Abstract
One of the monumental environmental and public health concerns of our time lies in the use of toxic preservatives in wood treatment plants. In this investigation, we report the results of potentially toxic heavy metals from wood treatment plants in Uasin Gishu County. A 20 g ground and sieved soil sample from each sampling site Cheplaskei (CK), Outspan (OS) and Sukunanga (SK) was weighed and treated with 0.5 M nitric acid for 2 h. The sample was then analyzed for potentially toxic metals using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The statistical treatment of the data was done using Principal component analysis (PCA) and Pearson’s correlation. The highest concentration of copper was observed at SK, which contributed approximately 37% of all the potentially toxic heavy metal content analyzed, whereas the concentration of lead was found to be about 33% of the total heavy metal content analyzed at the same sampling site. Furthermore, the most abundant metal in the sampled sites is manganese, which was found to be 390.0 ± 8.63, 279.0 ± 8.05and 44.5 ± 2.95 ppm in OS, CK, and SK, respectively. PCA showed that the heavy metals in the sample sites originated from two independent sources—natural and anthropogenic. Evidently from the concentration profile data, all the potentially toxic heavy metals had concentrations above the World Health Organization (WHO) acceptable limits, although, based on the contamination factors determined, the wood treatment sites are less polluted; however, there is need for regular monitoring to ensure adherence to proper public and environmental health practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The contamination of soil caused by human activities is a long-standing problem that presents a significant challenge to eliminate [1]. For instance, toxic heavy metals and potentially hazardous organic contaminants are recalcitrant and remain in the soil for many decades [1, 2]. Presently, one of the key threats to soil and plant quality is contamination by presumably contaminating elements such as lead and cadmium [1,2,3]. Their levels above the acceptable limits are harmful to all living organisms, including higher order animals such as humans. Heavy metals exist naturally in the environment, but enter water bodies, air and soil due to human activities, resulting in one of the major global challenges of our time [4, 5]. They are equally toxic to both plants and animals as most of them have no role once inside the plant and human tissues [4]. Therefore, research is focused on studying ways to not only accurately identify unwanted risk elements in the soil but also minimize their hazards. Healthy soil is not only the foundation of human health but also desirable for crop production [6]. Furthermore, soil is critical as a water storage system, and decarbonizes the environment in order to enhance climate adaptation and mitigate climate change.
Wood treatment is a vital activity carried out to enhance the durability of timber and wood with the sole purpose of protecting them from marine borers, insects and minimizing rotting [7]. The oxides of Cr (IV) (47.5%), Cu (18.5%), and inorganic As (34%) are mixed in water to prepare wood preservative, referred to as chromated copper arsenate (CCA) [8, 9]. Copper, a potentially toxic heavy metal, is responsible for protecting the wood against microorganism decay, such as fungi and bacteria. Arsenic exhibits insecticidal characteristics and is therefore ideal for wood treatment [8, 10]. Moreover, chemical pesticide preservation enables relatively non-resistant wood products to be used in outdoor building establishments. The most widespread approach used to preserve wood is pressure treatment with CCA [10]. Accordingly, there is increasing concern about the use of CCA in timber treatment because of the possible leaching of toxic metals into the soil environment and ultimately into the water systems [11]. In contrast to alternative construction materials, wood has attractive features including favorable tensile strength, good machinability, better insulation properties compared to other materials; however, it requires preservation because of its biodegradable properties especially under harsh conditions [12]. Accordingly, wood preservation strategies should be ecofriendly, safe and affordable [8, 12]. Conventional wood treatment chemicals such as the use of CCA have been forbidden in a number of developed countries including European countries as well as the United States because of their toxic components—arsenic and chromium [12].
Generally, potentially toxic heavy metals are well-established environmental contaminants because of their refractory nature in the environment and their ability to bio-accumulate in human tissues to cause serious etiological problems [5, 13, 14]. Toxic heavy metal pollution is a precursor for deleterious environmental and public health consequence [5, 15]. Potentially toxic heavy metals originate from two main sources—anthropogenic and natural [5, 11]. Once ingested into the body system, heavy metals bio-accumulate in plant tissues, and may affect ecological processes such as photosynthesis and mineral uptake [4]. On the other hand, they have the potential to damage organs such as the kidney, liver in animals, and are precursors for serious diseases such as cancer [4, 16]. Nevertheless, health risks caused by heavy metal toxicity are dose, time and concentration dependent [4]. It is essential to note that soils absorb and sequester potentially toxic metals, which might be released into water systems, and may be cause serious etiological risks to both animals and plants [17].
The use of wood treatment preservatives, especially in developing countries is highly unregulated, and this has led to serious environmental health problems. Ultimately, the ripple effect is the bio-magnification of the toxic heavy metals up the food chain leading to serious health consequences. Nonetheless, some heavy metals, such as zinc, iron and copper are required by many organs both in plants and humans for biological functions but in very low concentrations; however, they become toxic when their concentrations exceed the recommended levels [4]. The findings obtained in this study can inform the development of utilization possibilities for CCA-treated timber wastes [11]. Accordingly, soil protection policies should focus on the use of eco-friendly wood treatment practices in order to foster better climate adaptation and better environmental health. This study is important especially because Uasin Gishu is an agricultural county, dubbed the Kenyan food basket. Therefore, any soil contamination as a result of potentially toxic heavy metals can be detrimental to public health and food safety, and may have a significant impact on health facilities and the general provision of healthcare. Accordingly, there is a need for environmental management authority to continuously monitor wood treatment plants to ensure compliance to environmental protection laws.
