Abstract
Black pod disease causes yield losses in cocoa production, and copper-based fungicides are used to control the disease. The study investigated the extent of accumulated copper from copper-based fungicides and interaction with soil chemical properties and microbial biomass in three communities: Akaasu, Kyeikrom, and Tuntum in the Bibiani–Anhwiaso–Bekwai District. Soil samples were collected at 0–15 cm and 15–30 cm. The fresh soil samples were divided into two, sieved and stored under different conditions; 4 °C and room temperature. Physicochemical (soil pH, Organic Carbon, Total Nitrogen) and microbial analyses (Microbial biomass was determined by the chloroform fumigation method and extraction and soil microbial mass of phosphorus) were conducted on the samples. Tuntum had the highest concentration of total copper (286.54 mg/kg), while the Akaasu cocoa plantation had the lowest total copper concentration (215.63 mg/kg). Extractable and total copper vary significantly (p < 0.05) in the three cocoa farms' (Akaasu, Kyeikrom and Tumtum) top and subsoils compared to the reference values. However, soil pH, nitrogen and organic matter from soils of the cocoa farms did not vary significantly (P > 0.05) from their reference soils. The correlation analysis revealed that extractable and total Cu at different soil depths (0–15 cm and 15–30 cm) correlated negatively with organic matter contents. There was negative correlation between extractable and total Cu with microbial biomass though not significantly different at p > 0.05 in all the study locations. Generally, the concentrations of copper in the soil at all sites have not reached their critical levels of 800 mg/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There is an increasing demand for cocoa beans on the world market, but diseases and pests could limit production and supply to consumers [1]. Cocoa swollen shoot virus disease, black pod, capsid which damage the trees and crops, stem canker, pink disease and thread blight are some of the diseases and pests that affect cocoa in Ghana [2, 3]. However, Black pod disease is Ghana's major cocoa disease and it possess significant challenges to cocoa production [4, 5]. There is an increasing demand for cocoa beans on the world market, but diseases and pests could limit its production and supply [1]. World losses of 20–25% in cocoa production are due to black pod disease, an estimated 700,000 metric tons on a global scale, reducing global cocoa production and losses up to 63% in pod rot and stem canker up to 10% death of trees annually have been reported in Karkar Island and Papua New Guinea [4]. Phytophthora palmivora and Phytophthora megakarya are the causes of the most prevalent fungal diseases of cocoa in Ghana and are differentially spread in the country's six cocoa-growing regions (Western, Brong Ahafo, Eastern, Ashanti, Central, and Volta) [2, 6]. The spread and level of infection by black pod disease in these regions are influenced by climatic conditions [7]. According to [8], researchers and farmers have used several methods to control Phytophthora spp. Chemical biocide to control fungal diseases and insect-plant pests has helped increase crop yield and food production in conventional agricultural practices [9]. In Ghana, copper-based fungicides are recommended for controlling black pod disease [7]. These fungicides include Ridomil Gold Plus, Nordox, Funguran OH, Kocide 2000, Sidalco defender, Fungikill and Champion. Prolonged usage of copper-based fungicides may, however, have adverse effects on soil microorganism, human health, animals and non-target organisms [5]. According to [10], Cu accumulation occurs in the soil profile with the most significant biological activity. High levels of Cu, when found in soils, may be toxic to plants and soil microorganisms and lead to lower biological activity and, eventually, loss of soil fertility.
