied MPs’ presence of MPs in more than 100 branded worldwide salt and revealed non-uniform concentrations of MPs across different regions. The highest accumulation of MPs in table salt was observed in Croatia (145.6–208), followed by Indonesia (145.6), Italy (168.4–844.6), the United States (51–816), and China (561–693 items kg1). A recent study reported MPs’ presence of MPs in seawater sources and found that Asia had the highest abundance of MPs, followed by Australia, France, Iran, Japan, Malaysia, Portugal, Africa, and Spain (Fig. 2).
Presence of MPs in Spanish salt [18]
In another study, Yang et al. [35] reported the abundance, size, and type of MPs from various sources in China. They reported that China had the highest contribution to plastic waste deposition in the sea [36]. The concentration of MPs in salt has been reported to be 550–681 particles kg1 in sea salts, 43–364 particles kg1 in lake salts, and 7–204 particles kg1 in rock/well salts. Among the several types of salt, sea salt exhibits the highest concentration of MPs, which is three times higher than that of lake salt and seven times higher than that of rock salt. Other types of plastics include polyethylene terephthalate (PET), polyester (PES), polyethylene (PE), poly (1-butene) (PB), polypropylene (PP), and cellophane (CP). Additionally, micro-Raman spectroscopy analyses conducted by Karami et al. [37] identified polymers in 17 brand salts from eight countries: PP accounted for 40%, followed by PE (33.3%), polyethylene terephthalate (PET) (6.66%), polyisoprene/polystyrene (6.66%), polyacrylonitrile (10.0%), and polyamide-6 (nylon-6, NY6; 3.33%); however, in Spain and Turkey, PET and PE are the most prevalent polymers [14, 26]. Kim et al. [39], expand on these finding by investigating MPs presence in 39 different salt brands of 16 countries, including 28 sea salt brands. The amount of MPs extracted is high in sea salt, followed by lake and rock salt. Among sea salt, the highest concentration of MPs is present in Asian countries, implying that Asia is a hot spot of plastic pollution. In addition to tap water, the presence of MPs has been reported in drinking beer and table salt. This study further stated that the average synthetic debris ingestion by ordinary people from these three sources (beer, tap water, and table salt) is 5800 particles per annum [40].
In India, the presence of MPs in salt has been reported in several studies. Seth and Shriwastav [41] analysed the presence of MPs in table salts using contaminated seawater. Sivagami et al. [42], reported MPs presence in 10 branded salt in India and further evaluate their in vitro toxicity. Selvam et al. [43], concluded the average size of MPs found in Indian salt was less than 100 μm, and most of the polymer was PP, PE, nylon, and cellulose. In another study, the consumption of MPs presents in salt had a chance increased exposure to toxic heavy metals, as MPs surfaces can adhere to many heavy metals, including Arsenic, Iron, and nickel (Table 1).
4.2 Microplastics in water
Water, an indispensable renewable resource vital for sustaining life, faces increasing pollution due to anthropogenic activities such as urbanization, agriculture, industrialization, and population growth. This process is essential for all living organisms [44]. In addition to pollutants such as heavy metals, hydrocarbons, and excessive nutrients, MPs have emerged as contaminants detected in various water sources, such as drinking water bottles, tap water, and open ocean water [45, 46].
Both surface water and groundwater are essential for drinking water resources [47], with complex interconnections between them [48]. Anthropogenic activities and human influence have led to the presence of various pollutants in both surface and groundwater, as documented by numerous studies. Despite extensive research on MPs in freshwater systems, there remains a lack of comprehensive data and delayed global recognition of their environmental impact. The issue of MPs pollution has raised more questions than it has answered. The sources of MPs in freshwater include wastewater treatment, cargo shipping, fishery activities, human waste from beaches, urban runoff [21], fragmentation of large particles or product wear [49], discharge from wastewater plants, atmospheric deposition [50], and transfer and transport from agricultural activities [51]. According to Eerkes et al. [52], a possible route for the entry of MPs into freshwater includes effluent from washing machines, spillage from industrial activities, cosmetic items, and plastic items used in physical wear, which are degraded through environmental degradation and agricultural activities. Other potential sources of MPs in freshwater include weather phenomena such as typhoons. Wang et al. [53], reported that MPs intensity in freshwater increases after typhoons than before (Table 2).
Danopoulos et al. [61], and Schymanski et al. [62], reported the presence of MPs in potable drinking and bottled mineral waters. MPs sources are suspected of consumer product disintegration in treated drinking water, including packaging and textiles, bottle caps, and sealing [63]. The transport of drinking water and the use of plastic products during the purification of drinking water is also a route for MPs exposure. On the other hand, the detection of MPs in mineral drinking water might come from the water filling process, bottle cap, or from the polymer bottle itself. Among the different mineral water bottles, single-use water bottles reported lower concentrations of MPs, whereas reuse water bottles reported the presence of eight times higher concentrations of MPs than single-use plastic bottles because the re-utilization of the water bottle is under more stress. It is plausible that the rigid glass caused extra wear on the soft plastic material from the bottle cap and sealing, and thus MPs particles were released due to abrasion. Furthermore, the concentration of carbon dioxide in water bottles also affects the MPs concentration, and higher concentrations of carbon dioxide lead to increased water pressure and stress on plastic water bottles, resulting in the release of more plastic particles [62].
The presence of MPs in groundwater was reported by Panno et al. [64], but the mechanism of MPs contamination is still unknown. Furthermore, they reported that MPs sources in groundwater may originate from septic tanks and surface runoff. Studies also reported the presence of MPs in the groundwater. According to him, the possible entry of MPs into the groundwater system includes wastewater treatment plants or greywater discharge, septic tank outflows, aquifer recharge through the direct injection of contaminated water, loss of streams, and infiltration through soil pores [65]. In addition to groundwater contamination, MPs particles in groundwater act as sites for the translocation of hazardous heavy metals and harmful bacteria [66]. The concentration of MPs in groundwater is always lower than that in freshwater [67].
5 Pathways and accumulation of MPs in the human body
Ingestion and inhalation are the primary routes through which MPs are enter into humans. The gastrointestinal exposure to MPs can occur through various sources, including table salt, drinking water [46], seafood, mouthing of dirty toys by children, licking hands, dust containing MPs [9, 61], and airborne suspended MPs [7, 21, 68]. Additionally, dermal contact with plastic-associated goods can contribute to exposure [70]. Vianello et al. [71], reported that potential exposure to MPs in indoor environments were food, drink, and air and polyesters MPs reported as the most abundant in indoor environments. And the most significant concern for human exposure to MPs may result from consumption of contaminated food by MPs or inhalation of fugitive atmosphere particles [72, 69].
MPs are ubiquitous and distributed across the entire planet, from the highest peaks of Mount Everest to the deepest oceans, and from artic snow to alpine soils. After ingestion or inhalation, similar to particulate matter (PM) 2.5, MPs can penetrate various human organs, and their penetrating capability depends on their shape, size, and settling velocity [73]. Studies have reported the presence of MPs in lung tissue [31] and their persistence in lung fluid without notable changes in their shape and size even after 180 days [74]. A recent study reported the presence of MPs in the human placenta. MPs, such as nano-sized particles (5–10 μm), have the capability of penetrating into the bloodstream, resulting in reaching the placenta. However, there is much confusion about how nano-sized MPs particles penetrate the bloodstream [53]. MPs that are not sorbed and pass through the gastrointestinal tract are excreted through the human stool [75]. In addition, 90% of the desorbed MPs pass through the human excretory system [76].
5.1 Trophic transfer, bioaccumulation, and biomagnification of MPs
MPs are small particles (> 5 mm) found in various environments, including aquatic bodies. Understanding the trophic transfer of MPs from one trophic level to the next is an important concern in today’s world; however, there is a paucity of data to illustrate or prove how trophic transfer, bioaccumulation, and biomagnification of MPs occur in living organisms, especially humans. However, this review paper provides insight into the mechanisms of trophic transfer, bioaccumulation, and biomagnification in humans.