2 Materials and methods
2.1 The study area
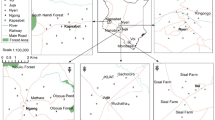
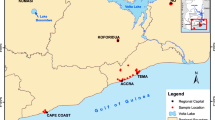
This study was conducted at some selected wood treatment sites in Uasin Gishu County, Kenya—located approximately 400 km west of Nairobi at an altitude of 2100 m. Geographically, the study area is located between 0° 25′ 0″ and 0\(^\circ \) 35′ 0″ North latitude, and 36\(^\circ \) 15′ 0″ and 36\(^\circ \) 20′ 0″ East longitude as shown in Fig. 1.
The study area focuses on wood treatment sites in Uasin Gishu County, which are a source of environmental health pollution. The study will capture selected wood treatment sites in Uasin Gishu County, which are located in SK, CK, and OS. The sampling points were located using a global information system (GIS). The use of wood treatment chemicals is a potential source of potentially toxic heavy metals in the soils around these treatment sites. This has affected the quality of the soils around the wood treatment facilities and ultimately the water ecosystem.
2.2 Reagents and chemicals
The purity of 10% nitric acid was ≥ 99%. Reagents and materials used in this study were acquired from Kobian Scientific, Kenya, Ltd Company—a subsidiary of Sigma-Aldrich Ltd., South Africa [18]. All the reagents were of analytical grade unless otherwise specified [19]. The reagents and analytical procedures that were utilized in this study are reported in literature [20].
2.3 Sample collection and sample handling
The soil sample was collected during the dry season in August 2023 from three sampling sites in replicates. A total of 12 samples—four from each wood treatment site were collected. Samples were screened for stones and debris, and transported to the laboratory in sterilized polythene bags where they were air dried at 25 °C in a dark room for 96 h, followed by grinding, and sieving using a molecular sieve of 45 μm. A 20 g sample was then weighed using an analytical balance and treated with 0.5 M nitric acid for 2 h on a hot plate set at 70 °C.
2.4 Recovery test and method validation
Calibration plots were constructed from the pure standards of the target metals—Cu, Fe, Cr, Mn, Zn and Pb. The correlation coefficient for the linear plots was, \(r\ge 0.989\). For accurate determination of ICP-AES, soil samples were spiked and relative recoveries of between 95.2% and 107.4% were obtained showing that the method used was remarkably accurate. The percentage recoveries were evaluated using Eq. 1.
where, C denotes concentration.
The limit of quantitation of ICP-AES was determined by computing the minimum limits of quantitation (MLD) – limit of detection (LOD) and limit of quantification (LOQ) using blank solutions. Accordingly, the MLD were evaluated following the procedures adopted by the International Union of Pure and Applied Chemistry (IUPAC) [21] using Eqs. 2 and 3.
Here, s denotes the slope of the plot for each target element while \(\delta \) is the standard deviation of the measured blank solution.
In order to assess the linearity of the method, calibration plots for each target heavy metal were prepared by plotting the peak area of the optimal emission lines as a function of the concentration of the standard solution. Ultimately, regression analysis was done to determine the y-intercept, the slope and the regression coefficients.
2.5 Analysis of heavy metals
Potentially toxic metals were analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES)–ICP-9000 according to the procedure adopted by Oyugi et al. [18]. Ultra-pure helium (99.999%) was used as the plasma, atomizer, and auxiliary gas. The nebulizer, plasma, and auxiliary gas flow rates were respectively, 0.90 L/min, 10.0 L/min and 0.60 L/min. The sensitivity of the instrument was 0.055 ppm, the detection limits of metals analyzed were Cu (0.004 ppm), Cr (0.001 ppm), Pb (0.005 ppm), Mn (0.004 ppm), Zn (0.002 ppm) and Fe (0.001 ppm), the plasma power was 1300 W and the optimum working range of the instrument was between 0.001 and 20.0 ppm. A gold coating of 9 nm (prepared using 6 nm by 1.5 nm layers) was applied and adhered to aluminium stubs to reduce charging of the non-electrically conductive particles. The grids were allowed to dry in air before putting them into the analysis chamber of a Zeiss Ultra Plus (Germany) field emission gun scanning electron microscope (FEG SEM) [22]. To aid in the identification of the sample, Noran system, version 3.1 ultra-dry SSD X-ray detector (EDX) was used to perform elemental analysis. For enhanced image quality, a second sample was coated with a 3 nm gold layer in order to obtain higher resolution images. All images were taken at an angle of 45o for a better definition of surface topology [23]. All the analyses were conducted in triplicate.
2.6 Soil size and distribution analysis
Particle soil size distribution analysis was conducted from various SEM micrographs and measured using Image J computer software [24]. Measurements were conducted three times on each soil sample image to guarantee reproducibility. Igor ver. 5.0 (Wave metrics, Lake Oswego, OR, USA) graphing software program was used to estimate the mean soil particle diameter [24].
