Abstract
Whilst the importance of fungal primary biological aerosol particles (PBAPs) has been recognised, few studies have empirically assessed how land-use patterns influence them. Here, we show the impacts of different land-use patterns on fungal PBAPs within the Brazilian Atlantic Forest biodiversity hotspot. Spanning a distance of ca. 600 km within this biome, we collected fungal bioaerosols in the following land-use patterns: a 25-year-old coffee plantation, a 2.5-year-old Eucalyptus plantation, a 3-months-old maize crop, a 2-year-old and another 7-year-old native forest reforestation area, and a native forest fragment. Using the portable Burkard air sampler, a total of 14 morphotype-species were found. Cladosporium sp. comprised as much as about 95% of all fungal spores collected, being present in all samples (frequency of 100%). Forest systems had as much as 5-to-19-fold more fungal spores in the air than maize crops. Sampling height and time (morning vs. afternoon) did not influence fungal concentration and number of species. In addition, using data derived from an eddy covariance tower in the Eucalyptus site, we estimated the emission rate to be 6649 spores m−2 s−1. Our study confirms that land-use patterns affect fungal PBAPs, and that replacing large areas of native Atlantic Forest by monospecific stands, a homogenisation of airborne fungi is occurring, with unknown consequences for climate regulation.
Similar content being viewed by others
1 Introduction
Primary biological aerosol particles (hereafter, PBAPs) such as bacteria, fungal spores, pollen, as well as fragments of animals and plants, are all known to affect local and regional climate, for example, by means of ice nucleation and cloud formation [1,2,3]. Being important drivers of local climate, PBAPs might also interfere with climate regulation at a global level [4]. Given their nature, the formation and emission of PBAPs depend on land-use, with shifts in PBAPs patterns (i.e., concentration and fluxes) occurring after the conversion of natural ecosystems to human-shaped landscapes [5]. Changes in land-use, therefore, impact on PBAPs, which, in turn, can affect local, regional, and global climate [6, 7], and might even, albeit unknown, partially mitigate the consequences of climate change. Even though land-use might exert important impacts on PBAPs budgets, few studies have empirically studied how land-use affects PBAPs worldwide.
Fungi and their spores are major contributors to atmospheric aerosols [8,9,10,11]. Due to the condensation and/or ice nucleation activity of some fungi, they may influence cloud, rain, and hail formation [12,13,14]. While climate regulation research mediated by fungi is developing globally [3, 11], little is known about this process in Brazil that is home to some of the world’s largest forests. The situation is even more concerning in the Atlantic Forest, the most populated Brazilian biome that is also considered as a global biodiversity hotspot [15]. The biome, where more than half of the country’s population lives, is highly fragmented and degraded, which poses significant threats to the environment and to our ability to mitigate climate change consequences [16].
Land-use changes are known to alter fungal communities [17]. Most of these changes in the fungal communities are harmful and alter soil physiochemistry, microclimatic conditions and vegetation structure [18]. It has been shown, for instance, that shifts in land-use in the Amazon Forest reduce the number of fungal species [19] and favour generalist fungi to the detriment of other (more specialist) species [20]. The majority of studies investigating the impacts of land-use on fungi has, however, placed a great emphasis on soil fungal communities, given their role in biogeochemical cycles and litter decomposition [21, 22]. As a consequence, studies focusing on the relationship between land-use patterns and fungal PBAPs are rare and scarce, and even more needed in highly-fragmented biomes as the Atlantic Forest.
Considering the Atlantic Forest, this important biome has received less attention than the Amazon Forest concerning in situ PBAPs research [23]. Emygdio et al. [24] found that Cladosporium sp. was the most frequent and abundant fungal species regardless season and microclimatic conditions. In addition, higher concentrations of airborne fungi occur in the afternoon due to spore release [25]. Moreover, in a recent study, Mantoani et al. [26] have found that high altitudes (above 150 m) and rainfall occurrence impact on fungal spores, reducing their concentration in the atmosphere. Whilst all those studies are unveiling the relationship between fungal PBAPs and climate in the biome, more research is needed to boost our comprehension of macroscale human-induced effects in the Atlantic Forest.
In light of this lack of information, the aim of this study was to investigate the impacts of different land-uses on fungal PBAPs’ concentration and number of species within the Brazilian Atlantic Forest biome. Since land management impacts fungal communities [27, 28], study sites compared forests of different ages and management practices with annual and perennial crops. Spanning three Brazilian states and a distance of ca. 600 km, our sites allowed us to assess fungal spore diversity within different land-use contexts. We addressed the following hypotheses: (1) higher abundance and richness (i.e., number of species) of fungal PBAPs are found in more diverse areas (i.e., native forest reforestations and forest fragment) in comparison to monocultures (i.e., maize, coffee, and Eucalyptus) [29]; (2) there is a higher diversity of fungal aerosols in forested areas in comparison to crops due to the structure of the vegetation [6]; (3) higher sampling heights have lower concentrations of fungal spores than levels closer to the ground due to the ground cover being a source of the aerosols [26]; and (4) there is a higher abundance and richness of fungal PBAPs at the end of the day (afternoon) in comparison to the morning, since warmer temperatures favour spore release and given that some fungal species present diel cycles [25, 30].