Generally, MPs have a large surface area and tend to adhere to the surrounding surfaces, including phytoplankton. According to Nolte et al. [77], negatively charged MPs have less affinity for adherence to the surrounding surfaces, whereas positively and neutrally charged MPs do [77]. Adherence of MPs has been reported on the surfaces of various aquatic planktons (e.g., Fucus vesiculosus, Chlorella pyremoidosa, Phaeodactylum tricornutum, and Nodularia spumigena) [78¸ 79] and is the main food source for numerous aquatic herbivorous species. These aquatic phytoplankton (primary producers) and others are produced in large quantities by aquatic herbivorous species (e.g., Dorosoma cepedianum, Myloplus schomburghii, and Pimphales promelas) [80]. Therefore, MPs are introduced into the food chain. Again, after huge consumption of these MPs adhered to aquatic phytoplankton by various aquatic organisms, studies have shown that some herbivorous fish have some quantity of MPs stored inside their bodies, due to the low egestion rate of MPs as compared to their ingestion rate [81]. Similarly, secondary consumers consume these primary consumers in huge quantities. In this manner, MPs move from one trophic level to the next.
The amount of microplastics (MPs) attached to aquatic phytoplankton is barely detectable. This raises the question: can these few MPs lead to bioaccumulation or biomagnification at the next trophic level? Despite the low number of MPs attached per phytoplankton consumed by primary consumers, the potential for MPs to accumulate in higher trophic levels remains significant due to the continuous and extensive consumption of phytoplankton by primary consumers, followed by secondary consumers, and so on [80]. Indeed, Bhatt and Chauhan [82] reported the potential transfer of MPs from one trophic level to another, causing bioaccumulation and biomagnification in numerous varieties of aquatic organisms. Furthermore, they mentioned that the average amount of MPs accumulated in predatory fish was higher (6.09), followed by omnivorous fish (5.85), and herbivorous fish (1.88, MPs particles per fish) (carnivorous > omnivorous > herbivorous), which clearly indicates the successful transfer of MPs from the primary trophic level to higher levels (Fig. 3).
MP-accumulated fish enter the human food chain through supermarkets, direct fishing, etc. Curren et al. [83], collected some seafoods from Singapore supermarkets and examined the amount of MPs present and reported that numerous amount of MPs are accumulated in various seafood. We, human beings, consume those foods, without thinking or not being aware of MPs. Similarly, there are many ways to introduce MPs into the human body through water, indoor and outdoor air, and salt.
Now, another question arises: after ingestion, do all these MPs pass through the human excretory system? Smith et al. [76], studied the MPs content in human excreta and found that only around 90% of ingested MPs are egested, with the remaining 10% accumulating inside the human body. Studies have also detected MPs in various human organs, including the lungs [73], placenta [84], bloodstream [85], and other organs. This suggests that MPs can accumulate inside the human body, yet there is currently no comprehensive study documenting the effects of MPs or any associated diseases in humans. However, MPs are xenobiotic substances that can cause unwanted problems in the human body. For instance, MPs are well-known carriers of several toxic metals and possess carcinogenic properties (e.g., plasticizers and flame retardants). Wardrop et al. [86], studied the effects of plastic additives (polybrominated diphenyl ethers, bisphenol A) on aquatic organisms. The authors revealed that there was an increased effect on the organisms when associated with MPs compared to the control. Additionally, MPs are well-known for their large hydrophobic surface area, which tends to sorb hydrophobic toxic substances, act as vectors for carrying various toxic substances, and affect aquatic organisms. Thus, MPs have a Trojan Horse” effect on aquatic organisms, and further research is needed to elucidate the potential health implications of MPs on humans.
5.2 Human health risk
In the twenty-first century, the scientific understanding of MPs in human organs and their potential effects on human health remains challenging owing to the context and multifaceted nature of these particles [81, 82, 88]. Human health risks associated with MPs can arise from their monomeric constituents (e.g., Bisphenol A), additives (e.g., plasticizers), or a combination thereof [89]. Given the pervasive use of plastic-containing items in daily life, individuals may unintentionally consume approximately 80 g of MPs per day [90]. Human exposure to MPs can adversely affect various biological systems, including particle toxicity, oxidative stress, inflammatory lesions, and increased translocation uptake [63, 91].
Inhalation of MPs can lead to respiratory symptoms such as dyspnea due to airway and interstitial inflammatory responses [92]. The tenacious nature of MPs have unique properties, such as hydrophobicity, which can exacerbate toxicity through inflammation via entanglement or MPs ingestion [87]. In vitro studies have demonstrated that cytotoxic effects are induced in the human brain and epithelial cells (T98G and HeLa cell lines) following exposure to nanoparticles, such as silver nanoparticles (AgNPs), carbon nanoparticles (e.g., graphene), polyethylene, and polystyrene [93].
Bladder cancer, elevated liver enzyme levels, anemia, breast cancer, reproductive problems, and low platelet counts are more common in plastic workers than in ordinary people [94]. And patient suffering from inflammatory bowel disease is highly susceptible to plastics [3]. Again, the presence of MPs in the placenta requires careful consideration because the placenta represents the interface between the foetus and the mother [95].
Moreover, plastics are a chemical cocktail product, and the release of these additives from the plastics additive during the recycling and recovery process poses additional health risks [96]. Most additives associated with plastics are potentially carcinogenic or endocrine disruptors [97]. Many chronic kidney diseases (CKD) have been associated with compounds such as phenol and indoles, while additives such as Bisphenol A (BPA) can interfere with the development of newborn animals and their immune system [98].
6 Effect of MPs in earthworms
Earthworms, a terrestrial invertebrate belonging to the phylum Annelida, inhabit various soil types and ecosystems, including forests, grasslands, arable lands, orchards, greenhouses, and plant nurseries. Some species are adapted to hygrophilicity, while others are endure harsh conditions, such as living under snow in high mountains [99]. Recognized as an architect of fertile soil and friend of agriculture and ecological engineering [100], it has traditionally been used in China since ancient times [101].
Despite their crucial role in soil health and ecosystem functioning, earthworms are susceptible to various xenobiotic substances, such as pesticides [102], detergents [103], and heavy metals [104]. In addition to these pollutants, MPs have been identified as emerging pollutants with detrimental effects on earthworms, and the degree of effect of MPs is dependent on the concentration and type of MPs. Studies have shown that low concentrations of MPs (≤ 0.5%W/W) have minimal effects on earthworm fitness, whereas higher concentrations can inhibit growth and increase mortality rates by compromising the earthworm’s self-defence mechanism [105]. In vivo cytotoxicity, experiment results show that earthworms exposed to MPs reduce the percentage of coelomocytes and cellular viability, whereas coelomocytes and cellular viability is an important earthworm immunological response [106]. Additionally, earthworms exposed to MPs affect their reproductive system and are more pronounced in males than in females. Hematoxylin and Eosin (H&E) staining assay revealed that spermatogenesis is affected by a reduction in the number of mature sperm cells along with improper arrangement of germ cells and loose tissue structure [106].
The comet assay is a sensitive biomarker for determining and evaluating the genotoxicity of pollutants in invertebrates and is commonly used to measure DNA damage caused by pollutants. Jiang et al. [107], reported that polystyrene MPs have been found to be increase in DNA damage in a dose dependent manner, with large particle exhibit greater genotoxicity than the smaller one. Moreover, the effect exerted by MPs on earthworms depends on the types of MPs and MPS, as nylon (90–150 μm) can reduce the reproduction rate by up to 25%, but not in PVC, whereas PVC is the most toxic polymer owing to the presence of its plasticizer [108]. Overall, MPs can affect earthworms at the cellular and organ levels, highlighting the importance of understanding their ecological impact.
6.1 Effect of MPs on plants
Plant growth is influenced by various environmental factors, particularly soil quality for terrestrial plants and water quality for aquatic plants. MPs in the environment are unable to penetrate plant cells owing to their large size, can adhere to the plant surface, and can be absorbed through the root. On the other hand, carbon nanomaterials can be absorbed by roots. However, small MPs can translocate from the roots to the shoots via endocytosis and capillary action [109]. Depending on the content of the growing medium, the presence of MPs in the environment may affect plant growth in a unique manner. Soil associated with MPs has been found to hinder seed germination by obstructing the testa of seeds such as Lepidium sativum and Glycine max, potentially impeding water absorption and root growth [110]. The negative effects of MPs on plant growth depend on their concentration and size, with large and concentrated MPs exerting greater harm [111]. The presence of MPs inside plants causes a lack of cell connection, cell wall pores, and hindrance of nutrient uptake, resulting in reduced shoot growth.