2.7 Statistical analysis
The statistical analyses reported in this study were conducted using the statistical package for social science (SPSS) ver. 25.0. The mean and standard deviation were determined for purposes of descriptive analysis. Variations in f target heavy metal concentration in various wood sampling sites was subjected to multivariate statistical analysis—the Pearson correlation analysis and the principal component analysis (PCA), in order to predict the sources of the potentially heavy metals in the wood treatment facilities.
3 Results and discussion
Potentially toxic heavy metals have increased in the environment, and their etiological effects have been a considerable health concern globally because they are phototoxic and can act as micro-pollutants [25,26,27,28]. Cadmium, lead, nickel, chromium, and arsenic present serious challenges to human health and natural ecosystems. Potentially toxic heavy metals undergo neither microbial nor chemical degradation, unlike organic contaminants, which are easily degraded by microbial activities. The non-biodegradability of toxic heavy metals results in their bioaccumulation in food chains with disastrous effects on animals and humans. High concentrations of essential elements such as Mn, Zn, or Cr can cause morphological, biochemical, and physiological alterations in plants and animals [29,30,31,32]. Waste disposal of wood-preservation effluent is a scientific challenge, despite the several studies that have been conducted over the years to investigate the effects of heavy metals on natural ecosystems [33,34,35].
3.1 Heavy metal concentration profiles
According to Table 1, the highest concentration of copper was observed at SK, which contributed approximately 37% of all the heavy metal content analyzed in the wood treatment site. Also, the concentration of lead was found to be about 33% of the total heavy metal content analyzed in the same sampling site. The most abundant trace metal at the sampled sites is manganese which was found to be 39.0 ± 2.58, 279.0 ± 8.05and 44.5 ± 2.95 ppm in OS, CK, and SK, respectively. It is evident from the data that all the heavy metals determined had concentrations way above the WHO acceptable limits. For instance lead was 118, 283 and 7510 times higher than the acceptable WHO limits in CK, OS and SK wood treatment sites, respectively. On the other hand, Chromium was, respectively 780, 270 and 64 above the WHO acceptable limits. Also, copper exceeded the WHO allowable limits by approximately 8.2, 3.9 and 42.5, in CK, OS and SK, respectively. These results show that the soils around the selected wood treatment sites are highly toxic and not suitable for any agricultural enterprise. During rainy seasons, the heavy metals can be washed down the nearby River Sosiani, and this may lead to serious contamination of the river.
Zinc, manganese, and iron are beneficial to the human biological system. Nevertheless, excess concentrations may have serious health impacts. For example, manganese is an essential nutrient that is important for metabolism, development, and the antioxidant in human health, but excessive ingestion can result in manganism, a neurodegenerative condition that can result in Parkinsonian disease-like disorder [16]. Elevated Mn in the body can damage the nervous system and lead to motor impairment [13, 16]. Generally, nine potentially toxic heavy metals such as manganese, cobalt, iron, chromium, vanadium, molybdenum, copper, nickel and zinc play essential roles as building blocks for organic biomolecules as well as in regulating biological functions [13, 15]. It is important to note also that, the antioxidant, membrane-protective, enhancing immunity, hormonal functions of trace metals, and anti-bacterial, and anti-viral properties of copper and its compounds is critical in biological functions, and the sustainability of life [15].
Trace elements account for about 0.1% of the human biological system and are also essential for the existence of lower organisms, being necessary for proper development, cell metabolism, reproduction, and immune function [36]. Excess iron deposits may also cause serious disorders such as organ failure. The disease associated with excess iron is called hemochromatosis, affects the liver and pancreas, and occurs when there are high pathologic levels of iron accumulation in the body [37]. Moreover, zinc has been reported to be an essential trace metal necessary for the growth of organisms; however, its toxicity may present several symptoms such as fever, breathing difficulty, nausea, chest pain, and cough [38].
3.2 Contamination factor, degree of contamination and pollution load index
Health risk assessment of potentially toxic heavy metals in soils associated with human and environmental health may furnish information relating to exposure to contaminants [17, 39]. The contamination factor (CF) which accounts for contamination by a single heavy metal was determined using Eq. 4.
Here, \({C}^{i}\) is the concentration of the potentially toxic heavy metals in soil sample while \({C}_{n}^{i}\) is the standard pre-industrial standard concentrations in ppm—70 for Pb, 90 for Cr, 50 for Cu, 175 for Zn, 850 for Mn [40, 41]. The calculated contamination factors for potentially toxic metals obtained from the wood treatment sites are reported in Table 2.
It is evident from Table 2 that \({C}_{f}^{i}\) of most metals is \(\le 1\), generally implying low level of pollution. Nevertheless, the \({C}_{f}^{i}\) of Cu and Pb in SK wood treatment facility were \(<3\ge 1\), suggesting medium pollution [41, 42]. The sum of the \({C}_{f}^{i}\) for the elements investigated gives the degree of contamination \(Cdeg\) which is determined by Eq. 5.
The data associated with \(Cdeg\) is reported in Table 2. Evidently, Cr, Zn, Mn and Pb exhibit low degree of contamination because \(Cdeg<1.5.\) On the other hand, \(2<Cdeg<4\), implying average level of contamination [41, 42].