High application of the Bordeaux mixture has resulted in a significant build-up of Cu in surface and subsurface soils in India [11]. The prolonged application of copper-based fungicides could lead to their accumulation in soil, affecting soil quality and human health. There is the possibility of occurrence of this situation because the government of Ghana, through the Ghana Cocoa Board, has, over the past thirteen years, been assisting cocoa farmers in the country to spray their farms against capsids and black pod disease under the Cocoa Diseases and Pest Control (CODAPEC) popularly known as “Cocoa Mass spraying” programme [12]. The Cocoa farmers in Bibiani–Anhwiaso–Bekwai District often use fungicides [13]. Most of these farmers have benefited from the Government of Ghana's free cocoa mass spraying programme over the past thirteen years [14]. All organisms require Cu as an essential element, so deficiency may result in reduced microbial activity and decreased soil fertility and possible death [5]. However, when present in soils, in high levels may be toxic to plants and soil microorganisms and could lead to reduced biological activity and loss of soil fertility [5]. High levels of Copper have been shown to damage mycorrhizal associations [15] and reduce microbial activity and functions [16]; the significant adverse effects of Copper residues on fertile agricultural soils range between 20 and 400 mg/kg. Copper residues are also known to restrict soil bioturbation and be toxic to soil organisms, resulting in the accumulation of organic materials due to the death of the soil organisms [5]. Therefore, this study investigates the potential accumulation of Cu in soil following prolonged application of copper-based fungicides in three selected communities (Akaasu, Kyeikrom and Tuntum) in the Bibiani–Anhwiaso–Bekwai District of Ghana. The research will provide information on the status of the quality of the soil and the impact of continued usage of fungicides on the soil.
2 Materials and methods
2.1 Study area
The study was carried out in the Bibiani–Anhwiaso–Bekwai District in the Western North Region of the Republic of Ghana. The district is located between latitude 5 \(^\circ\) 54’N and 6 \(^\circ\) 70’N and longitude 2 \(^\circ\) 06’W and 2 \(^\circ\) 27’W. It is bounded to the south by Asankragua District, north by Asunafo South District, west by Sefwi Wiawso District and east by Atwima Nwabiagya District [17]. The area experiences a wet semi-equatorial climate with a mean annual temperature of about 26 °C. The site is marked by a bimodal rainfall regime, with the major rainfall regime from May to July and the minor rainfall regime from August to September. Mean rainfall is between 1,250 mm and 1,750 mm per annum. The dry season begins in October and through the early part of March [17].
2.2 Experimental design
2.2.1 Selected cocoa farms and reference forests
Cocoa farms and reference forests were selected from three communities, Akaasu (C1), Kyeikrom (C2) and Tuntum (C3), in the district, as shown in Fig. 1. Three farms were sampled in Akaasu and Kyeikrom, while four were in Tuntum. Uncultivated forests about 500 m from these plantations were also selected as references. The reference forests were labeled as reference forests 1 (F1), 2 (F2) and 3 (F3) for Akaasu, Kyeikrom and Tuntum, respectively. The sampling points were obtained using a Geographic Positioning System (GPS) device.
2.2.2 Soil sampling
Soil samples were randomly taken from ten (10) selected cocoa farms sprayed with copper-based fungicide seasonally for 15 years in July, which is recommended for spraying cocoa farms in Ghana. A pair of new, non-powered, disposable gloves were used for taking soil samples. The gloves were changed during the sampling when cleanliness was compromised [18]. Soils from forests close to the farms which have never been sprayed with these fungicides were selected as control. A soil auger was used for the sampling. Soil samples were collected at two distinct depths, thus, 0–15 cm and 15–30 cm. For each depth, six cores were taken and mixed thoroughly in a plastic bowl into a composite sample. Two composite samples were taken from the ten (10) selected farms and labeled, giving 20 samples at each depth. The fresh soil samples were divided into two. One was immediately sieved through 4 mm mesh and stored at 4 °C for the soil microbial test. The other half was air dried, sieved through a 2 mm mesh and stored in plastic bags for the chemical analysis. All soil samples were sent for analysis at the Soil Research Institute of the Council for Scientific and Industrial Research (CSIR) at Kwadaso, Kumasi.