Moreover, MPs also affect plant photosynthesis in both terrestrial and aquatic plants, affecting parameters such as stomatal conductance, transpiration rate, intracellular CO2 concentration, chlorophyll content (chlorophyll a and chlorophyll b), and Rubisco activity [120]. This leads to a reduction in the photosynthetic rates of terrestrial plants and decreased chlorophyll content and photosynthetic efficiency in aquatic plants. The flowers of various terrestrial plants have been found to be contaminated with MPs, potentially affecting their fertilization [121]. Nano-sized MPs may reach the ovary through flower styles, interfering with pollination, as their size can mimic pollen grains [122, 123]. Overall, interactions between MPs and plants have significant implications for plant growth, development, and ecosystem functioning [99, 100] (Table 3).
6.2 Effect of MPs on soil physicochemical properties and microbial diversity
In the soil environment, MPs infiltrate through various pathways, such as atmospheric deposition, irrigation and flooding, composting, street runoff and littering, sewage sludge, application of biosolids, and plastic mulch films to improve plant growth and reduce moisture loss [124]. The introduction of MPs into soil profoundly affects soil physicochemical properties, microbial diversity, soil enzyme activities, and nutrient cycling [125].
Changes in soil physical parameters are contingent on factors such as MPs type, concentration, tenacity, soil type, and environmental conditions. Owing to their large surface area and small size, MPs in the soil allow them to occupy the interstitial space between the soil particles. The filling of these porous spaces between soil particles affects soil aggregation, increases soil compaction, and reduces porosity. The aggregation or accumulation of MPs in the soil creates a microhabitat, leading to changes in the soil microbial diversity [126]. Additionally, the presence of MPs in soil alters the soil water-holding capacity by replacing porosity, thereby demising soil water-retention properties [127]. Moreover, impeding soil porosity due to MPs also affects soil aeration and interferes with the movement of gases between the soil particles [128].
Oxygen is important for aerobic microbial processes, as it reduces the amount of oxygen due to changes in soil porosity, which affects soil microbial diversity. Owing to their large surface area, MPs have the potential to influence soil surface reflectance and thermal properties. The dark colouration of MPs on the soil surface increases the absorption of solar radiation, resulting in increased soil temperature, which in turn affects microbial diversity and plant growth, etc. [126].
6.3 Effect of MPs in soil microbial diversity
Soil microbial diversity and biomass are the main criteria for maintaining fundamental ecosystem functions, including organic matter decomposition, nutrient cycling, and carbon and gas flux [129]. The incorporation of MPs into soil ecosystems also exerts a notable effect on soil microbial diversity, primarily by altering microbial populations.
Upon entering soil environments, MPs can affect soil physicochemical properties and create a microhabitat within the soil matrix. These microhabitats influence the distribution and diversity of soil microorganisms by providing a niche space that favours the proliferation of certain microbial taxa while inhibiting others [130]. Due to their large surface area, MPs have a tendency to bind nutrients on their surface area, resulting in localized nutrient availability on MP-contaminated surrounding areas and decreased nutrient content in the surrounding soil, leading to changes in soil microbial diversity. Similarly, studies have reported a reduction in alpha diversity (richness, evenness, and diversity) of microbial communities on the surface of MPs compared with other substrates [131].
Moreover, MPs can serve a habitat on the surface of MPs after making thin a biofilm on the surface of MPs which is also known as “Plastisphere.” Biofilms provide a niche for specific microorganisms that can inhabit this plastisphere, resulting in reduced microbial diversity. For instance, MPs (PE) have been found to inhibit the growth of Proteobacteria, whereas the same MPs increase the growth of Actinobacteria. Similarly, some studies have reported that MPs such as polyvinyl chloride (PVC) and polyethylene (PE) can alter the relative abundance of microbial taxa, suppressing the growth of certain groups, such as sphingomonadaceae and xanthobacteriaceae, while enhancing the relative abundance of Burkholderiaceae [132].
Furthermore, degraded MPs can release additives and chemicals (such as plasticizers and flame retardants) into the surrounding soil. These additives and other chemical compounds are known for their toxic effects, thereby contributing to changes in the soil microbial diversity. These mechanisms collectively illustrate how MPs influence microbial diversity within soil ecosystems.
6.4 Effect of MPs in nutrient cycling
Nutrient cycling is a fundamental process in ecosystems that involves the movement and transformation of various nutrients within the environment and among biotic and abiotic entities [133]. However, due to human anthropogenic and other activities can disrupt the functions of natural ecosystems, thereby affecting nutrient cycling. Among these disturbances, MPs are emerging as significant pollutants that affect nutrient cycling, including nitrogen, carbon, phosphorus, and some other micronutrient cycles [110, 111]. Nitrogen cycling is a complex natural process in which nitrogen compounds undergo continuous transformation and transfer through nitrification and denitrification, and each process is controlled by the respective microorganisms and enzymes [133]. The incorporation of MPs into soil interferes with nitrogen cycling at different stages. For example, the intrusion of MPs affects soil microbial diversity, thereby affecting enzyme activity and N cycling [134]. However, the effect of MPs on nitrogen cycling depends on the type of MPs and their additives.
Certain MPs, such as PLA, are easier to degrade and serve as a source of carbon, leading to an increase in inorganic carbon and thus stimulating the growth of certain bacteria such as Cyanobacteria, Bradyrhizobium, and o-Frankiales, resulting in enriched N fixation [135]. Similarly, studies have reported an increase in the enzyme activity of the nifH gene in biofilms of agricultural residual plastics. In contrast, the authors further mentioned that there was a decrease in the enzymatic activity of amoA and nirK, which are responsible for ammonia monooxygenase and nitrite reductase, respectively [136]. However, the effects of MPs on the nitrogen cycle are dependent on the type of MPs and their concentration, exposure, and environmental conditions. For instance, MPs, such as low-density polyethylene (LDPE), have been found to increase the abundance of genes associated with nitrogen cycling, such as nifH, AOB, amoA, and nirK [137]. In contrast, the presence of MPs such as PVC may inhibit ammonium oxidation while potentially enhancing the denitrification process [138]. Similarly, studies have reported that the presence of MPs, such as PS, in soil has a negative potential effect on microbial biomass and leucine aminopeptidase activity [139], and MPs, such as PVC, inhibit ammonium oxidation, whereas there is a positive effect on denitrification (Fig. 4).
Functional genes involved in essential nitrogen cycling change in in 30 days treatment of various plastic i.e., control, cPVC_p (PVC plastic without plasticizer), and PVC (PVC plastic with plasticizer). Reprinted from The Lancet, Vol. number 427, (Zhu et al. 2022), Copyright 2021, with permission from Elsevier
In aquatic environments, the intrusion of MPs can lead to the formation of biofilms on the surface of MPs, increasing porosity and accelerating the conversion of NH4+–N and NO2−–N oxidation, as well as the denitrification process [140]. Overall, the influence of MPs on nitrogen cycling is multifaceted and depends on various factors, necessitating further research to elucidate their effects comprehensively.
6.5 Effect of MPs in carbon cycle
The carbon cycle is a fundamental natural process involved in the continuous transformation of carbon- and carbon-containing compounds in the Earth’s atmosphere, ocean, and living organisms. With the escalating concern over global warming, attention has intensified to the emission of greenhouse gases, mainly CO2 [141]. Intrusion of MPs profoundly affects the carbon cycle in terrestrial and ocean environments in numerous ways, for instance, by changing the soil physicochemical properties, microbial diversity and their function, growth of plants, and litter decomposition [142]. However, the intricate mechanism underlying the disturbance of the soil carbon cycle by MPs is complex and depends on many factors, including the type, size, and concentration of plastics, soil type, and local environmental conditions.