The pollution load index (PLI) is calculated in order to assess the level of contamination of potentially toxic heavy metals in a sample. The values of the PLI range from zero to 10, which represent non-contaminated and highly contaminated, respectively [42]. Generally, values of PLI < 1 suggest non-contamination, while values of PLI > 1 imply contamination. PLI can be determined from Eq. 6.
The calculated PLI values for the metals investigated were 0.10, 0.13 and 0.15 respectively, for CK, OS and SK. These values give the impression that the site quality is good, and no significant contamination is observed. It should be noted the contamination factor and pollution load index of Fe was not determined because it is not suitable in this context [41].
3.3 Multivariate analysis of heavy metals in wood treatment sites
In order to postulate the source of the potentially toxic heavy metals investigated, PCA was applied to the data set obtained. The data extracted from PCA is given in Table 3. The first two principal components (PCs) with eigenvalues greater than one accounted for 99.99% of the total variance. The heavy metals including Mn, Cr, and Fe demonstrated considerable positive loading values ≥ 0.91 in PC1which accounted for 81.70% of the total variance.
PC2 shows 18.29% of the total variability for metals such as Cu, Pb, and Zn with high rotated positive loading values ≥ 0.40, as observed in Fig. 2. This further demonstrates that metals, Mn and Cr, originate from the same source possibly human activities such as wood preservation chemicals such as CCA. However, the data reported in Table 3 indicates that PC2 is negatively loaded with Mn and Cr. The component scree plots as depicted in Fig. 3 show that the potentially toxic heavy metals from the sample sites may have originated from two sources—natural and anthropogenic.
3.4 Pearson correlation analysis
Pearson correlation analysis was used to determine the combinations of the metal species in wood treatment sites and the results are displayed in Table 4. It is an effective tool for assessing the correlation between variables particularly the metal species. In other words, high Pearson correlation coefficients between heavy metals suggest that target metals may have a similar origin. The positive and negative correlation coefficient data indicate a significantly strong correlation among the concentrations of heavy metals. For instance, Pb showed the highest correlation with Cu suggesting they originate from a similar source. On the other hand, Mn and Fe exhibited a strong positive correlation with Cr, suggesting a similar origin. Also, Mn and Fe showed a strong negative correlation with Pb implying independent sources in the wood treatment plants.
The PCA and the highest positive correlation values of 0.9996 between Cr and Mn (cf. Fig. 2); with coefficient values of 0.9952 between Pb and Cu as reported in Table 3, evidently demonstrate that the two metals are derived from the same source. Considerable correlations were noted between target heavy metals within the same principal components, indicating that the data obtained from Pearson’s correlation analysis and PCA are in agreement.
3.5 Surface topology and elemental analysis of soil samples in the study area
Among several microstructural analyzes, SEM is very important, because the soil microstructure formed by clay particles and additives can be observed in detail [43]. It can also be shown that the contrast of elemental peak height ratios analysed using EDX is important for rapid screening of soil samples, particularly when combined with other soil qualities such as composition, fabric, shapes and surface texture of individual particles within the soil traces [44]. Nevertheless, better resolution and more accurate elemental data analysis methods such as ICP-AES are employed.
Figures 4, 5, 6 show the surface morphology of the soil samples collected from CK, OS and SK, respectively. The micrographs were obtained using a scanning electron microscope. This was done in order to assess the microstructure of a soil at the different wood treatment plants. It was observed that the soil texture and particle distribution at the three wood treatment sites were different. For instance, the soil texture in CK (Fig. 4) is compact and may suggest that, clay soil is dominant in the wood treatment site. On the other hand, the soil texture in OS is dominated by particulates suggesting a porous type of soil, probably a loam type of soil or a mixture of sand and loam soils. For this reason, it is expected that wood treatment waste may percolate into the soil and easily wash away as run-off during rainy seasons. Also, the water holding capacity may be low, and this may be attributed to the coarse sand particles. Nonetheless, wood treatment waste can still stick to the soil particles.
The shape of the soil particles and particle arrangement display the soil microstructure and macroscopic properties such as permeability, compressibility and strength. Reduced pore spaces harden the soil structures, increase strength and decreases the hydraulic conductivity coefficient [43]. This phenomenon can be observed at the OS wood treatment site according to Fig. 5. The soil texture observed in Fig. 6 is intermediate between compact and porous, characteristic of a mixture of loam and clay soils.
According to Table 5, the SEM–EDX outcomes depicted an approximate percent elemental composition as: C (17%), O (42%), Si (17%), Al (14%), Fe (7%), K (1.3%), As (0.46%), Ti (0.04%), and Cr (0.46%) for CK. The other sampled sites reported varying percentage compositions of the elements reported in CK wood treatment plant. The highest percentage of carbon (35.89%) was reported in the SK wood treatment plant, suggesting that the soils around the site may contain high levels of organic contaminants.
Notably, the relative composition of Cr reported in OS (8.91%), the highest in comparison to other wood treatment sites, is consistent with the concentration reported in Table 1. Therefore, it is clear that OS wood treatment sites could be using CCA-based preservatives to treat wood. Based on the data reported in Table 5, SEM–EDX outcomes show that the elements determined may be derived from silicified quartz, feldspar, and iron-bearing minerals that originated from anthropogenic activities, and the presence of carbon in high percent composition may indicate the use of organic-based preservatives in the wood treatment plants.