2 Material and Methods
2.1 Study Sites
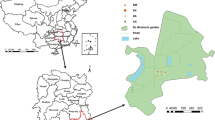
2.1.1 Londrina, Paraná state
A maize plantation (ca. 1.80 m tall) and a 7-year-old native species reforestation (ca. 8 m tall) adjacent to an Atlantic Forest fragment (“Mata dos Godoy” State Park), in the city of Londrina, in the northern part of the state of Paraná, (23°44′23.23″S, 51°25′83.61″W), were investigated. Sites are ca. 500 m apart and the elevation of both sites is ca. 600 m a.s.l. The climate is humid subtropical (Cfa), presenting warm and rainy summers and mild dry winters with uncommon frosts, with an average annual temperature of 21 °C and an average annual rainfall of ca. 1,600 mm. Soil at the studied sites is classified as Eutroferric Red Nitosol [31]. The 7-year-old native species restoration site (thereafter referred to as REF7) is ca. 4 ha, has a spacing of 2 × 3 m, and it is dominated by Alchornea cf. glandulosa, Heliocarpus popayanensis, Phytolacca dioica, Schinus terebinthifolius, and Senna multijuga, native tree species that make up approximately 80% of all species used in the initial plantation. The maize plantation (thereafter referred to as MAIZE) planted on March 2021 (i.e., 3-months-old) was in a pre-flowering phenological state, had a spacing of 0.50 × 0.50 m and is ca. 65 ha.
2.1.2 Arceburgo, Minas Gerais state
We collected fungal PBAPs at Fazenda Cachoeira (21°23′36.50″ S; 46°55′15.87″ W), located in Arceburgo, a city in the south region of the state of Minas Gerais. For that, a 25-year-old coffee plantation (i.e., Coffea arabica; thereafter referred to as COFFEE) was compared with a native forest fragment (thereafter referred to as FOR). Distance between sites was 50 m, elevation is ca. 700 m a.s.l. and the soil is classified as Eutrophic Red-Yellow Argisol. The climate is humid subtropical climate (Cwa), also presenting warm and rainy summers and mild dry winters. The coffee plantation (spacing of 3 × 1.20 m) covers an area of 40 ha, is 25-year-old, and has an average tree height of ca. 2.50 m. The native forest fragment is ca. 40 ha and has a canopy of ca. 8 m dominated by the species of Bastardiopsis densiflora, Cabralea canjerana, Guarea kunthiana, Lonchocarpus muehlbergianus, and Trichilia claussenii.
2.1.3 Itatinga, São Paulo state
Two contrasting forest ecosystems located close to Itatinga, at the central-south region in the São Paulo state, both belonging to the Forest Science Experimental Station ((23°04′79.04’’S; 48°63′76.77′′W) of the “Luiz de Queiroz” College of Agriculture (ESALQ), University of São Paulo, were also studied. The 2-year-old native forest restoration plantation (840 m. a. s. l.; thereafter referred to as REF2) covers an area of 30 ha and had an average tree height of ca. 2 m. Six native tree species composed the plantation: Cariniana estrellensis, Cecropia pachystachya, Guazuma ulmifolia, Hymenaea courbaril, Handroanthus impetigionosus, and Syagrus romanzoffiana. The 2.5-year-old clonal plantation of Eucalytpus urophylla (750 m. a. s. l.; thereafter referred to as EUC) had an average tree height of ca. 16 m and an area totalling 90 ha. Distance amongst sites is ca. 10 km, soil is classified as Dystrophic Red-Yellow Latosol and the climate is humid subtropical climate (Cfa) as the other two sites.
2.2 Experimental Design and Data Collection
We collected fungal PBAPs using a portable Burkard air sampler (Burkard Manufacturing Co., Hertfordshire, UK) at each of the six sites. Data was collected on 11 May 2021 at Londrina, on 26 May 2021 at Arceburgo, and on 3 May 2021 at Itatinga. Three sampling heights were assessed (1 m, 5 m, and 10 m above-ground level) with the aid of an adapted telescopic pole attached to a holder that secured the Burkard air sampler (Supplementary Fig. 1). The exception was the Eucalyptus plantation at Itatinga, in which the canopy height was above the others, and thus, sampling heights were 1 m, 5 m, and 20 m, and data were collected using the levels of the eddy covariance tower located at that site. We have also collected data in the morning (ca. 09:00) and afternoon (ca. 17:00) to check for diel differences. Moreover, heights were sampled consecutively and sampling of the three heights was completed within 20 min for each site and sampling time.
The portable Burkard air sampler sampled the air for 5 min and a total volume of 50 L of air was collected for each slide (10 L/min) [25, 26]. Slides were prepared with a “Melinex” tape coated with an adhesive and sampling was carried out during sunny days with calm wind conditions. One slide per height per sampling time per site was collected, totalling 6 slides per site and 36 slides analysed for the whole experiment (i.e., 1 slide × 3 heights × 2 sampling times × 6 land-use patterns = 36 slides). The Portable Burkard air sampler has a theoretical cut-off size of 2.52 µm and a 2.3–2.4 µm experimental cut-off size [32]. More information regarding the portable Burkard air sampler can be found in Aizenberg et al. [32].
Following the procedures of Rogers and Muilenberg [33], sampled slides were fixed using glycerine jelly to cover the entire slide. Slides were analysed thoroughly using a microscope at a 1000×magnification. Fungal PBAPs' abundance and richness were determined for each slide by means of analysis and classification by a specialist following Emygdio et al. [24, 25] and Mantoani et al. [26]. For that, all fungal spores were counted and identified as per Haines et al. [34]. As presented in Mantoani et al. [26], to infer fungal concentration per cubic metre of air (spores m−3), we divided the total number of spores counted by the total air volume sampled by the portable air Burkard sampler using Eq. 1. Finally, the frequency of fungal spores (expressed as a percentage) was determined by dividing the number of times each morphotype-species appeared in any slide by the total number of slides (for instance, if a morphotype-species has frequency of 50%, it appeared in 18 out of 36 slides).