MPs are mainly composed of carbon; for instance, MPs, such as PE and PS, contain more than 90% carbon [143]. Owing to their versatile properties, MPs prevent microbial decomposition, immobilization, and binding to soil inorganic or organic compounds [144]. These MPs are locked within soil particles and change soil physicochemical properties, as described above [144]. Thus, MPs function as sources of carbon in the soil environment (i.e., they do not originate from plants). PE-based MP are hydrophobic in nature, reduce the moisture content in the surrounding soil, and create an aerated environment around the MPs surface [138], thus accelerating soil organic matter mineralization [145], thus accelerating soil organic matter mineralization [146]. Similarly, some studies have also stated that the addition of MPs to soil increases the soil dissolved organic carbon content [147]. For instance, studies have reported that there is an increase in total organic carbon in river water mixed with nanoplastics (21.21 mg/L) than in normal river water (9.78 mg/L), which leads to changes in carbon storage in aquatic environments (i.e., an increase in the accumulation of carbon) [148]. Thus, the intrusion of MPs in soil increases the carbon in soil (SOM and DOC), but further studies are needed to make a scientific sense.
Again, incorporation of MPs in the soil also affected the distribution of photosynthetic carbon in the aboveground and belowground parts. The addition of MPs such as PVC at a low rate (1% of MPs) increases the allocation of photosynthetic carbon in the shoot part and slightly changes the root-to-shoot ratio [149]. The low-rate addition of MPs hampers root growth or produces phytotoxicity in the root, which may result in decreased allocation of photosynthetic carbon in the plant root [150]. However, increasing the concentration of MPs led to changes in soil physicochemical properties (i.e., increased aeration, water holding capacity, and reduced bulk density, resulting in turnover of organic matter and reduction of nutrient limitation) and nitrogen immobilization, which might counteract the phytotoxic effect in roots, thus increasing the distribution of photosynthetic carbon allocation in roots. For instance, 1–5% PVC powder increases the allocation of photosynthetic carbon in the roots of wheat plants [149]. Similarly, another study reported that the allocation of photosynthetic carbon in roots also increased with the addition of 10% PE and PVC powder [151]. The increased allocation of carbon in roots (rhizodeposition) is particularly important for root-association fungi, which play a significant role in carbon sequestration. Root carbon sequestration through rhizodeposition plays a crucial role in microbial function, global carbon cycle, and ecosystem stability [151]. However, the effects or alterations in root-fungal associations are dependent on the type of MPs; therefore, further study is required to draw a strong conclusion.
6.6 Effect of MPs in phosphorus cycle
Phosphorus, an essential element for all the living organisms, play a pivotal role in regulating nutrient dynamics within the ecosystems. The intrusion of MPs into the soil environment exerts discernible effects on the phosphorus cycle. Notably, studies indicate that MPs, particularly those derived from polyvinyl chloride (PVC) and polystyrene (PS), have a demonstrable impact on total phosphorus levels in soil, as evidenced by notable reductions [152]. However, the presence of plasticizers, such as PVC MPs, has been observed to augment the accessibility of phosphorus in acidic red soil, promoting soil P cycling [153]. These processes involved inorganic P solubilization and organic P mineralization, which contributed to the augmentation of readily available P in the soil [154].
7 Strategic management and future recommendations
Microplastic (MP) pollution is a pervasive and emerging environmental concern worldwide. The initiative measure of MPs pollution in our environment is simply to avoid or reduce the production of plastic debris, prevent debris from entering our ecosystem, develop clean-up technologies, and bioremediation [155]. In addition to these scientific management techniques, some political, economic, and social factors play a very important role in implementing efficient processes to control plastic waste problems and MPs pollution [156].
Currently, the main strategies to solve the problem of MPs pollution should focus on source reduction, cleaning technology, and remediation strategies. Strategies for the management of MPs pollution are described below.
-
I.
Removing the MPs from personal care products.
The removal of MPs from personal care products can help reduce MPs pollution. MPs are often used in various products, such as body wash, facial scrubbing, and toothpaste. Many countries, such as Canada and Australia, have started to phase out or ban the use of plastics and have gained momentum in various countries and regions. Similarly, in 2015, the U.S. government started effectively banning the sale of personal care products containing plastic beads in 2017 [157]. By eliminating MPs from various personal care products, or if consumers can prefer plastic beads free, there might be a significant reduction in the amount of MPs entering various ecosystems.
-
II.
Use of biodegradable plastics.
Biodegradable plastics are polymers that undergo mineralization processes that facilitate specific microorganism and enzyme activities, resulting in the production of carbon dioxide, methane, water, inorganic compounds, or biomass [158]. Currently, bioplastics are emerging in the market, offering a potential substitute for conventional plastics for diverse applications. Their integration holds promise in alleviating the challenges associated with MPs pollution [159].
-
III.
Improved reuse, recycling, recovery, and wastewater treatment plants.
Enhancing the infrastructure and management systems pertaining to solid waste holds promise for mitigating the influx of plastic debris into surface water bodies. The recycling of plastics is another effective approach for reducing MPs pollution. Similarly, the use of waste plastics as a source of energy and the recovery of waste plastics as synthetic crude and valuable products will also reduce MPs pollution. Augmenting water treatment facilities by implementing advanced filtration technologies is an effective measure for the interception and prevention of MPs ingress into surface water reservoirs such as rivers and oceans [157].
-
IV.
Development of cleanup and bioremediation technologies.
The escalating concern surrounding plastic pollution has catalysed efforts to identify economically viable and environmentally sustainable methods for plastic degradation. Recent advancements in research and technologies have unveiled a spectrum of microbes that have the potential for biodegradation of plastics such as PE, PS, and PET. Bacterial strains such as Enterobacter asburiae and Exiguobacterium were isolated from the guts of Plodia interpunctella and Tenebrio molitor larvae and showed the potential for degradation of plastics [155]. Similarly, bacteria such as Ideonella sakaiensis 201-F6 produce the enzyme terephthalic acid, which helps in converting PET efficiently into its two environmentally benign monomers, terephthalic acid and ethylene glycol [160].
8 Conclusions
Over the last couple of decades, the proliferation of MPs across various environmental matrices has emerged as a pressing global concern. Their presence in air, water, beer, salt, fish aquatic organisms, and both aquatic plants and terrestrial plants affects microbial diversity, nutrient cycling, and soil physicochemical properties. Furthermore, owing to their large surface area and resistant to microbial degradation, MPs act as vectors for various toxic metals, potentially leading to bioaccumulation and subsequent effects on the next trophic level. The formation of plastispheres help in change the microbial diversity and further alters ecosystem dynamics by altering various ecosystem functions, such as nutrient supply and enzyme activities. Additionally, MPs impeding soil pose various problems for soil such as soil structure, water retention capacity, and dwelling organism activities, such as earthworms.
MPs are ubiquitous in different environmental components and cause serious effects on various organisms and the functioning of ecosystems by entering various organisms and ecosystem processes through various sources, such as table salt, drinking water, atmospheric fallout, and soft drinks. Through trophic transfer, these MPs finally enter the human body and cause breathing or ingestion problems. Presence of MPs has already been detected in deep human lung cells, and therefore, these tiny particles are creating serious concern to human health worldwide, which requires meticulous strategies to overcome this problem. Similarly, the presence of microplastic in aquatic organisms causes serious problems to different aquatic organisms such as reduce in reproduction rate, increase the mortality rate etc. again, the presence of MPs in soil effects the soil microbial community as well as hindrance in the growth of plant.
As of now, many questions remain unanswered regarding MPs, for example, the pathways of MPs at the cellular level, accumulation of MPs in tissue, effects of long-term exposure, and its potential effect on human health are still unrevealed. A vast amount of knowledge has been gained, and the possible impact of microplastics on human health is only speculated after referring to animal testing results. Nevertheless, all the additives associated with plastics are potentially carcinogenic and endocrine disruptors. Similarly, there is limited research based on the effect of MPs in aquatic organism and terrestrial organism, soil physiochemical properties and microbial diversity. More studies are needed to better understand the distribution, occurrence, and effects on various environment and human health.