3.6 Estimation of soil size distribution
The soil size distribution was estimated using Image J software and presented in Figs. 7, 8, 9. Soil size distribution and composition is key in examining the physical properties of soil such as fractal dimensions that can affect different soils and soil fertility [45]. The average estimated soil particle distribution for CK wood treatment facility was found to be 156.20 ± 23.13 nm whereas those of OS and SK were 93.45 ± 17.34 nm and 100.70 ± 16.04 nm, respectively. The particle size distribution curve, obtained from an analyzer such as image J, is largely determined by the media and form of soil particle mobility, mechanical processes and land use. Given various matrices of particle mobility, form and media, the size of the transported particles assumes a normal distribution pattern [46] as can be observed in Figs. 7, 8, 9.
Pollutant bioaccessibility on a given soil size fraction is dependent on potentially toxic elements (PTE), therefore PTE contaminated soils can directly harm humans through oral ingestion, inhalation and skin contact and exposure to polluted soils, that may result in serious health problems [47]. In spite of adsorption or distribution characteristics, fine soil particles largely exhibit a higher capacity to combine heavy metals; although, some studies have observed a contrary opinion, according to which heavy metals are more enriched in coarser particles [47, 48]. This is the case in SK where the soil particles are coarser than those observed in OS sampling sites, yet they contains the relatively higher concentration of heavy metals explored in this work.
Generally, soil properties and composition determine soil erosion, water movement, and particle migration, including retention of microscopic pollutants [49]. Accordingly, the more contaminated the soil is, the heavier the metals that leach into lower genetic levels of the soil [3, 47]. This is consistent with the heavy metal distribution in the SK wood treatment plant.
4 Eco-friendly strategies in wood preservation
Wood being one of the most versatile and green building materials, needs to be treated to prolong its lifespan and durability. With the increasing environmental awareness and the use of low carbon construction methods, the position of wood products in the construction industry has gained significant acceptance [50, 51]. Therefore, significant efforts are being made globally to device alternative wood preservatives based on natural products of low toxicity [12]. Although most of the wood privatives used currently are hazardous, there have been attempts to devise eco-friendly wood treatment approaches that can minimize harm to the environment and public health. As one of the most environmental-friendly approaches to modification, wood silification has been inspired by the petrified wood naturally formed via the permeation of silicic acid in wood over millions of years. Therefore, wood could be silicified through the use sol–gel technology or silica sol penetration [51]. Natural product extracts from plants such as linseed oil, proanthocyanidins or polyflavonoid tannins, diols, and chitosan have been identified to have antifungal and antimicrobial properties that can be used to preserve wood [12, 52]. Wood extractives can protect wood through various mechanisms, such as free radical antioxidants, fungicides, and metal chelation [53]. Nonetheless, combining natural products and synthetic wood preservatives such as biocides can be synergistic in wood preservation and can promise long term protection of wood [54, 55]. Furthermore, thermal and chemical modifications also provide biological resistance and promote the dimensional properties of wood although more knowledge needs to be explored, including safety for the environment [15, 56]. Potentially toxic heavy metals for instance Hg, Cr, As and Fe exhibit reduction and oxidation processes, and therefore, bioremediation strategies convert them into their corresponding soluble and mobile phases [57, 58]. Bioremediation can reduce Fe (III) to Fe (II), As (V) to As (III)and Hg (II) to Hg (0) [59]. Fe (0) nano particles have been effectively used to degrade potentially toxic heavy metals and organic pollutants in soils [57]. This is important in the remediation of contaminated soils around wood treatment plants.
5 Limitations of the study
The wood treatment facilities sampled in this work were limited to three that were within important wetland ecosystems and the major river in the county (River Sosiani), and should have been extended to other wood treatment plants in the entire Uasin Gishu County, especially because lumbering is one of the most important economic activities in the county. Furthermore, the samples were collected during the dry season and therefore the data analyzed cannot completely provide the true status of toxic heavy metal pollution in the wood treatment facilities. Temporal and seasonal variations in heavy metal distribution in the wood treatment facilities were not considered. This was largely necessitated by resource constraints such as funding considering the fact that this was a self-sponsored project. The point of wood waste discharge into nearby streams and swamps common in the study area was not sampled for analysis to determine the extent of heavy metal pollution occasioned by wood treatment facilities in the area because this is part of ongoing research and will be featured in future publications. Nonetheless, this study is detailed, and has included a comprehensive soil analysis of the selected wood treatment sites. Also, a complete analysis of elemental composition has been conducted.