2.3 Chemical analyses
2.3.1 Soil pH
The pH meter was measured in a 1:1 soil–water (w/v) ratio using a pH meter (H19017) Microprocessor, Hanna Instruments. The pH meter was calibrated before the measurement was taken. 25 g of soil was weighed into a 50 mL polyethylene beaker and 25 mL of distilled water was added and the solution was stirred thoroughly and allowed to stand for 30 min. The pH was measured by immersing the electrode in the upper part of the soil solution.
2.3.2 Soil organic carbon
Determination of soil organic carbon was done by the modified, as described by [19]. It involves the oxidation of the organic matter with potassium dichromate. The excess dichromate was titrated against ferrous sulphate after the reaction. Air-dried sample of 1.0 g was weighed into a clean and dry 250 mL Erlenmeyer flask. The 10 mL 0.1667 M potassium dichromate (K2Cr2O7) solution was accurately dispensed into the flask using the custom laboratory dispenser. The flask was swirled gently so that the sample was made wet. Afterward, 100 mL of distilled water was added and mixed well and 20 mL of concentrated sulphuric acid (H2SO4) was dispensed rapidly into the soil suspension using an automatic pipette and swirled vigorously for 1 min and allowed to stand on a porcelain sheet for about 30 min. The addition of 10 mL of ortho-phosphoric acid and 1 mL of diphenylamine indicator was added and titrated by adding 1.0 M ferrous Sulphate from a burette until the solution turned dark green at the end-point from an initial purple colour. About 0.5 mL 0.1667 M K2Cr2O7 was added to restore excess K2Cr2O7 and by adding FeSO4 drop-wise to attain a stable end-point. The volume of the FeSO4 solution used was recorded.
The soil organic carbon content was calculated as:
where
M = molarity of ferrous Sulphate solution.
V1 = mL of ferrous Sulphate solution required for blank.
V1 = mL of ferrous Sulphate solution required for the sample.s = weight of dried soil samples in grams.
0.39 = 3 × 0.001 × 100% × 1.3 (3 = equivalent mass of carbon)
1.3 = a compensation factor for the incomplete combustion of the organic carbon.
Using the formula, % organic matter was calculated as;
2.3.3 Determination of total nitrogen
Determination of total nitrogen was done by the Kjeldahl digestion and distillation method [20]. 2.0 g soil was weighed into a Kjeldahl digestion flask and 5 mL distilled water was added. The flask was placed on a Kjeldahl digestion apparatus and heated initially gently and vigorously for at least 3 h. Selenium tablets and 5 mL of concentrated H2SO4 were added to the soil sample. After a clear mixture was obtained, the flask was removed and then allowed to cool. About 40 mL of distilled water was added to the digested material and transferred into a 100 mL distillation tube. 20 mL of 40% NaOH was added to the solution and then distilled using the Tecator Kjeltec distiller. The digested material was distilled for four min. and the distillate was received into a flask containing 20 mL of 4% boric acid (H3BO3) prepared with PT5 (bromocresol green) indicator producing approximately 75 mL of the distillate. After distillation, the colour change was from pink to green, after which the flask's content was titrated with 0.02 M HCl from a burette. The volume of 0.02 M HCl used was recorded and %N was calculated at the end-point when the solution changed from weak green to pink. A blank distillation and titration were also carried out to take care of traces of nitrogen in the reagents and the water used.
The percentage of nitrogen in the sample was expressed as:
where
M = concentration of hydrochloric acid used in the titration.a = volume of hydrochloric acid used in sample titrationb = volume of hydrochloric acid used in the blank titration.s = weight of dried soil sample in grams.