Data availability
Data files will be made available from the corresponding author upon reasonable request. No datasets were generated or analysed during the current study.
References
Mulder KF. Sustainable consumption and production of plastics?—The case of clean technologies. Technol Forecast Soc Change. 2017. https://doi.org/10.1016/S0040-1625(97)00129-7.
Bhat MA, Eraslan FN, Gaga EO, Gedik K. Scientometric analysis of microplastics across the globe. In: Microplastics in the ecosphere: air, water, soil, and food. Hoboken: Wiley; 2023. p. 3–13. https://doi.org/10.1002/9781119879534.ch1.
Rubio L, Marcos R, Hernández A. Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J Toxicol Environ Health Part B. 2019;23:1–18. https://doi.org/10.1080/10937404.2019.1700598.
Barra R, Leonard SA, Whaley C, Bierbaum R. Plastics and the circular economy. Scientific and Technical Advisory Panel to the Global Environment Facility. 2018. p. 1–26.
Bhat MA, Gedik K, Gaga EO. Atmospheric micro (nano) plastics: future growing concerns for human health. Air Qual Atmos Health. 2023;16(2):233–62. https://doi.org/10.1007/s11869-022-01272-2.
Sarmah R, Dutta R, Baishya S, Borah S. Microplastic pollution: an emerging environmental issue. J Entomol Zool Stud. 2018;6(6):340–4.
Lehtiniemi M, Hartikainen S, Näkki P, Engström-Öst J, Koistinen A, Setälä O. Size matters more than shape: ingestion of primary and secondary microplastics by small predators. Food Webs. 2018;17: e00097. https://doi.org/10.1016/j.fooweb.2018.e00097.
Bhat MA, Gedik K, Gaga EO. A preliminary study on the natural aging behavior of microplastics in indoor and outdoor environments. Int J Environ Sci Technol. 2024;21(2):1923–36. https://doi.org/10.1007/s13762-023-05319-4.
Bhat MA. Unveiling the overlooked threat: macroplastic pollution in indoor markets in an urban city. Case Stud Chem Environ Eng. 2024;9: 100558. https://doi.org/10.1016/j.cscee.2023.100558.
Bergmann M, Gutow L, Klages M. Marine anthropogenic litter. Cham: Springer Nature; 2015.
Zhang Q, Xu EG, Li J, Chen Q, Ma L, Zeng EY, et al. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54(7):3740–51.
Benny D, Ashraf PM, Thomas SN. Microplastics in the edible and inedible tissues of pelagic fishes sold for human consumption in Kerala, India. Environ Pollut. 2020;266: 115365. https://doi.org/10.1016/j.envpol.2020.115365.
Sathish MN, Jeyasanta I, Patterson J. Occurrence of microplastics in epipelagic and mesopelagic fishes from Tuticorin, Southeast coast of India. Sci Total Environ. 2020;720: 137614. https://doi.org/10.1016/j.scitotenv.2020.137614.
Ferraz M, Bauer AL, Valiati VH. Microplastic concentrations in raw and drinking water in the Sinos River, southern Brazil. Water. 2020;12:1–10.
Sruthy S, Ramasamy EV. Microplastic pollution in Vembanad Lake, Kerala, India: the first report of microplastics in lake and estuarine sediments in India. Environ Pollut. 2016;222:1–8. https://doi.org/10.1016/j.envpol.2016.12.038.
Ding J, Jiang F, Li J, Wang Z, Sun C, Wang Z, et al. Microplastics in the coral reef systems from Xisha Islands of South China Sea. Environ Sci Technol. 2019;53(14):8036–46.
Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, et al. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health. 2018;1:1–5. https://doi.org/10.1016/j.coesh.2017.10.002.
Iñiguez ME, Conesa JA, Fullana A. Microplastics in Spanish table salt. Sci Rep. 2017;7(1):1–7.
Lachenmeier DW, Kocareva J, Noack D. Microplastic identification in German beer—an artefact of laboratory contamination? Dtsch Lebensm-Rundsch. 2015;111(10):437–40.
Bhat MA. A comprehensive characterization of indoor ambient microplastics in households during the COVID-19 pandemic. Air Qual Atmos Health. 2024. https://doi.org/10.1007/s11869-024-01559-6.
Mrowiec B. Plastic pollutants in water environment. Environ Prot Nat Res. 2017;28(4):51–5. https://doi.org/10.1515/oszn-2017-0030.
Jin M, Wang X, Ren T, Wang J, Shan J. Microplastics contamination in food and beverages: direct exposure to humans. J Food Sci. 2021;86(7):2816–37. https://doi.org/10.1111/1750-3841.15802.
Dris R, Gasperi J, Saad M, Mirande C, Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull. 2016;104(1–2):290–3. https://doi.org/10.1016/j.marpolbul.2016.01.006.
Amato-lourenço LF, Galvão S, De Weger LA, Hiemstra PS, Vijver MG, Mauad T. Mini-review of microplastics in the atmosphere and their risks to humans. Sci Total Environ. 2020;703: 135504. https://doi.org/10.1016/j.scitotenv.2019.135504.
Bhat MA. Indoor microplastics: a comprehensive review and bibliometric analysis. Environ Sci Pollut Res Int. 2023;30(58):121269–212129. https://doi.org/10.1007/s11356-023-30902-0.
Bhat MA, Eraslan FN, Gedik K, Gaga EO. Impact of textile product emissions: toxicological considerations in assessing indoor air quality and human health. In: Ecological and health effects of building materials. Cham: Springer; 2021. https://doi.org/10.1007/978-3-030-76073-1_27.
Sheraz M, Kim J, Kim J. Nano/microplastics in indoor air: a critical review of synthesis routes for toxicity testing and preventative measure strategies. Process Saf Environ Prot. 2023;180:274–304. https://doi.org/10.1016/j.psep.2023.10.002.
Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2019;151:56–68. https://doi.org/10.1016/j.freeradbiomed.2020.01.179.
Öztürk M, Khan SM, Altay V, Efe Egamberdieva RD, Khassanov FO. Biodiversity, conservation and sustainability in Asia. Cham: Springer; 2022. p. 1–1089. https://doi.org/10.1007/978-3-030-73943-0.
Verla AW, Enyoh CE, Verla EN, Nwarnorh KO. Microplastic–toxic chemical interaction: a review study on quantified levels, mechanism and implication. SN Appl Sci. 2019;1(11):1–26.
Amato-lourenço LF, Galvão S, De WLA, Hiemstra PS, Vijver MG, Mauad T. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci Total Environ. 2020;749: 141676. https://doi.org/10.1016/j.scitotenv.2020.141676.
Peixoto D, Pinheiro C, Amorim J, Oliva-teles L, Natividade M. Estuarine, coastal and shelf science microplastic pollution in commercial salt for human consumption: a review. Estuar Coast Shelf Sci. 2019;219:161–8. https://doi.org/10.1016/j.ecss.2019.02.018.
Carapeto C, Brum S, Rocha MJ. Which table salt to choose? J Nutr Food Sci. 2018;8(3):8–11.
Durack E, Alonso-gomez M, Wilkinson MG. Salt: a review of its role in food science and public health. Curr Nutr Food Sci. 2008;4(4):290–7.
Yang D, Shi H, Li L, Li J, Jabeen K, Kolandhasamy P. Microplastic pollution in table salts from China. Environ Sci Technol. 2015;49(22):13622–7.
Li J, Yang D, Li L, Jabeen K, Shi H. Microplastics in commercial bivalves from China. Environ Pollut. 2015;207:190–5. https://doi.org/10.1016/j.envpol.2015.09.018.
Karami A, Golieskardi A, Keong Choo C, Larat V, Galloway TS, Salamatinia B. The presence of microplastics in commercial salts from different countries. Sci Rep. 2017;7:1–11.
Gündoğdu S. Contamination of table salts from Turkey with microplastics. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35(5):1006–14. https://doi.org/10.1080/19440049.2018.1447694.