2.3 Determination of Fungal PBAPs Flux and National Emissions
A flux-gradient method was used to estimate fungal spore fluxes at the Eucalyptus sampling site (EUC) deriving data from a gradient of concentrations using two heights (1 and 20 m). The method follows the Monin–Obukhov similarity theory [35] and assumes that in the atmospheric surface layer the flux of a scalar is a function of the aforementioned gradient, the sampling heights, and a transport velocity that is dependent on atmospheric turbulence and stability. For further details about the application of this method to culturable airborne microorganisms see Carotenuto et al. [36] and references therein. Due to the lack of an eddy covariance system in the other land-use patterns/sites, we have utilised the results achieved at the Eucalyptus site to infer fungal spore emissions elsewhere. The limitation of this flux calculation (and extrapolation) is that we were only able to use one sampling time (09:00) for a single site, deriving its flux estimation results to others. Consequently, estimation on fungal spore emissions should be interpreted with care.
To infer annual fungal spore emissions by land-use type for the entire country, we have used the following approach. First, we have calculated the total area of each land-use type at the municipality level within the Brazilian Atlantic Forest biome. For that, data from the 2021 Brazilian Institute of Geography and Statistics (IBGE) [37] agricultural census were used to obtain the coffee and maize area for each Brazilian municipality. Data for the other land-use types were obtained using two different products of the MapBiomas project [38], which produces annual land-cover mappings with data from the Landsat satellite series (30 m spatial resolution) from 1985 onwards. The first product used was the land cover map for 2021, from which the “Forest Plantation” class was extracted to represent areas with Eucalyptus in Brazil. Although Eucalyptus composes more than 75% of all planted trees in Brazil, ranking in first [39], other tree species planted for commercial purposes (e.g., Pinus sp.) are also included in this land product. Since this type of data is gathered altogether, we were unable to dissociate Eucalyptus land-cover area from other species’, which is a limitation of our study.
The second product used was the “Deforestation” and “Secondary Vegetation” maps for 2019, which was used to estimate areas of forest fragments and reforestation sites. The 2019 product was used in this second case, as this is the most recent product available in the MapBiomas platform. After estimating the total area of each land-use type within the whole Atlantic Forest biome, we have used a straightforward calculation to estimate fungal spore emission for each of the other five land-use types without an eddy covariance tower. For this, data was derived by using the estimation obtained for the Eucalyptus site (spores m−2 s−1) to the other five sites. Subsequently, we have scaled that up to hectares (spores ha−1 s−1), multiplied it by the total area of each land-use in the whole of Brazil (spores per million km2 s−1), and converted the final number to estimate annual spore emissions in billions of spores per million of km2 (i.e., spores × 109 km2 × 106 y−1) for the entire country.
2.4 Culturable Fungal Sampling
At Itatinga, São Paulo state, in both the 2.5-year-old Eucalyptus plantation and in the 2-year-old native forest restoration area, in addition to fungal spore sampling using the portable Burkard air sampler, a Microbial Air Monitoring System (MAS100, Merck KGaA, Darmstadt, Germany) was used to collect culturable fungi. As per Mantoani et al. [26], the instrument sampled a total volume of 250 L of air into sterile Petri dishes with plates containing a modified Dicloran Rosa Bengal culture medium [40]. Plates were immediately put inside a thermal box once sampling was done, and then stored at 4 °C pending analysis. The samples were incubated at 30º ± 2 ºC for up to 7 days for isolation and identification at the Adolfo Lutz Institute Mycology Laboratory, in São Paulo, Brazil. A total of 12 plates were collected, one plate for each sampling height (i.e., 1, 5, and 20 m, at the Eucalyptus site, and 1, 2, and 5 m, at the restoration site, respectively) for each sampling time (morning, ca. 09:00, and in the afternoon, ca.17:00). Fungi were cultured and a Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS; Bruker Daltonics, Billerica, Massachusetts, USA) was used to classify fungal samples at the genus level [41]. Finally, frequency of fungi (expressed as percentage) was determined dividing the number of times each morphotype-species appeared in any plates by the total number of plates (for instance, if a morphotype-species has frequency of 50%, it appeared in 6 out of 12 plates).
2.5 Statistical Analyses
Data on fungal spore concentration and number of species from the Burkhard samplers were log-transformed to satisfy the assumptions of normality (Shapiro–Wilk’s test) and homoscedasticity (Levene’s test). Analysis of Variance (ANOVA) followed by Tukey’s HSD post-hoc test was used to check differences in spore concentration and number of species at different six land-uses (i.e., coffee plantation, Eucalyptus plantation, maize crop, two native forest reforestation types, and a forest fragment), different sampling heights (1 m, 5 m, and 10/20 m above ground level), and distinct sampling hours (i.e., mornings, 09:00 h, or afternoons, 17:00 h). In parallel, we calculated the differences (i.e., ‘delta values’) in spore concentration between successive sampling heights and estimated the 95% confidence interval around their mean to check for the existence of PBAPs gradient. All analyses were performed with a significance level of α = 0.05, using Statistica v. 14.0.0.15. In contrast, analysis of culturable fungi is solely descriptive due to the low number of samples collected.
3 Results
3.1 Properties of the Pool of Airborne Fungal Spores above the Atlantic Forest biome
3.1.1 Fungal Spores Composition Analysis
A total of 14 morphotype-species were identified using the portable Burkard air sampler. These were: Alternaria sp., Aspergillus/Penicillium-like (hereafter, Asp/Pen-like), Cladosporium sp., Curvularia sp., Drechslera-like, Epicoccum sp., Ganoderma sp., Leptosphaeria-like, other Mitospores, Pithomyces sp., Spegazzinia sp., Tetraploa sp., Torula sp., and Venturia-like (Fig. 1A). Asp/Pen-like, Cladosporium sp. and Torula sp. were the only morphotype-species that were present above all land-use patterns analysed. Concerning their frequency, Cladosporium sp. were the only fungi present in all samples (100%). Asp/Pen-like spores were present in more than 60% of samples and Torula sp. reached 50%, with all the other morphotype-species having varied levels of frequency. Ganoderma sp., Leptosphaeria-like, other Mitospores, and Tetraploa sp. only appeared in one sample each.