2.3.4 Digestion of soil samples for total copper and extractable copper
2.0 g of each soil sample was placed in a beaker and the metal contents were extracted by adding 15 mL of 50% HNO3 and placed on a hot plate with a watch glass cover and, heated at 95 °C for 15 min. The heating continued with partial covering without boiling till the solution was reduced to about 5 ml and allowed to cool. 2 mL of distilled water and 3 mL of 30% H2O2 was then added and heated gently. The addition of 5 mL followed by concentrated HCl and 10 ml distilled water and refluxed again for 15 min. The solution was filtered after cooling and the filtrate was transferred into a 50 ml volumetric flask and topped up to the mark with distilled water [21]. A blank sample was also treated in the same way. Each was filtered using a Whatman filter paper (Cat No 1001 110) grade 1.10 g of soil sample was weighed into a shaking bottle. 30 ml of ammonium acetate and ethylenediaminetetraacetic acid (EDTA) were added to the sample and then shaken for 2 h. on a reciprocating shaker. Using Whatman filter paper No.42 the samples were filtered into a flask. 5 mL of the filtrate was pipetted into a test tube and then 10 mL of lanthanum chloride (LaCl3) solution was added. For the total copper the digestion was done using HClO4, HNO3, HF. The metal concentrations were determined using the Atomic Absorption Spectrophotometer (AAS) [22].
After digestion, the solutions obtained were analyzed for total and extractable metals using AAS (Buck Scientific AAS, Model 210 VGP). Separate calibration curves were prepared for copper by running different concentrations of standard solutions. The instrument was set to zero by running the respective reagent blanks. The digested solutions were aspirated individually and atomized in an air-acetylene flame. All samples were run in triplicates and average mg/kg values were taken for each determination [22] and compare to the detection limit for copper in the soil in Table 1.
2.3.5 Soil microbial biomass carbon and nitrogen
Microbial biomass was determined by the chloroform fumigation method and extraction (FE), as described by [24]0.10 g of sieved field moist soil sample was put in a crucible and placed in a desiccator. A shallow dish containing 30 mL of alcohol-free chloroform was placed in the desiccator. A 10 g soil sample was also placed in a separate desiccator without chloroform as a control. The desiccators were covered and allowed to stand at room temperature for 5 days [25]. After fumigation, 50 mL of 0.5 M K2SO4 solution was added immediately to the soil samples to extract microbial carbon and nitrogen from the lysed microorganism. The total nitrogen in the extract was determined by the Kjeldahl method. The amount of microbial carbon in the extract was determined using the colorimetric method. An aliquot 5 mL of the extract was pipetted into a 250 mL Erlemeyer flask. To this were added 5 mL of 0.17 M potassium dichromate and 10 mL concentrated Sulphuric acid. The resulting solution was allowed to cool for 30 min. after which 10 mL of distilled water was added. A standard series was developed concurrently with carbon concentrations ranging from 0, 2.5, 5.0, 7.5, and 10.0 mg/m. These concentrations were obtained when volumes of 0, 5, 10, and 20 ml of a 50 mg/mL C stock were pipetted into labeled 100 mL volumetric flasks and made up to the mark with distilled water. The absorbances of the standard and sample solutions were read on a spectronic 2ID spectrophotometer at a wavelength of 600 nm.
A standard curve was obtained by plotting absorbance values of the standard solution against their corresponding concentration. Extracted carbon concentration of the samples was determined from the standard curve. For biomass C and N calculations, k-factors of 0.35 and 0.45 [24] were used, respectively. The following equations [24] were used to estimate the microbial C and N from the extracted C and N, respectively:
where
E = the extracted nitrogen produced following fumigation,
Ec = the extracted carbon produced following fumigation;k = the fraction of the killed biomass extracted as carbon or nitrogen under standardized conditions.
2.3.6 Soil microbial biomass phosphorus
Microbial biomass P level was determined using 5.0 g of field-moist soil weighed into a crucible and fumigated in a desiccator with 30 mL of alcohol-free chloroform for 5 days.