Kim J, Lee H, Kim S, Kim H. Ecotoxicology and human environmental health global pattern of microplastics (MPs) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution. Environ Sci Technol. 2018;52(21):12819–28.
Kosuth M, Mason SA, Wattenberg EV. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE. 2018;13(4):1–18.
Seth CK, Shriwastav A. Contamination of Indian sea salts with microplastics and a potential prevention strategy. Environ Sci Pollut Res. 2018;25(30):30122–31.
Sivagami M, Selvambigai M, Devan U, Joseph AA, Karmegam N, Biruntha M, et al. Extraction of microplastics from commonly used sea salts in India and their toxicological evaluation. Chemosphere. 2021;263: 128181. https://doi.org/10.1016/j.chemosphere.2020.128181.
Selvam S, Manisha A, Venkatramanan S, Chung SY, Paramasivam CR, Singaraja C. Microplastic presence in commercial marine sea salts: a baseline study along Tuticorin Coastal salt pan stations, Gulf of Mannar, South India. Mar Pollut Bull. 2019;2020(150): 110675. https://doi.org/10.1016/j.marpolbul.2019.110675.
Singh RM, Gupta A. Water pollution-sources, effects and control water pollution-sources, effects and control. Res Gate. 2017;5(3):1–17.
Singh J, Yadav P, Pal AK, Mishra V. Water pollutants: origin and status. In: Sensors in water pollutants monitoring: role of material. Springer: Singapore; 2020. p. 5–20.
Bhat MA, Janaszek A. Evaluation of potentially toxic elements and microplastics in the water treatment facility. Environ Monit Assess. 2024;196(5):475. https://doi.org/10.1007/s10661-024-12651-w.
Grönwall J, Danert K. Regarding groundwater and drinking water access through a human rights lens: self-supply as a norm. Water. 2020;12(2):419.
Ezugwu CN, Apeh S. Ground water and surface water as one resource: connectivity interaction. IOSR J Mech Civ Eng. 2017;14(3):54–9.
Koelmans AA, Mohamed Nor NH, Hermsen E, Kooi M, Mintenig SM, De France J. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019;155:410–22. https://doi.org/10.1016/j.watres.2019.02.054.
Ravit B, Cooper K, Moreno G, Buckley B, Yang I, Deshpande A, et al. Microplastics in urban New Jersey freshwaters: distribution, chemical identification, and biological affects. AIMS Environ Sci. 2017;4(6):809–26.
Crossman J, Hurley RR, Futter M, Nizzetto L. Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci Total Environ. 2020;724: 138334. https://doi.org/10.1016/j.scitotenv.2020.138334.
Eerkes-Medrano D, Leslie HA, Quinn B. Microplastics in drinking water: a review and assessment. Curr Opin Environ Sci Health. 2019;7:69–75. https://doi.org/10.1016/j.coesh.2018.12.001.
Wang J, Lu L, Wang M, Jiang T, Liu X, Ru S. Typhoons increase the abundance of microplastics in the marine environment and cultured organisms: a case study in Sanggou Bay, China. Sci Total Environ. 2019;667:1–8. https://doi.org/10.1016/j.scitotenv.2019.02.367.
Singh S, Trushna T, Kalyanasundaram M, Tamhankar AJ, Diwan V. Microplastics in drinking water: a macro issue. Water Supply. 2022;22(5):5650–74. https://doi.org/10.2166/ws.2022.189.
Tong H, Jiang Q, Hu X, Zhong X. Occurrence and identification of microplastics in tap water from China. Chemosphere. 2020;252: 126493. https://doi.org/10.1016/j.chemosphere.2020.126493.
Feld L, Da Silva VH, Murphy F, Hartmann NB, Strand J. A study of microplastic particles in Danish tap water. Water. 2021;13:15. https://doi.org/10.3390/w13152097.
Li Y, Meng Y, Qin L, Shen M, Qin T, Chen X, Duan X. Occurrence and removal efficiency of microplastics in four drinking water treatment plants in Zhengzhou, China. Water. 2023;16:1–15. https://doi.org/10.3390/w16010131.
Shen M, Zeng Z, Wen X, Ren X, Zeng G, Zhang Y, Xiao R. Presence of microplastics in drinking water from freshwater sources: the investigation in Changsha, China. Environ Sci Pollut Res. 2021;28(31):42313–24. https://doi.org/10.1007/s11356-021-13769-x.
Li H, Zhu L, Ma M, Wu H, An L, Yang Z. Occurrence of microplastics in commercially sold bottled water. Sci Total Environ. 2023;867: 161553. https://doi.org/10.1016/j.scitotenv.2023.161553.
Sami SM, Al-Fatlawi AH. Partial and temporal variations in concentration of micro plastic in drinking water of Al-Hilla river. Ecol Eng Environ Technol. 2023;24(5):1–10. https://doi.org/10.12912/27197050/162707.
Danopoulos E, Twiddy M, Rotchell JM. Microplastic contamination of drinking water: a systematic review. PLoS ONE. 2020;15(7):1–23. https://doi.org/10.1371/journal.pone.0236838.
Schymanski D, Goldbeck C, Humpf HU, Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–62. https://doi.org/10.1016/j.watres.2017.11.011.
Johnson AC, Ball H, Cross R, Horton AA, Jürgens MD, Read DS, et al. Identification and quantification of microplastics in potable water and their sources within water treatment works in England and Wales. Environ Sci Technol. 2020;54(19):12326–34.
Panno SV, Kelly WR, Scott J, Zheng W, Mcneish RE, Holm N, et al. Microplastic contamination in karst groundwater systems. Groundwater. 2019;57(2):189–96.
Re V. Shedding light on the invisible: addressing the potential for groundwater contamination by plastic microfibers. Hydrogeol J. 2019;27(7):2719–27.
Selvam S, Jesuraja K, Venkatramanan S, Roy PD, Kumari VJ. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J Hazard Mater. 2021;402: 123786. https://doi.org/10.1016/j.jhazmat.2020.123786.
Mintenig SM, Löder MGJ, Primpke S, Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ. 2019;648:631–5. https://doi.org/10.1016/j.scitotenv.2018.08.178.
Bhat MA. Microplastics in indoor deposition samples in university classrooms. Discov Environ. 2024;2(1):23. https://doi.org/10.1007/s44274-024-00054-0.
Kadac-Czapska K, Knez E, Grembecka M. Food and human safety: the impact of microplastics. Crit Rev Food Sci Nutr. 2024;64(11):3502–21. https://doi.org/10.1080/10408398.2022.2132212.
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020;702: 134455. https://doi.org/10.1016/j.scitotenv.2019.134455.
Vianello A, Jensen RL, Liu L, Vollertsen J. Simulating human exposure to indoor airborne microplastics using a breathing thermal manikin. Sci Rep. 2019;9(1):1–11. https://doi.org/10.1038/s41598-019-45054-w.
Abbasi S, Turner A. Human exposure to microplastics: a study in Iran. J Hazard Mater. 2021;2021(403): 123799. https://doi.org/10.1016/j.jhazmat.2020.123799.
Pauly JL, Stegmeier SJ, Allaart HA, Cheney RT, Zhang PJ, Mayer AG, et al. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol Biomark Prev. 1998;7(5):419–28.
Law BD, Bunn WB, Hesterberg TW. Solubility of polymeric organic fibers and manmade vitreous fibers in gambles solution. Inhal Toxicol. 1990;2(4):321–39.
Schwabl P, Ko S. Detection of various microplastics in human stool. Ann Intern Med. 2019;171(7):453–8.
Smith M, Love DC, Rochman CM, Neff RA. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018;5(3):375–86.
Nolte TM, Hartmann NB, Kleijn JM, Garnæs J, van de Meent D, Jan Hendriks A, et al. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat Toxicol. 2017;2017(183):11–20. https://doi.org/10.1016/j.aquatox.2016.12.005.
Helmersson K. Effects of microplastic leachates on phytoplankton: a laboratory study on Nodularia spumigena and Phaeodactylum tricornutum. 2011;78.
Gutow L, Eckerlebe A, Giménez L, Saborowski R. Experimental evaluation of seaweeds as a vector for microplastics into marine food webs. Environ Sci Technol. 2016;50(2):915–23.