6 Conclusions
This study has found that wood preservatives can leach into the soil to form harmful chemical species, including potentially toxic heavy metals and possible organic contaminants. Herein, we report that the highest concentration of copper was observed at the SK wood treatment plant, which contributed approximately 37% of all the heavy metal content analyzed at the wood treatment site. Also, the concentration of lead was found to be about 33% of the total heavy metal content analyzed at the same sampling site. Furthermore, the most abundant trace metal in the sampled sites is manganese which was found to be 39.0 ± 2.58, 279.0 ± 8.05and 44.5 ± 2.95 ppm in OS, CK, and SK, respectively. It is evident from the data that all the heavy metals determined had concentrations way above the WHO acceptable limits. For instance, lead was approximately118, 283 and 7510 times higher than the acceptable WHO limits in CK, OS and SK wood treatment sites, respectively. On the other hand, chromium was, respectively, 780, 270 and 64 above the WHO acceptable limits. Copper exceeded the WHO allowable limits by approximately 8.2, 3.9 and 42.5, in CK, OS and SK, respectively. It was also noted that the average estimated soil particle distribution for the CK wood treatment facility was 156.20 ± 23.13 nm whereas those of OS and SK were, correspondingly, 93.45 ± 17.34 nm and 100.70 ± 16.04 nm. PCA showed that the heavy metals in the sample sites originated from two independent sources—natural and anthropogenic. Generally, fine soil particles largely exhibit a higher capacity to adsorb heavy metals; although, some studies have observed a contrary opinion, according to which heavy metals may be more enriched in coarser particles, as is the case in this work, where SK had a higher metal concentration than OS. From elemental composition analysis, it was observed that the highest percentage of carbon (35.89%) was reported in the SK wood treatment plant, suggesting that the soils around the site may contain high levels of organic contaminants. During rainy seasons, the heavy metals may be washed down the nearby River Sosiani, and this may lead to serious contamination of the river, and crop produce along the river bank. Ultimately, the ripple effect is the bio-magnification of the toxic heavy metals up the food chain leading to serious health consequences. Clearly, from the results reported, SK wood treatment facility is contaminated with potentially heavy toxic metals, although, based on the contamination factors determined, the wood treatment sites are less polluted; nevertheless, there is need for regular monitoring to ensure adherence to proper public and environmental health practices. Moreover, soil protection policies in wood treatment facilities in the County should focus on the use of eco-friendly wood treatment practices that can enhance food safety, environmental health and climate adaptation.
Data availability
The data associated with the findings of this study are available from the corresponding author upon reasonable request.
References
Odum PU, Ekere NR, Abugu HO, Ihedioha JN, Nwoke SU, Ezike CC, Eze SI. Potential toxic elements load and their health risk assessment in vegetables grown in Nsukka Area of South-Eastern Nigeria. Toxicol Int. 2021;28(2):103–14. https://doi.org/10.18311/ti/2021/v28i2/26074.
Egwuonwu PF, Ihedioha JN, Abugu HO, Ekere NR. Impact of some beverage industries on the physicochemical characteristics of Ajali River in Enugu, Nigeria. Environ Monit Assess. 2021;193(3):136. https://doi.org/10.1007/s10661-021-08912-7.
Pikuła D, Stępień W. Effect of the degree of soil contamination with heavy metals on their mobility in the soil profile in a microplot experiment. Agronomy. 2021. https://doi.org/10.3390/agronomy11050878.
Rehman AU, Nazir S, Irshad R, Tahir K, Ur Rehman K, Islam RU, Wahab Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J Mol Liquids. 2021;321: 114455. https://doi.org/10.1016/j.molliq.2020.114455.
Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA, Simal-Gandara J. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci. 2022;34(3): 101865. https://doi.org/10.1016/j.jksus.2022.101865.
Münzel T, Hahad O. Soil and water pollution and human health: what should cardiologists worry about? Cardiovasc Res. 2023;119(2):440–9. https://doi.org/10.1093/cvr/cvac082.
Li W, Ren D, Zhang X, Wang H, Yu Y. The furfurylation of wood: a nanomechanical study of modified wood cells. BioResources. 2016;11(2):3614–25.
Morais S, Fonseca H, Oliveira SMR. Environmental and health hazards of chromated copper arsenate-treated wood: a review. Int J Environ Res Public Health. 2021;18(11):5518. https://doi.org/10.3390/ijerph18115518.
Chen AY, Olsen T. Chromated copper arsenate-treated wood: a potential source of arsenic exposure and toxicity in dermatology. Int J Women’s Dermatol. 2016;2(1):28–30. https://doi.org/10.1016/j.ijwd.2016.01.002.
Decker P, Cohen B, Butala JH, Gordon T. Exposure to wood dust and heavy metals in workers using CCA pressure-treated wood. AIHA J. 2002;63(2):166–71. https://doi.org/10.1080/15428110208984700.
Liu Y, Du J, Dong Z, Rahman MM, Gao Y, Yan K, Naidu R. Bioavailability and risk estimation of heavy metal(loid)s in chromated copper arsenate treated timber after remediation for utilisation as garden materials. Chemosphere. 2019;216:757–65. https://doi.org/10.1016/j.chemosphere.2018.10.141.
Khademibami L, Bobadilha GS. Recent developments studies on wood protection research in academia: a review. Front For Glob Change. 2022. https://doi.org/10.3389/ffgc.2022.793177.
Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V, Kanthasamy AG. Manganese-induced neurotoxicity: new insights into the triad of protein misfolding, mitochondrial impairment, and neuroinflammation. Front Neurosci. 2019;13: 465315.
Ekere NR, Ugbor MCJ, Ihedioha JN, Ukwueze NN, Abugu HO. Ecological and potential health risk assessment of heavy metals in soils and food crops grown in abandoned urban open waste dumpsite. J Environ Health Sci Eng. 2020;18(2):711–21. https://doi.org/10.1007/s40201-020-00497-6.