Another crucible containing 5 g sample was placed in a separate desiccator without fumigation as control. Both unfumigated and fumigated samples were shaken with 35 mL Bray’s No.1 extracting solution (0.03 M NHF + 0.025 M HCI) for 10 min. and filtered. Correction for adsorption of P during fumigation was made by simultaneously equilibrating unfumigated soil with a series of P containing standard solutions followed by extraction with the Bray-1 solution. The amount of P was determined according to the relationship between P added (from standard solution or microbial lysis) and P extracted by the Bray-1 solution [24]. At equilibrium phosphorus adsorption is described by the following equation [26]
where
Extp = Pi concentration (mg/L) extracted after equilibration with different amount of P added; Ext0 = Pi concentration extracted without P addition,b1, b2 = coefficients estimated by non-linear regression of mean values of Extp against Pad,
Pad = amount of P added (0–20 mgkg−1).
Chloroform released is calculated from the equation, P corresponds to a P addition and
where
Pchl = chloroform released P (mgkg−1).
Extchl = Pi concentration in extracts of fumigated samples.
The amount of microbial P is estimated by assuming a kp factor of 0.4
The pollution level was estimated using the soil enrichment factor and Index geo-accumulation of Cu to provide information on the current levels of Cu pollution in the study area [27].
2.3.7 Statistical analysis
The statistical tools used to analyze data were Analysis of Variance (ANOVA) techniques and the correlation model. These statistical procedures were computationally implemented using the STATA (version 12) software package (2011) at a significance level of 5% (P < 0.05). Descriptive statistics (mean and standard deviation) were used to describe the concentrations of soil chemicals (pH, organic matter) and microbial biomass. Ordinary Least Square (OLS) correlation coefficient was used in testing the strength of relationships (±) between the Cu (extractable and total) deposits and soil microbial biomass concentrations, while R2 established the extent (proportion) of variability explained by the Cu deposit in these relationships.
3 Results
3.1 Soil chemical properties recorded at soil depth 0–15 cm
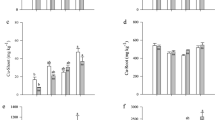
Mean values of soil pH, nitrogen, organic matter extractable copper, and total copper recorded in the top soil (0–15 cm) from Akaasu, Kyeikrom and Tuntum cocoa plantations and the reference forests are presented in Table 2. Generally, the pH from the sample farms was higher than the reference soil samples due to the accumulation of copper. Results from ANOVA showed that there were high significant differences (P < 0.05) between the cocoa farms’ soil samples and the reference forests’ soil samples in relation to extractable and total copper in the top soils of all three study locations. Analysis of variance (ANOVA) showed that the pH of cocoa farms soil samples selected from Akaasu, Kyeikrom and Tuntum did not differ significantly (P > 0.05) from soils of the reference forests. Mean values of nitrogen in the top soils of cocoa farms selected from Akaasu, Kyeikrom and Tuntum with their reference forests did not show significant differences (P > 0.05) from the analysis of variance. Similarly, organic carbon and organic matter showed no significant differences (P > 0.05) with their reference forests soils in all the three study locations.
Table 2 shows the soil pH, nitrogen, organic carbon, organic matter, extractable copper, and total copper recorded at soil depth of 15–30 cm from cocoa farms located within the study areas and the three reference forests. Generally, the concentrations of extractable and total copper recorded from sub-soils of all the selected cocoa farms were higher, compared to the reference forests soil samples. Results from ANOVA showed that there were significant differences (P < 0.05) between cocoa farms soil samples and the reference forest soils samples in relation to extractable and total copper in the sub-soils of all the three study locations. Analysis of variance (ANOVA) showed that pH from selected cocoa farms soil samples at Akaasu, Kyeikrom and Tuntum did not differ significantly (P > 0.05) from soils of their reference forests in the sub soils. Mean values of nitrogen in the sub-soils of cocoa farms selected from Akaasu, Kyeikrom and Tuntum with their reference forests also did not show significant differences (P > 0.05) from the analysis of variance. Similarly, organic carbon and organic matter showed no significant differences (P > 0.05) with their reference forest soils in all three sub-soil study locations.