Desforges JPW, Galbraith M, Ross PS. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch Environ Contam Toxicol. 2015;69(3):320–30.
Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut. 2014;185:77–83.
Bhatt V, Chauhan JS. Microplastic in freshwater ecosystem: bioaccumulation, trophic transfer, and biomagnification. Environ Sci Pollut Res. 2023;30(4):9389–400. https://doi.org/10.1007/s11356-022-24529-w.
Curren E, Leaw CP, Lim PT, Leong SCY. Evidence of marine microplastics in commercially harvested seafood. Front Bioeng Biotechnol. 2020;8:1–9.
Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C, et al. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers. 2022;14(13):1–14.
Zhang Q, Liu L, Jiang Y, Zhang Y, Fan Y, Rao W, et al. Microplastics in infant milk powder. Environ Pollut. 2023;323: 121225. https://doi.org/10.1016/j.envpol.2023.121225.
Wardrop P, Shimeta J, Nugegoda D, Morrison PD, Miranda A, Tang M, et al. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ Sci Technol. 2016;50(7):4037–44.
Campanale C, Massarelli C, Savino I, Locaputo V. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health. 2020;17(4):1212.
Bhat MA, Gedik K, Gaga EO. Environmental toxicity of emerging micro and nanoplastics. In: Nanotechnology interventions in food packaging and shelf life. Boca Raton: CRC Press; 2022. p. 311–38.
Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;21(31):179–94.
Ebere EC, Wirnkor VA, Ngozi VE. Uptake of microplastics by plant: a reason to worry or to be happy? World Sci News. 2019;131:256–67.
Eraslan FN, Bhat MA, Gedik K, Gaga EO. The single-use plastic pandemic in the covid-19 era. In: Microplastics in the ecosphere: air, water, soil, and food. Hoboken: Wiley; 2023. p. 65–75. https://doi.org/10.1002/9781119879534.ch4.
Prata JC. Airborne microplastics: consequences to human health ? Environ Pollut. 2018;234:115–26. https://doi.org/10.1016/j.envpol.2017.11.043.
Schirinzi GF, Pérez-pomeda I, Sanchís J, Rossini C. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ Res. 2017;159:579–87. https://doi.org/10.1016/j.envres.2017.08.043.
Abu ME, Abdel GM, Mahrous OA, Hendy OM, Allam HK, Saad A, et al. Health disorders among workers in a plastic factory in Egypt Health disorders among workers in a plastic factory in Egypt. Menoufia Med J. 2017;30(1):81–6.
Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, et al. Plasticenta: first evidence of microplastics in human placenta. Environment. 2021;146: 106274.
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–99. https://doi.org/10.1016/j.jhazmat.2017.10.014.
Alabi OA, Ologbonjaye KI, Awosolu O, Alalade OE. Public and environmental health effects of plastic wastes disposal: a review. J Toxicol Risk Assess. 2019;5(021):1–3.
González-Parra E, Herrero JA, Elewa U, Bosch RJ, Arduán AO, Egido J. Bisphenol a in chronic kidney disease. Int J Nephrol. 2013;2013:1.
Julka JM. Earthworm resources and vermiculture. Zool Surv India. 1993;51–6.
Ramsay JA, Hill S. Earthworms the agriculturalist’s friends. EAP Publication-6. Macdonald J. 1978;39(10):6–8.
Zhou Y, He RQ. Earthworm fibrinolytic enzymes. In: Handbook of proteolytic enzymes. Oxford: Academic Press; 2013. p. 2953–8.
Garcia M, Römbke J, de Brito MT, Scheffczyk A. Effects of three pesticides on the avoidance behavior of earthworms in laboratory tests performed under temperate and tropical conditions. Environ Pollut. 2008;153(2):450–6.
Thomas A, Rakhi TV, Sunish KS. A study on the effect of detergent on megascolexkonkanensis. Int J Innov Res Sci Eng Technol. 2019;8(8):9020–8.
Parihar K, Kumar R, Singh M, Shefali S. Impact of heavy metals on survivability of earthworms impact of heavy metals on survivability of earthworms. Int Medico-legal Report J. 2019;26:2.
Cao D, Wang X, Luo X, Liu G, Zheng H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf Ser Earth Environ Sci. 2017;61(1):6–10.
Kwak JI, An YJ. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J Hazard Mater. 2021;402: 124034.
Jiang X, Chang Y, Zhang T, Qiao Y, Klobučar G, Li M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ Pollut. 2020;259: 113896.
Lahive E, Walton A, Horton AA, Spurgeon DJ, Svendsen C. Microplastic particles reduce reproduction in the terrestrial worm Enchytraeus crypticus in a soil exposure. Environ Pollut. 2019;255: 113174.
Husen A, Siddiqi KS. Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol. 2014;12(1):1–10.
De Silva YSK, Rajagopalan UM, Kadono H. Microplastics on the growth of plants and seed germination in aquatic and terrestrial ecosystems. Glob J Environ Sci Manag. 2021;7(3):347–68.
Bosker T, Bouwman LJ, Brun NR, Behrens P, Vijver MG. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere. 2019;226:774–81. https://doi.org/10.1016/j.chemosphere.2019.03.163.
Yu H, Zhang X, Hu J, Peng J, Qu J. Ecotoxicity of polystyrene microplastics to submerged carnivorous Utricularia vulgaris plants in freshwater ecosystems. Environ Pollut. 2020;265: 114830. https://doi.org/10.1016/j.envpol.2020.114830.
Dong Y, Gao M, Qiu W, Song Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol Environ Saf. 2021;211: 111899. https://doi.org/10.1016/j.ecoenv.2021.111899.
Li B, Huang S, Wang H, Liu M, Xue S, Tang D. Effects of plastic particles on germination and growth of soybean (Glycine max): a pot experiment under field condition. Environ Pollut. 2021;272: 116418. https://doi.org/10.1016/j.envpol.2020.116418.
Lehmann A, Leifheit EF, Feng L, Bergmann J, Wulf A, Rillig MC, et al. Microplastic fiber and drought effects on plants and soil are only slightly modified by arbuscular mycorrhizal fungi. Soil Ecol Lett. 2020;4:1–3.
Qi Y, Yang X, Mejia A, Huerta E, Beriot N, Gertsen H, et al. Macro- and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci Total Environ. 2018;645:1048–56. https://doi.org/10.1016/j.scitotenv.2018.07.229.
Abbasi S, Moore F, Keshavarzi B, Hopke PK, Naidu R, Mahmudur M, et al. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci Total Environ. 2020;744: 140984. https://doi.org/10.1016/j.scitotenv.2020.140984.
Li S, Wang T, Guo J, Dong Y, Wang Z, Gong L. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J Hazard Mater. 2021;5(415): 125614.
Li Z, Li Q, Li R, Zhou J, Wang G. The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ Sci Pollut Res. 2021;28(13):16042–53.
Gao M, Liu Y, Song Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere. 2019;237: 124482. https://doi.org/10.1016/j.chemosphere.2019.124482.
Chen Y, Li Y, Liang X, Lu S, Ren J, Zhang Y, Han Z, Gao B, Sun K. Effects of microplastics on soil carbon pool and terrestrial plant performance. Carbon Res. 2024;3:1–23. https://doi.org/10.1007/s44246-024-00124-1.
Oliveira M, Ameixa OMCC, Soares AMVM. Are ecosystem services provided by insects “bugged” by micro (nano)plastics? TrAC Trends Anal Chem. 2019;113:317–20. https://doi.org/10.1016/j.trac.2019.02.018.
Mühlschlegel P, Hauk A, Walter U, Sieber R. Lack of evidence for microplastic contamination in honey. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34(11):1982–9. https://doi.org/10.1080/19440049.2017.1347281.
Bläsing M, Amelung W. Plastics in soil: analytical methods and possible sources. Sci Total Environ. 2018;612:422–35. https://doi.org/10.1016/j.scitotenv.2017.08.086.