A Total frequency (as a percentage of appearance in all samples) of all the 14 fungal morphotype-species identified using the portable Burkard air sampler in the six land-use types studied, and B, total frequency of all the 8 culturable fungal morphotype-species identified using the Microbial Air Monitoring System (MAS100) in the 2.5-year-old Eucalyptus site and the 2-year-old native forest restoration plantation only, Itatinga, São Paulo state
Regardless of the sampling location (i.e., different land-use patterns), sampling height (i.e., 1, 5, or ≥ 10 m), and sampling time (i.e., mornings, 09:00 h, or afternoons, 17:00 h), Cladosporium sp. constituted as much as 95% of all fungal spores collected (Table 1). Torula sp. was the second most abundant with ca. 3%, and Asp/Pen-like the third with ca. 1%. All the other 11 morphotype-species taken together constituted ca. 1%. However, the contribution to the fungal budget varied amongst land-use types when they were accounted for separately. A greater contribution (above 10%) of Asp/Pen-like was registered in the maize plantation and in the 7-year-old restoration site, both in the region of Londrina (Paraná state). Moreover, virtually all spores collected at the Eucalyptus plantation (99.58%) and at the 2-year-old reforestation using native species (98.59%), both at Itatinga (São Paulo), were identified as Cladosporium sp.
3.1.2 Fungal PBAPs and Land-use Patterns
The 25-year-old coffee plantation exhibited the highest spore concentration (141,707 ± 82,161 spores m−3), followed by the 2-year-old native species reforestation site (80,850 ± 18,296 spores m−3), and the 2.5-year-old Eucalyptus plantation (51,897 ± 34,083 spores m−3; Fig. 2). The forest fragment had an average of 38,330 ± 15,517 spores m−3 and the 7-year-old native species reforestation 22,343 ± 15,670 spores m−3. Maize was the land-use pattern with the lowest concentration of fungal PBAPs, with an average of 4,193 ± 984 spores m−3 only (Fig. 2), significantly smaller than coffee (F5,30 = 4.851; P = 0.002; 95% CI = − 2.242, − 0.235) and the 2-year-old restoration area (F5,30 = 4.851; P = 0.002; 95% CI = -2.290, -0.283).
Total concentration (spores m−3 × 1000) of fungal spores collected at the different sampling sites (n = 6 ± SE), regardless sampling time or height. COFFEE, 25-year-old coffee plantation at Arceburgo; EUC, 2.5-year-old Eucalyptus plantation at Itatinga; MAIZE, 3-months-old maize plantation at Londrina; REF2, 2-year-old native species reforestation at Itatinga; REF7, 7-year-old native species reforestation at Londrina; FOR, forest fragment at Arceburgo
Consequently, forest systems had as much as 5-to-19-fold more fungal spores than maize crops. The number of fungal species was also higher at the coffee plantation (8.17 ± 0.95) in comparison to maize (2.17 ± 0.60; F5,30 = 6.974; P < 0.001; 95% CI = 0.203, 1.055) and Eucalyptus (2.00 ± 0.26; F5,30 = 6.974; P < 0.001; 95% CI = 0.190, 1.042). Moreover, fungal richness was also higher at the forest fragment (6.50 ± 1.41) in comparison to maize (95% CI = 0.027, 0.878) and Eucalyptus (95% CI = 0.014, 0.865). Thus, maize crops and Eucalyptus plantations showed the lowest numbers of fungal morphotype-species amongst all the six land-use types investigated in our study (Fig. 3).
Total number of fungal species (richness) collected in the different sampling sites (n = 6 ± SE), regardless sampling time or height. COFFEE, 25-year-old coffee plantation at Arceburgo; EUC, 2.5-year-old Eucalyptus plantation at Itatinga; MAIZE, 3-months-old maize plantation at Londrina; REF2, 2-year-old native species reforestation at Itatinga; REF7, 7-year-old native species reforestation at Londrina; FOR, forest fragment at Arceburgo
3.1.3 Sampling Height and Diel Time
There were no differences in the concentration (F2,33 = 2.900; P = 0.069) and number of fungal species (F2,33 = 0.826; P = 0.447) with increasing sampling height, and no interactions between land-types and sampling heights for spore concentration (F10,18 = 1.64; P = 0.174) and fungal richness (F10,18 = 1.45; P = 0.237) were detected as well. Despite this, we have detected the existence of an actual gradient on fungal spores since there were significant differences between ‘delta values’ when considering successive sampling heights for samples collected in morning (P < 0.05; Mean = 72,840; CI = 1,946; 143,733). In addition, there were no differences between the concentrations (F1,34 = 0.391; P = 0.845) and richness of fungal spores (F1,34 = 0.003; P = 0.956) when morning was compared to the afternoon, and no interactions between treatments and diel time was detected for spore concentration (F5,24 = 0.972; P = 0.454) or fungal richness (F5,24 = 1.06; P = 0.405).