Table 3 shows correlation coefficients relating soil chemical properties and extractable copper in the top soil from cocoa farms at Akaasu, Kyeikrom and Tuntum. Soil pH showed negative relationship with extractable copper but did not correlate significantly (P > 0.05). Organic matter correlated negatively but not significant (P \(<\) 0.05) with extractable copper. Additionally, with multiple coefficient of determination R2 = 0.559, 0.297 and 0.205 for Akaasu, Kyeikrom and Tuntum, respectively. This indicates that pH and organic matter relate more than half the proportion of the total variability in the content of extractable copper in the top soils of cocoa farms located at Akaasu, but the rest of the farms were less than half of the proportion.
Correlation coefficients relating soil chemical properties and total copper in the top soil from cocoa farms at Akaasu, Kyeikrom, and Tuntum are presented in Table 4. Soil pH and organic matter showed negative correlation to total copper in the top soils of cocoa farms located at all the farms selected at the three communities. However, Kyeikrom p-value was significant indicating that the chemical properties can be influenced by the total copper accumulated in the soil.
Mean values of soil microbial biomass carbon, nitrogen and phosphorus recorded in top soil (0–15 cm) from Akaasu, Kyeikrom and Tuntum cocoa plantations and their reference forests soil samples are presented in Table 5. Results from analysis of variance showed that soil microbial biomass carbon values recorded in soil samples from Akaasu cocoa farms did not differ significantly (P > 0.05) from the reference forest soil samples. Microbial biomass carbon values recorded in soil samples from Kyeikrom were also not significantly different (P > 0.05) from the reference forest soil samples. Similarly, Microbial biomass carbon values from Tuntum cocoa farms showed no significant difference (P > 0.05) from the reference forest soil samples. From Table 6, the ANOVA results showed no significant difference (P > 0.05) between microbial biomass N2 values and reference forest soil samples from cocoa farms at Akaasu. The microbial biomass N2 recorded at Kyeikrom cocoa farms was also not significant (P > 0.05) compared with the reference forest soil samples. Microbial biomass N2 values recorded from cocoa farms at Tuntum and the reference forest soil samples did not differ significantly (P > 0.05). Microbial biomass phosphorus recorded in soil samples from Akaasu cocoa farms and the reference forest soil samples were not significantly different (P > 0.05). Microbial biomass phosphorus recorded in soil samples from Kyeikrom cocoa farms did not differ significantly when compared with soil samples from reference forest (P > 0.05). Again, microbial biomass phosphorus in the top soils of Tuntum cocoa farms did not differ significantly (P > 0.05) from that of reference forest soil samples.
The results presented in Table 6 shows correlation coefficients relating extractable copper and microbial biomass carbon in the top soils of cocoa farms located at Akaasu, Kyeikrom, and Tuntum. Extractable copper showed negative relationship with microbial biomass carbon but not significant at (P > 0.05) levels at all the selected farms in the various communities.
The results presented in Table 7 shows correlation coefficients relating extractable copper and microbial biomass carbon in the top soils of cocoa farms located at Akaasu, Kyeikrom, and Tuntum. Extractable copper showed negative relationship with microbial biomass nitrogen but was not significant at (P > 0.05) levels at all the selected farms in the various communities.
The results presented in Table 8 shows correlation coefficients relating extractable copper and microbial biomass carbon in the top soils of cocoa farms located at Akaasu, Kyeikrom, and Tuntum. Extractable copper showed negative relationship with microbial biomass nitrogen but was not significant at (P > 0.05) levels at all the selected farms in the various communities.