Tariq M, Iqbal B, Khan I, Khan AR, Jho EH, Salam A, Zhou H, Zhao X, Li G, Du D. Microplastic contamination in the agricultural soil—mitigation strategies, heavy metals contamination, and impact on human health: a review. Plant Cell Rep. 2024;43(3):1–15. https://doi.org/10.1007/s00299-024-03162-6.
Chia RW, Lee JY, Jang J, Kim H, Kwon KD. Soil health and microplastics: a review of the impacts of microplastic contamination on soil properties. J Soils Sediments. 2022. https://doi.org/10.1007/s11368-022-03254-4.
An L, Liu Q, Deng Y, Wu W, Gao Y, Ling W. Sources of microplastic in the environment. In: The handbook of environmental chemistry. Berlin: Springer; 2024. p. 143–59. https://doi.org/10.1007/698_2020_449.
Yu Y, Li J, Song Y, Zhang Z, Yu S, Xu M, et al. Stimulation versus inhibition: the effect of microplastics on pak choi growth. Appl Soil Ecol. 2022;177: 104505. https://doi.org/10.1016/j.apsoil.2022.104505.
Bastida F, Eldridge DJ, García C, Kenny Png G, Bardgett RD, Delgado-Baquerizo M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021;15(7):2081–91. https://doi.org/10.1038/s41396-021-00906-0.
Yu H, Zhang Y, Tan W. The, “neighbor avoidance effect” of microplastics on bacterial and fungal diversity and communities in different soil horizons. Environ Sci Ecotechnol. 2021;8: 100121. https://doi.org/10.1016/j.ese.2021.100121.
Miao L, Wang P, Hou J, Yao Y, Liu Z, Liu S, et al. Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ. 2019;650:2395–402. https://doi.org/10.1016/j.scitotenv.2018.09.378.
Hou J, Xu X, Yu H, Xi B, Tan W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ Int. 2021;149: 106398. https://doi.org/10.1016/j.envint.2021.106398.
Wijesooriya M, Wijesekara H, Sewwandi M, Soysa S, Rajapaksha AU, Vithanage M, et al. Microplastics and soil nutrient cycling. In: Microplastics in the ecosphere: air, water, soil, and food. Hoboken: Wiley; 2023. p. 321–38.
Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut. 2019;254: 112983. https://doi.org/10.1016/j.envpol.2019.112983.
Zhang S, Pei L, Zhao Y, Shan J, Zheng X, Xu G, et al. Effects of microplastics and nitrogen deposition on soil multifunctionality, particularly C and N cycling. J Hazard Mater. 2023;451: 131152. https://doi.org/10.1016/j.jhazmat.2023.131152.
Qian H, Zhang M, Liu G, Lu T, Qu Q, Du B, et al. Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut. 2018;229(8):1–11.
Rong L, Zhao L, Zhao L, Cheng Z, Yao Y, Yuan C, et al. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci Total Environ. 2021;773: 145640. https://doi.org/10.1016/j.scitotenv.2021.145640.
Seeley ME, Song B, Passie R, Hale RC. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat Commun. 2020;11(1):2372. https://doi.org/10.1038/s41467-020-16235-3.
Qin R, Su C, Liu W, Tang L, Li X, Deng X, et al. Effects of exposure to polyether sulfone microplastic on the nitrifying process and microbial community structure in aerobic granular sludge. Bioresour Technol. 2020;302: 122827. https://doi.org/10.1016/j.biortech.2020.122827.
Chen H, Wang Y, Sun X, Peng Y, Xiao L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere. 2020;243: 125271. https://doi.org/10.1016/j.chemosphere.2019.125271.
Wang X, Xing Y, Lv M, Zhang T, Ya H, Jiang B. Recent advances on the effects of microplastics on elements cycling in the environment. Sci Total Environ. 2022;849(August): 157884. https://doi.org/10.1016/j.scitotenv.2022.157884.
Rillig MC, Leifheit E, Lehmann J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021;19(3):1–9. https://doi.org/10.1371/journal.pbio.3001130.
Rillig MC. Microplastic disguising as soil carbon storage. Environ Sci Technol. 2018;2:6079–80.
De Souza Machado AA, Lau CW, Kloas W, Bergmann J, Bachelier JB, Faltin E, Becker R, Görlich AS, Rillig MC. Microplastics can change soil properties and affect plant performance. Environ Sci Technol. 2019;53(10):6044–52.
Guo QQ, Xiao MR, Ma Y, Niu H, Zhang GS. Polyester microfiber and natural organic matter impact microbial communities, carbon-degraded enzymes, and carbon accumulation in a clayey soil. J Hazard Mater. 2021;5:405.
Lützow MV, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Euro J Soil Sci. 2006;57(4):426–45.
Liu H, Yang X, Liu G, Liang C, Xue S, Chen H, et al. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere. 2017;185:907–17.
Hu D, Shen M, Zhang Y, Zeng G. Micro (nano) plastics: an un-ignorable carbon source? Sci Total Environ. 2019;20(657):108–10.
Zang H, Zhou J, Marshall MR, Chadwick DR, Wen Y, Jones DL. Microplastics in the agroecosystem: are they an emerging threat to the plant–soil system? Soil Biol Biochem. 2020;148(July): 107926. https://doi.org/10.1016/j.soilbio.2020.107926.
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L. Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–86.
Zhou J, Wen Y, Marshall MR, Zhao J, Gui H, Yang Y, et al. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci Total Environ. 2021;787: 147444. https://doi.org/10.1016/j.scitotenv.2021.147444.
Yu H, Fan P, Hou J, Dang Q, Cui D, Xi B, et al. Inhibitory effect of microplastics on soil extracellular enzymatic activities by changing soil properties and direct adsorption: an investigation at the aggregate-fraction level. Environ Pollut. 2020;267: 115544. https://doi.org/10.1016/j.envpol.2020.115544.
Yan Y, Chen Z, Zhu F, Zhu C, Wang C, Gu C. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull Environ Contam Toxicol. 2021;107(4):602–9. https://doi.org/10.1007/s00128-020-02900-2.
Qu Q, Zhang Z, Peijnenburg WJGM, Liu W, Lu T, Hu B, et al. Rhizosphere microbiome assembly and its impact on plant growth. J Agric Food Chem. 2020;68(18):5024–38.
Yang J, Yang Y, Wu WM, Zhao J, Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol. 2014;48(23):13776–84.
Calero M, Godoy V, Quesada L, Martín-Lara MÁ. Green strategies for microplastics reduction. Curr Opin Green Sustain Chem. 2021;28: 100442. https://doi.org/10.1016/j.cogsc.2020.100442.
Wu WM, Yang J, Criddle CS. Microplastics pollution and reduction strategies. Front Environ Sci Eng. 2017;11(1):1–4.
Singh SS, Thakur A, Sandilya S, Kumar A. Recent advances in bioplastics: synthesis and emerging perspective. Iran J Chem Chem Eng. 2022;41(8):2704–27.
Sandilya S, Thakur A, Suresh Singh S, Kumar A. Recent advances, synthesis and characterization of bio-based based polymers from natural sources. Plant Cell Biotechnol Mol Biol. 2021;22:70–93.
Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science. 2016;351(6278):1196–9.
Acknowledgements
The authors are thankful to the Department of Forestry, Mizoram University for providing all possible support to carry out this work.
Funding
The corresponding author extends gratitude towards the Department of Science & Technology, New Delhi for providing financial support in the form of a major research project no. DST/CCP/MRDP/193/2019(G).
Author information
Authors and Affiliations
Contributions
Salam Suresh Singh conceptualized the idea, carried out the experiment, analyzed and interpreted the data, and wrote the paper. Shri Kant Tripathi acquired funding, supervised and helped in writing the manuscript. Rajdeep Chanda, Ngangbam Somen Singh, Ramthar Mawi, Ningthoujam Ranjana Devi, Khoisnam Vramari Devi and Keshav Kumar Upadhyay assisted in writing, reviewing and proofreading of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Singh, S.S., Chanda, R., Singh, N.S. et al. Microplastic pollution: exploring trophic transfer pathways and ecological impacts. Discov Environ 2, 103 (2024). https://doi.org/10.1007/s44274-024-00139-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00139-w