3.1.4 Fungal Spore Fluxes at the Eucalyptus site and Estimated National Emissions
Regarding the flux of fungal spores at the Eucalyptus site, we estimated a total of 6649 spores m−2 s−1 to be emitted in the morning (09:00 h) of the sampling day. When extrapolating this to the other land-use types at the national level, the annual spore emission rate estimated for each of the six land-use types in the entire biome ranked (from the highest emission rate to the smallest) as the following: 7-year-old native forest restoration > forest fragment > 2-year-old native forest restoration > coffee > Eucalyptus > maize (Table 2; Fig. 4).
Total annual spore emission estimates (in billions of spores per million hectares) categorised by land-use type for Brazilian municipalities within the Atlantic Forest biome. COFFEE, 25-year-old coffee plantation at Arceburgo; EUC, 2.5-year-old Eucalyptus plantation at Itatinga; MAIZE, 3-months-old maize plantation at Londrina; REF2, 2-year-old native species reforestation at Itatinga; REF7, 7-year-old native species reforestation at Londrina; FOR, forest fragment at Arceburgo; Total, the sum of estimated emissions for the evaluated land-use types in the whole Atlantic Forest biome
3.2 Properties of the Pool of Airborne Culturable Fungi above the Atlantic Forest biome
In total, eight culturable fungal species were identified in the Eucalyptus stand and the 2-year-old native forest restoration plantation (Fig. 1B). Cladosporium sp. and Fusarium sp. were present in all samples (frequency of 100%). Neurospora sp. and Penicillium brevicompactum were only present in the 2.5-year-old Eucalyptus site. On the other hand, Mucor circinelloides and Curvularia lunata appeared only in the 2-year-old restoration area.
4 Discussion
Both the first hypothesis on a higher diversity (i.e., abundance and richness) of fungal PBAPs linked to more diverse and structured areas (i.e., forest sites composed by native species) opposed to monocultures (i.e., maize, coffee, and Eucalyptus), and the second hypothesis that forested areas benefit fungal species more than crops, can be only partially accepted. The reason for this is that, unexpectedly, the coffee plantation had the highest abundance and richness of fungal spores, indicating that whilst this is a site composed mainly by a single plant species, it still can accommodate a high diversity of fungi. It is important to notice that the coffee plantation we evaluated was the ‘standard’ used in Brazil (i.e., full-sun) [42], in which in between plantation lines there is some growth of perennial herbs and grass species. Whilst that might influence the budget of airborne fungal spores, this would be minimal since coffee plants cover most of the studied area, reducing the grow of other species to a minimum. Furthermore, although coffee planted underneath trees and polyculture are also used and might represent some of the data we analysed using the MapBiomas system, we cannot dissociate those from the full-sun coffee, which is a limitation of our study.
The atmosphere above the monoclonal Eucalyptus plantation exhibited similar (and not statistically distinct) abundance of fungal spores in comparison to the other forest systems composed by native species. Fungal species richness, on the other hand, appears to be more sensitive to shifts in land-use in comparison to fungal spore concentration [19, 20]. For instance, maize and Eucalyptus had the lowest numbers of species (average of 2), being composed mainly by Cladosporium sp. and Asp/Pen-like, whereas the other land-use patterns also presented other morphotype-species. Leptosphaeria-like and Tetraploa sp., for example, only appeared in sites dominated by native plant species, which could imply that these fungal species, like as many other fungi, could be considered as environmental indicators for habitat quality shifts [43]. In addition, Silva et al. [44] also found a lower diversity of macrofungi species in planted forest in comparison to native forest areas in Central Portugal, indicating that even a shift of dominant tree species while retaining similar ecosystem structure is sufficient to alter fungal communities.
In disagreement with our third hypothesis, and other studies [25, 45], the total abundance and richness of fungal spores did not decrease with increasing height. This might indicate that below 20 m there are no distinct patterns in fungal PBAPs and only at higher altitude levels (i.e., above 150 m) these differences become clearer [26]. Our fourth hypothesis that sampling time would impact fungal spores was also not confirmed. This could partially be explained by the low replication in our study, as other studies have identified that some fungal species do have diel effects and are impacted, for instance, by air humidity [30, 45, 46].
Interestingly, the high number of spores being emitted at the Eucalyptus site (6,649 spores m−2 s−1) indicate that even though these ecosystems appear to host and emit a reduced number of fungal species, they still have a huge numerical contribution to the fungal atmospheric budget. While our sampling refers to only one site and sampling hour, the rate that we observed was 100-fold bigger compared to the annual mean emission rate of ca. 60 spores m−2 s−1 across the USA [11]. Furthermore, our results are comparable to fungal fluxes estimated by Crawford et al. [47] in a Colorado pine forest (− 6000 to + 6000 spore-like m−2 s−1) and by Taha et al. [48] in composting windrows (8.3–11.1 × 1000 Colony Forming Units m−2 s−1 of Aspergillus fumigatus). When extrapolating this result to a basis of total area in the entire country for each of the six land-use types studied, managed crops (i.e., coffee, Eucalyptus, and maize) have shown a much smaller contribution (i.e., up to 148-fold less) to the fungal atmospheric budget than restoration areas or the native forest fragment. This is probably related to land management since the cultivation areas have undergone fertilisation and herbicide spraying for a long time amongst the use of other chemical. Obviously, a more intense land management should have impacted fungal communities [27, 28] and their ability to produce spores, reducing, in the case of the maize and Eucalyptus sites, the fungal airborne communities as an indirect effect of herbicide-treatment [49, 50].