4 Discussion
Results from the study indicate that the concentrations of extractable copper in both the top and sub-soils were higher in the cocoa plantations than in the reference soils. Similarly, total copper concentrations were significantly higher in the soils from the cocoa plantations than the reference forest soils. This finding is consistent with the work of [28, 29] in a study of the impact of copper-based fungicides application on the contamination of cocoa plants and soils. These differences might be due to the prolonged application of copper-based fungicides to control fungi in the cocoa plantations. [30, 31] found that the amount of micronutrients such as, manganese, zinc, and iron decreased as the copper content in the soils of grape farms increased due to the prolonged application of the Bordeaux mixture. Similarly, due to fungicide application the available phosphorus contents of the soils decreased at both surface and sub-surface layers. Immobilization of available phosphorus or fixation may be encouraged as a result of increasing base saturation of the soils with fungicide residues [32]. [29, 33] also found that the amount of phosphorus available to plants reduced with increasing copper content of soils in cocoa plantations and caused nutrient imbalance. The Tuntum cocoa plantations recorded the highest mean values of total copper in the top and sub-soils of 286.54 ± 69.26 mgkg−1 and 223.10 ± 30.29 mgkg−1 respectively. Though the concentrations of total copper have not exceeded the critical levels of 800 mgkg-1for crops receiving foliar copper-based fungicidal spray as noted by [30, 34], prolong usage of copper-based fungicides will gradually increase their levels and adversely affect soil biodiversity. Results of the study also showed that the concentration of copper was higher in the top soils in all the study locations than in their respective reference forests. This could be attributed to the high affinity of Cu for soil organic matter and hydrous oxides, which means that Cu is likely to build-up in top soils due to its strong interaction over a long period of time [34]. According to [35], in the soil profile where there is greatest biological activity Cu accumulation is likely to occur which corresponds to the zone in the top soils. Results from the correlation analysis revealed that pH and organic matter correlated negatively with extractable and total copper with multiple coefficient of determination which suggests that pH and organic matter relate more than half the total proportion of variability in the content of extractable and total copper in all the three study locations. The mean pH was generally higher in the top soils than the sub soils for all the study locations. Soil samples from cocoa plantations recorded higher values of soil pH in top and sub soils than the reference forests. Among the cocoa farms locations, Kyeikrom recorded the highest pH in the top soil, while Tuntum cocoa plantations recorded the highest pH in the sub soil. The more acidic or lower values of pH recorded in the reference forests suggests to the fact that, as pH decreased, the proportion of copper present in soil solution as Cu2+ increased. Movement of copper along the soil profile, phytotoxicity threshold for crops and bioavailability for root uptake depend on soil pH [36], quality of organic matter, soil texture, cation exchange capacity [37, 38]. The predominant factors influencing copper availability are organic matter and soil pH [34]. As organic matter in the soil increases, copper availability decreases. Organic matter binds copper more strongly than any other micronutrient [34]. This strong interaction reduces fixation by soil mineral and leaching, as well as its availability to crops. When the soil pH is increased, the amount of copper held by clay and organic matter increases, making copper availability to plants also to decreased [34]. The levels of organic carbon in the top soils were higher than the sub soils. Also, reference samples from Akaasu cocoa farms recorded the highest levels of organic carbon in both top and sub soils. According to [39] organic carbon correlates with organic matter in soils and that soil organic matter acts as the major sink and source of organic carbon with measured soil organic carbon content often serving as a proxy for soil organic matter. Results from the study also showed that organic matter contents were higher in the cocoa plantations than the reference samples except F1. This might be due to decomposition of materials from the trees (litters, residues of pod husks) from the cocoa plantations over periods under cultivation. The organic matter content also decreased with increasing soils depth. Findings from ordinarily least square regression revealed that extractable and total copper in the top correlated negatively with the levels of organic matter. This could be attributed to the fact that organic matter has the capability of binding copper, therefore making it difficult for it to be released for plant use. These findings are in line with studies done by [37] to determine the effect of soil organic matter levels in extractabilities of zinc, manganese, and copper in soil