Ermakov VV, Jovanović LN. Biological role of trace elements and viral pathologies. Geochem Int. 2022. https://doi.org/10.1134/S0016702922020045.
Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199–227. https://doi.org/10.1007/978-94-007-7500-8_7.
Obayomi OO, Sulaiman MB, Oluwasola HO, Sulaiman AB, Akpomie KG, Odewole OA, Otunomo II, David MK. Ecological risk assessment of potentially toxic elements in the bottom sediments of a stream in Oke-Ere, Kogi State, North Central Nigeria. Int J Environ Sci Technol. 2023;20(12):13107–18. https://doi.org/10.1007/s13762-023-04851-7.
Oyugi AM, Kibet JK, Adongo JO. Analysis of the concentration of heavy metals in Khat Grown in Meru County and the assessment of their associated health risks. Int J Anal Chem. 2024;2024:6688184. https://doi.org/10.1155/2024/6688184.
Chebet E, Mbui D, Kibet J, Kamau G. The speciation of selected trace metals in Nairobi river water, Kenya. Eurasian J Anal Chem. 2018;13:4.
Konieczka P, Namiesnik J. Quality assurance and quality control in the analytical chemical laboratory: a practical approach. Boca Raton: CRC Press; 2016.
Manousi N, Zachariadis GA. Development and application of an ICP-AES method for the determination of nutrient and toxic elements in savory snack products after autoclave dissolution. Separations. 2020;7(4):66.
Peksa AE, Wolf K-HAA, Slob EC, Chmura Ł, Zitha PLJ. Original and pyrometamorphical altered Bentheimer sandstone; petrophysical properties, surface and dielectric behavior. J Petrol Sci Eng. 2017;149:270–80. https://doi.org/10.1016/j.petrol.2016.10.024.
Poynton SD, Slade RC, Omasta TJ, Mustain WE, Escudero-Cid R, Ocón P, Varcoe JR. Preparation of radiation-grafted powders for use as anion exchange ionomers in alkaline polymer electrolyte fuel cells. J Mater Chem A. 2014;2(14):5124–30.
Maiyo AK, Korir BK, Kibet JK. The impact of puff frequency on respirable particulate matter in mainstream cigarette smoke. Clin Respir J. 2023;17(4):286–94. https://doi.org/10.1111/crj.13592.
Li G, Sun GX, Ren Y, Luo XS, Zhu YG. Urban soil and human health: a review. Eur J Soil Sci. 2018;69(1):196–215.
Tahir MB, Kiran H, Iqbal T. The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: a review. Environ Sci Pollut Res. 2019;26(11):10515–28.
Khan NA, Khan SU, Ahmed S, Farooqi IH, Yousefi M, Mohammadi AA, Changani F. Recent trends in disposal and treatment technologies of emerging-pollutants-a critical review. TrAC, Trends Anal Chem. 2020;122: 115744.
Werkneh AA, Rene ER. Applications of nanotechnology and biotechnology for sustainable water and wastewater treatment. In: Water and Wastewater Treatment Technologies. pp. 405–430. Springer, (2019)
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif M. Morphological, physiological and biochemical responses of different plant species to Cd stress. Int J Chem Biochem Sci. 2013;3(2013):53–60.
Kanwal U, Ali S, Shakoor MB, Farid M, Hussain S, Yasmeen T, Adrees M, Bharwana SA, Abbas F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ Sci Pollut Res. 2014;21(16):9899–910.
Maleki M, Ghorbanpour M, Kariman K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene. 2017;11:247–54.
Rai PK. Impacts of particulate matter pollution on plants: implications for environmental biomonitoring. Ecotoxicol Environ Saf. 2016;129:120–36.
Igiri BE, Okoduwa SI, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J Toxicol. 2018. https://doi.org/10.1155/2018/2568038.
Al Naggar Y, Khalil MS, Ghorab MA. Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc J Toxicol. 2018;3: 555603.
Pujari M, Kapoor D. Heavy metals in the ecosystem: sources and their effects. In: Heavy metals in the environment. pp. 1–7. Elsevier, (2021)
Gumienna-Kontecka E, Rowińska-Żyrek M, Łuczkowski M. The role of trace elements in living organisms. In: Recent advances in trace elements. pp. 177–206. (2018)
Ponka P. Rare causes of hereditary iron overload. Semin Hematol. 2002;39(4):249–62. https://doi.org/10.1053/shem.2002.35638.
Hussain S, Khan M, Sheikh TMM, Mumtaz MZ, Chohan TA, Shamim S, Liu Y. Zinc Essentiality, toxicity, and its bacterial bioremediation: a comprehensive insight. Front Microbiol. 2022;13: 900740.
Sulaiman MB, Adamu AM, Ali SB, Ezenobi UV, Gimba AM, Akinlotan OO, Abubakar A. Heavy metal contamination in medicinal plants: assessing carcinogenic and non-carcinogenic health risks. Discov Environ. 2024;2(1):11. https://doi.org/10.1007/s44274-024-00035-3.
Rahman MS, Ahmed Z, Seefat SM, Alam R, Islam ARMT, Choudhury TR, Begum BA, Idris AM. Assessment of heavy metal contamination in sediment at the newly established tannery industrial Estate in Bangladesh: a case study. Environ Chem Ecotoxicol. 2022;4:1–12. https://doi.org/10.1016/j.enceco.2021.10.001.
Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980;14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8.
Hamid E, Payandeh K, Karimi Nezhad MT, Saadati N. Potential ecological risk assessment of heavy metals (trace elements) in coastal soils of southwest Iran. Front Public Health. 2022;10: 889130. https://doi.org/10.3389/fpubh.2022.889130.
Ural N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: an overview. Open Geosci. 2021;13(1):197–218. https://doi.org/10.1515/geo-2020-0145.
Pye K, Croft D. Forensic analysis of soil and sediment traces by scanning electron microscopy and energy-dispersive X-ray analysis: an experimental investigation. Forensic Sci Int. 2007;165(1):52–63. https://doi.org/10.1016/j.forsciint.2006.03.001.
Qi F, Zhang R, Liu X, Niu Y, Zhang H, Li H, Li J, Wang B, Zhang G. Soil particle size distribution characteristics of different land-use types in the Funiu mountainous region. Soil Tillage Res. 2018;184:45–51. https://doi.org/10.1016/j.still.2018.06.011.
Yan B, Zhang Y, Zang S, Chen Q, Sun L. Distributions of particle sizes in black soil and their environmental significance in Northeast China. Sustainability. 2021. https://doi.org/10.3390/su13073706.
Li Y, Padoan E, Ajmone-Marsan F. Soil particle size fraction and potentially toxic elements bioaccessibility: a review. Ecotoxicol Environ Saf. 2021;209: 111806. https://doi.org/10.1016/j.ecoenv.2020.111806.
Wang J, Qin M, Huang T, Tu N, Li B. Particle size distribution and pollutant dissolution characteristics of road-deposited sediment in different land-use districts: a case study of Beijing. Environ Sci Pollut Res. 2021;28(29):38497–505. https://doi.org/10.1007/s11356-021-13426-3.
Hu H, Tian F, Hu H. Soil particle size distribution and its relationship with soil water and salt under mulched drip irrigation in Xinjiang of China. Sci China Technol Sci. 2011;54(6):1568–74. https://doi.org/10.1007/s11431-010-4276-x.
Järvinen J, Ilgın HE, Karjalainen M. Wood preservation practices and future outlook: perspectives of experts from Finland. Forests. 2022. https://doi.org/10.3390/f13071044.
Jiang J, Wang C, Ebrahimi M, Shen X, Mei C. Eco-friendly preparation of high-quality mineralized wood via thermal modification induced silica sol penetration. Ind Crops Prod. 2022;183: 115003. https://doi.org/10.1016/j.indcrop.2022.115003.
Gérardin P. New alternatives for wood preservation based on thermal and chemical modification of wood—a review. Ann For Sci. 2016;73(3):559–70. https://doi.org/10.1007/s13595-015-0531-4.
Schultz TP, Nicholas DD. Development of environmentally-benign wood preservatives based on the combination of organic biocides with antioxidants and metal chelators. Phytochemistry. 2002;61(5):555–60. https://doi.org/10.1016/s0031-9422(02)00267-4.
Schultz TP, Nicholas DD. Naturally durable heartwood: evidence for a proposed dual defensive function of the extractives. Phytochemistry. 2000;54(1):47–52. https://doi.org/10.1016/s0031-9422(99)00622-6.
De Vetter L, Depraetere G, Stevens M, Janssen C, Van Acker J. Potential contribution of organosilicon compounds to reduced leaching of biocides in wood protection. Ann For Sci. 2009;66(2):209–209. https://doi.org/10.1051/forest/2008091.
Rajput H, Rajan P, Li S, Stratton GW, Murray G, He Q. Development of wood preservatives to prevent biodeterioration. J Wood Chem Technol. 2023;43(6):371–89. https://doi.org/10.1080/02773813.2023.2284161.
Miranji EK, Kipkemboi PK, Kibet JK. A review of toxic metals and hazardous organics in wood treatment sites and their etiological implications. J Chem Rev. 2022;4(1):40–66. https://doi.org/10.22034/jcr.2022.326656.1140.
Jankowska A, Kwiatkowski A. Effectiveness of European oak wood staining with iron (II) sulphate during natural weathering. Maderas Ciencia y tecnología. 2022. https://doi.org/10.4067/S0718-221X2022000100415.
Pokhrel D, Viraraghavan T. Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res. 2006;40(3):549–52.
Acknowledgements
The Department of Chemistry & Biochemistry—University of Eldoret, and the Directorate of Research and Extension of Egerton University is appreciated for supporting this study. Albert Oyugi Morang’a—a Master of Chemistry student at Egerton University is appreciated for providing guidance during statistical treatment of data.
Funding
This study received no specific grants from any funding agency.
Author information
Authors and Affiliations
Contributions
EMK: Method development, statistical analysis, writing and editing. JKK: Conceptualization, editing and supervision. PKK: Visualization, Editing, and supervision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
This article has the consent of all the authors.
Competing interests
The authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miranji, E.K., Kibet, J.K. & Kipkemboi, P.K. Determination of potentially toxic heavy metals in selected wood treatment sites in Uasin Gishu County and their associated health concerns. Discov Environ 2, 70 (2024). https://doi.org/10.1007/s44274-024-00093-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00093-7