It is important to remember that maize and Eucalyptus were the land-use types with the smallest number of fungal species in the aerosol component, indicating that by replacing large areas of native forest by such monospecific stands, a homogenisation of airborne fungi is occurring, with unknown consequences for climate regulation. As for the distribution of spore emissions for coffee plantations, it is notable that while they exhibit high values, they are generally concentrated in specific areas. Particularly, noteworthy are the Brazilian states of Minas Gerais (10,023 km2) and Espírito Santo (3,890 km2), which encompass the largest coffee-growing regions in Brazil. On the other hand, the remaining forest fragments and reforestation types (REF7 and REF2) exhibit a relatively similar distribution throughout the biome, with municipalities generally sharing similar profiles, confirming the degradation of the Brazilian Atlantic Forest [16] and the urgent need to restore it [51]. Given that fungi are highly affected by environmental variations and specific conditions of temperature and humidity [30, 45, 46], and considering the environmental heterogeneity of the Atlantic Forest [16], our initial results (and extrapolations) should be taken with care. However, they point out the need to perform more research in this area in Brazil and elsewhere, since monospecific stands (i.e., coffee and Eucalyptus) within a biodiversity hotspot might still present relatively high rates of fungal spore emission, though they are much less diverse [44].
Finally, although a total of 14 and 8 morphotype-species were found using the portable Burkard air sampler or the MAS100, respectively, we are aware, however, that there is a much higher diversity of fungi in Brazil, with more than 3,000 species detected in the Atlantic Forest biome alone [52]. One explanation could be that some fungi species/genera were simply not detected in our study because they do not produce airborne spores (i.e., yeasts). Other genera, particularly of culturable fungi, are not so easily detected because culture on growth media can be problematic. Consequently, researchers should consider the techniques they are using to assess the fungal PBAP budget, since other approaches (e.g., eDNA) might lead to different results [53]. Moreover, sampling low volumes of air could be another explanation, specifically for the portable Burkard air sampler, since typically, more than 20 m3 of air are collected for aeromicrobiome analysis, and we analysed a total volume of 1.8 m3 (50 L of air per slide × 36 slides = 1800 L of air or 1.8 m3 of air).
That said, this would perhaps increase the number of fungal species but would not change the fact that Cladosporium sp. is much more abundant than any other type of fungi. It is important to note that Cladosporium sp. accounted for more than 95% of all fungal spores collected using the portable Burkard and it was present in all samples collected with both instruments. As a matter of fact, this indicates the ubiquity of this fungal genus that could be used as a proxy to evaluate other species in the Atlantic Forest [25, 26] and in other environments or even in global studies [54], warranting more research on this topic. This way, if researchers focus on this morphotype-species, they are evaluating most fungal spores that they could sample. This high number indicates that research may not be compromised if other fungal species are not included in the analysis, or if there is limitation on budget/time restriction, which is often the case.
5 Conclusions
The results of our study suggest that land-use profoundly affects the fungal PBAP profile in the boundary layer of the Atlantic Forest biome, having a large effect on the total number of fungal spore species. For example, forest systems (such as the reforestation using native species and the forest fragment) had as much as 5-to-19-fold more fungal spores than maize crops. Data has also shown that sampling height of up to 20 m and diel time (morning vs afternoon) did not influence fungal spore concentration and richness, and despite a low retrieval of only 14 morphotype-species using the portable Burkard air sampler, Cladosporium sp. corresponded to as much as 95% of all fungal spores collected, being present in all samples collected (frequency of 100%). In addition, Cladosporium sp. presented a frequency of 100% in all samples collected using the MAS100. This indicates that this fungus could be used as a proxy for other fungi and/or in studies that aim at relating fungal PBAPs with climate. Finally, the high flux rate of 6649 spores m−2 s−1 estimated above the Eucalyptus site, demonstrates that within the Atlantic Forest biome, even a monoclonal plantation, shows a significant contribution to fungal PBAPs. Nevertheless, our study confirms that land-use patterns affect fungal PBAPs, and that replacing large areas of native Atlantic Forest by monospecific stands, a homogenisation of airborne fungi is occurring, with unknown consequences for climate regulation.
Data availability
Data will be made available upon request.
References
Bauer H, Kasper-Giebl A, Löflund M, Giebl H, Hitzenberger R, Zibuschka F, Puxbaum H. The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmos Res. 2002;64:109–19.
Martinez-Bracero M, Markey E, Clancy JH, McGillicuddy EJ, Sewell G, O’Connor DJ. Airborne fungal spore review, new advances and automatisation. Atmosphere. 2022;13:308.
Šantl-Temkiv T, Amato P, Casamayor EO, Lee PKH, Pointing SB. Microbial ecology of the atmosphere. FEMS Microbiol Rev. 2022;46:1–18.
Després VR, et al. Primary biological aerosol particles in the atmosphere: a review Tellus B. Chem Phys Meteorol. 2012;64:15598.
Artaxo P, et al. Atmospheric aerosols in Amazonia and land use change: from natural biogenic to biomass burning conditions. Faraday Discuss. 2013;2013(165):203.
Li X, Chen H, Yao M. Microbial emission levels and diversities from different land use types. Environ Int. 2020;143: 105988.
Morais FG, et al. Relationship between land use and spatial variability of atmospheric brown carbon and black carbon aerosols in Amazonia. Atmosphere. 2022;2022(13):1328.
Ebert W, Taylor PE, Andreae MO, Pöschl U. Contribution of fungi to primary biogenic aerosols in the atmosphere: wet and dry discharged spores, carbohydrates, and inorganic ions. Atmos Chem Phys. 2007;7:4569–88.
Heald CL, Spracklen DV. Atmospheric budget of primary biological aerosol particles from fungal spores. Geophys Res Lett. 2009;36:L09806.
Šantl-Temkiv T, et al. Bioaerosol field measurements: challenges and perspectives in outdoor studies. Aerosol Sci Technol. 2020;54:520–46.
Janssen RHH, Heald CL, Steiner AL, Perring AE, Huffman JA, Robinson ES, Twohy CH, Ziemba LD. Drivers of the fungal spore bioaerosol budget: observational analysis and global modelling. Atmos Chem Phys. 2021;21:4381–401.
Sesartic A, Lohmann U, Storelvmo T. Modelling the impact of fungal spore ice nuclei on clouds and precipitation. Environ Res Lett. 2013;8: 014029.
Els N, Larose C, Baumann-Stanzer K, Tignat-Perrier R, Keuschnig C, Vogel TM, Sattler B. Microbial composition in seasonal time series of free tropospheric air and precipitation reveals community separation. Aerobiologia. 2019;35:671–701.
Kunert AT, et al. Macromolecular fungal ice nuclei in Fusarium: effects of physical and chemical processing. Biogeosciences. 2019;16:4647–59.
- TNC, The Nature Conservancy. 2023. tnc.org.br/. Accessed at: 20 June 2023.
Lima RAF, Oliveira AA, Pitta GR, Gasper AL, Vibrans AC, Chave J, ter Steege H, Prado PI. The erosion of biodiversity and biomass in the Atlantic Forest biodiversity hotspot. Nat Commun. 2020;11:6347.
Spurgeon DJ, Keith AM, Schmidt O, Lammertsma DR, Faber JH. Land-use and land-management change: relationships with earthworm and fungi communities and soil structural properties. BMC Ecol. 2013;13:46.
Waymouth V, Miller RE, Kasel S, Ede F, Bissett A, Aponte C. Riparian fungal communities respond to land-use mediated changes in soil properties and vegetation structure. Plant Soil. 2022;475:491–513.
Fracetto GGM, Azevedo LCB, Fracetto FJC, Andreote FG, Lambais MR, Pfenning LH. Impact of Amazon land use on the community of soil fungi. Sci Agric. 2013;70:59–67.
Mueller RC, Rodrigues JLM, Nüsslein K, Bohannan BJM. Land use change in the Amazon rain forest favours generalist fungi. Funct Ecol. 2016;30:1845–53.
Brinkmann N, et al. Intensive tropical land use massively shifts soil fungal communities. Sci Rep. 2019;9:3403.
Xu M, Li X, Cai X, Li X, Christie P, Zhang J. Land use alters arbuscular mycorrhizal fungal communities and their potential role in carbon sequestration on the Tibetan Plateau. Sci Rep. 2017;7:3067.
Mantoani MC, Martins JA, Martins LD, Carotenuto F, Šantl-Temkiv T, Morris CE, Rodrigues F, Gonçalves FLT. Thirty-five years of aerosol–PBAP in situ research in Brazil: the need to think outside the Amazonian box. Climate. 2023;11:17. https://doi.org/10.3390/cli11010017.
Emygdio APM, Degobbi C, Gonçalves FLT, Andrade MF. One year of temporal characterization of fungal spore concentration in São Paulo metropolitan area, Brazil. J Aerosol Sci. 2018;115:121–31.
Emygdio APM, et al. Bioaerosol vertical fungal spores profile in Minas Gerais State, Brazil. Aerobiologia. 2022;38:85–101.
Mantoani MC, et al. Rainfall effects on vertical profiles of airborne fungi over a mixed land-use context at the Brazilian Atlantic Forest biodiversity hotspot. Agric For Meteorol. 2023;331: 109352.
Durall DM, Gamiet S, Simard SW, Kudrna L, Sakakibara SM. Effects of clearcut logging and tree species composition on the diversity and community composition of epigeous fruit bodies formed by ectomycorrhizal fungi. Can J Bot. 2006;84:966–80.
Tomao A, Bonet JA, Castano C, de Miguel S. How does forest management affect fungal diversity and community composition? Current knowledge and future perspectives for the conservation of forest fungi. Forest Ecol Manage. 2020;457: 117678.
Marion ZH, Orwin KH, Wood JR, Holdaway RJ, Dickie IA. Land use, but not distance, drives fungal beta diversity. Ecology. 2021;102: e03487.
Gusareva ES, et al. Microbial communities in the tropical air ecosystem follow a precise diel cycle. PNAS. 2019;116:23299–308.
Mantoani MC, Torezan JMD. Regeneration response of Brazilian Atlantic forest woody species to four years of Megathyrsus maximus removal. Ecol Manage. 2016;359:141–6.
Aizenberg V, Reponen T, Grinshpun SA, Willeke K. Performance of Air-O-Cell, burkard, and button samplers for total enumeration of airborne spores. Am Industr Hygiene Assoc. 2000;61:855–64.
- Rogers C.; Muilenberg M. L. 2001. Comprehensive guidelines for the operation of hirst-type suction bioaerossol samplers. Pan-American Aerobiology Association, Standardized Protocols, 2001.
- Haines J.; Escamilla B.; Muilenberg M. L; Gallup J.; Levetin E. 2000. Mycology of the air. An introduction to the sampling and identification of airborne fungus spores. Tucson, Arizona.
Monin A, Obukhov A. Basic laws of turbulent mixing in the surface layer of the atmosphere. Contr Geophys Inst Acad Sci USSR. 1954;151:163–87.
Carotenuto F, Georgiadis T, Gioli B, Leyronas C, Morris CE, Nardino M, Wohlfahrt G, Miglietta F. Measurements and modeling of surface–atmosphere exchange of microorganisms in Mediterranean grassland. Atmos Chem Phys. 2017;17:14919–36.
- IBGE, Brazilian Institute of Geography and Statistics. Census 2021. https://ibge.gov.br. Accessed at: 22 June 2023.
Souza CM Jr, Shimbo JZ, Rosa MR, Parente LL, Alencar AA, Rudorff BFT, Hasenack H, Matsumoto M, Ferreira LG, Souza-Filho PWM, et al. Reconstructing three decades of land use and land cover changes in Brazilian biomes with landsat archive and earth engine. Remote Sens. 2020;12:2735.
- IBA, Indústria Brasileira de Árvores. Relatório Anual 2023. https://iba.org/datafiles/publicacoes/relatorios/relatorio-anual-iba2023-r.pdf. Accessed at: 6 Jan 2024.
e de Matos Castro Silva D, Santos DCS, Pukinskas SRBS, Oshida JTU, Oliveira L, Carvallho AF, Melhem MSC. A new culture medium for recovering the agents of Cryptococcosis from environmental sources. Braz J Microbiol. 2015;46:355–8.
Bizzini A, Greub G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin Microbiol Infect. 2010;16:1614–9.
Koutouleas A, Sarzynski T, Bordeaux M, Bosselmann AS, Campa C, Etienne H, Turreira-García N, Rigal C, Vaast P, Ramalho JC, Marraccini P, Ræbild A. Shaded-coffee: a nature-based strategy for coffee production under climate change? A Review Front Sustain Food Syst. 2022;6: 877476.
Soares DMM, et al. Fungal bioassays for environmental monitoring. Front Bioeng Biotechnol. 2022;10: 954579.
Silva LP, et al. Natural woodlands hold more diverse, abundant, and unique biota than novel anthropogenic forests: a multi-group assessment. Eur J Forest Res. 2019;138:461–72.
- Golan J. J.; Pringle A. 2016. Long-distance dispersal of fungi. Microbiol Spectrum, 5: FUNK-0047–2016.
Löbs N, et al. Aerosol measurement methods to quantify spore emissions from fungi and cryptogamic covers in the Amazon. Atmos Meas Tech. 2020;13:153–64.
Crawford I, Robinson NH, Flynn MJ, Foot VE, Gallagher MW, Huffman JA, Stanley WR, Kaye PH. Characterisation of bioaerosol emissions from a Colorado pine forest: results from the BEACHON-RoMBAS experiment. Atmos Chem Phys. 2014;14:8559–78.
Taha MPM, Pollard SJT, Sarkar U, Longhurst P. Estimating fugitive bioaerosol releases from static compost windrows: feasibility of a portable wind tunnel approach. Waste Manage. 2005;25:445–50.
Druille M, Cabello MN, Omacini M, Golluscio RA. Glyphosate reduces spore viability and root colonization of arbuscular mycorrhizal fungi. Appl Soil Ecol. 2013;64:99–103.
Wilkinson V, Lucas RL. Effects of herbicide on the growth of soil fungi. New Phytol. 1969;68:709–19.
Pinto LFG, Voivodic M. Reverse the tipping point of the Atlantic forest for mitigation. Nat Clim Chang. 2021;11:364–5.
Maia LC, et al. Diversity of Brazilian fungi. Rodriguésia. 2015;66:1033–45.
Fröhlich-Nowoisky J, et al. Biogeography in the air: fungal diversity over land and oceans. Biogeosciences. 2012;9:1125–36.
Grinn-Gofroń A, et al. Airborne Alternaria and Cladosporium fungal spores in Europe: forecasting possibilities and relationships with meteorological parameters. Sci Total Environ. 2019;653:938–46.
Acknowledgements
The authors thank the support received from local landowners and the COOXUPÉ association, particularly Mr. Éder Ribeiro dos Santos. We also thank the “Instituto Água e Terra” for granting permission to research at the “Mata dos Godoy State Park” in Londrina, and the fieldwork team for guidance during sampling at Itatinga. Authors also express their gratitude to Mrs. Solange Lima for helping with fungal taxonomy. The authors also thank the Funding Agency (FAPESP), NSJC, the editor and the anonymous reviewers for their contributions in previous versions.
Funding
This work was supported by FAPESP (“Fundação de Amparo à Pesquisa do Estado de São Paulo”; São Paulo Research Foundation; Grants: 2016/06160-8 and 2020/14143-1).
Author information
Authors and Affiliations
Contributions
MCM and FLTG conceived and designed the research; MCM and LCCG performed the experiment and collected fieldwork data; MCM, APR, and FC did the statistical analyses; MCM, LCCG, MFA, MAFSD, PLSD, FR, DMCS, VBDF, APR, JAM, LDM, JMDT, PHSB, JG, OCC, VP, FC, TŠ-T, CEM, and FLTG wrote and edited the manuscript; MCM and FLTG led the writing of the manuscript. Critical contribution to drafts and final approval for publication was given by all authors.
Corresponding author
Ethics declarations
Competing interests
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
44274_2024_49_MOESM1_ESM.docx
Supplementary file1 (DOCX 2279 KB) Figure 1 – Fungal PBAPs sampling using the portable Burkard air sampler at (A) 10 m in REF7, (B) 5 m in MAIZE, (C) 1 m in FOR, (D) 1 m in COFFEE, (E) 5 m in REF2, and (F) 5 m in EUC. Legend: REF7, 7 year old native species reforestation at Londrina; MAIZE, 3-months-old maize plantation at Londrina; FOR, forest fragment at Arceburgo; COFFEE, 25-yr old coffee plantation at Arceburgo; REF2, 2-yr old native species reforestation at Itatinga; EUC, 2.5 yea old Eucalyptus plantation at Itatinga
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mantoani, M.C., Guerra, L.C.C., Andrade, M.F. et al. Land-use patterns and fungal bioaerosols in the Brazilian Atlantic Forest biome. Discov Environ 2, 19 (2024). https://doi.org/10.1007/s44274-024-00049-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00049-x