solutions concentrations. Nitrogen levels in the top soils were also higher than the sub soils for all the study locations. The levels of nitrogen in the sub soils from all the cocoa farms were not different from the reference samples. Reference samples F1 from Akaasu recorded the highest nitrogen levels in the top soil. Total nitrogen of the soil correlates with the organic carbon content and a change in the content of the organic matter in soil whether by addition or by loss is usually accompanied by a change in the content of nitrogen according to Wild [35]. [34] also noted that nitrogen of the soil usually exists almost entirely in the organic matter. In this form, nitrogen is not available for use by plants directly but must first be transformed by soil bacteria (oxidation) to an available form such as nitrates which are soluble in water and which may be absorbed by plants. Extractable and total cadmium were higher in soil samples from the cocoa plantations than the reference samples. The higher values of cadmium observed in the cocoa plantations might be due to application of cocoa fertilizers (Asaasewura, Cocofeed) by farmers of the selected cocoa farms in the past years under cultivation of the land. The most likely origin of the excess cadmium in soils according to [31] is from heavy contaminated phosphate fertilizers. Results from the study showed that the levels of microbial biomass carbons, nitrogen and phosphorus were not significantly different from their respective reference forests in all the study locations. This suggests that soil microbial biomass was not adversely affected by the application of copper- based fungicides. The soil microbial biomass is both a source and sink of the nutrients contained in the organic matter. It acts as the transformation agent of the organic matter in soil [40]. However, the overall biological activity of the soil is been controlled by soil microbial biomass as agent. Findings from ordinarily least square regression revealed that extractable correlated negatively with concentrations of microbial biomass carbon, nitrogen and phosphorus in all the study locations. All organisms required copper as an essential element and deficiency may results in reduction in biological function and potentially death. However, high levels of copper when found in soils may be toxic to plants and soil microorganisms and can lead to lower biological activity and eventually loss of soil fertility [38]. [36] also found that high copper concentration reduces microbial activity and function in soils. Soil microorganisms have been impacted negatively by copper residues in avocado orchards according to [41]. The study has shown that the concentrations of copper in the soils of cocoa plantations will gradually increase with prolong application of copper-based fungicides which will eventually affect the activities of soil microorganisms. This phenomenon may pose a threat leading to reduced surface activity, greater litter build-up and consequent loss of soil fertility in cocoa plantations. It was observed that the selected cocoa farms from Tuntum had the highest accumulation of total copper amongst the three study locations.
5 Conclusion
The findings revealed that the levels of total Cu in the soils of cocoa plantations were higher than that of the forests. Therefore, there has been accumulation of Cu in the cocoa farms as a result of the use of the copper-based fungicides. Soils from the forest were more acidic than those of the cocoa plantation. It was found that the availability of extractable and total Cu in the soils of cocoa plantations decreased with increasing levels of soil pH. The availability of extractable and total Cu correlated negatively with the organic matter. insignificant correlation between extractable Cu and microbial biomass (C, N and P).The study indicated that the concentration of Cu in the soil of the investigated cocoa plantations had not reached its critical levels; however, further prolonged use of copper-based fungicides in the farms may increase their levels, eventually affecting soil microorganisms. Hence, further study can be conducted on this to affirm the results.
Data availability
Data are available upon request.
References
Etaware PM. Some identifiable factors responsible for the variation in cocoa production in Nigeria and other cocoa producing nations, adjudicated by their contributions to the global market. Front Agron. 2022;4:731019. https://doi.org/10.3389/fagro.2022.731019.
Omosebi TR, Adefulu AD, Cole AA. Agricultural credits and Cocoa production in selected local government areas of Ondo state. Int J Manag Sci Bus Res. 2021;10:38–53.
Akrofi AY, Amoako-Atta I, Assuah M, Kumi-Asare E (2014) Pink disease caused by Erythricium salmonicolor (Berk. & Broome) Burdsall: an epidemiological assessment of its potential effect on cocoa production in Ghana. J Plant Pathol Microbiol 5.
Adeniyi D (2019) Diversity of cacao pathogens and impact on yield and global production. In: Theobroma Cacao - employing science for sustainability of Global Cocoa Economy. IntechOpen. https://doi.org/10.5772/intechopen.81993.
Akrofi AY, Amoako-Atta A, Assuah M, Asare EK. Black pod disease on cacao (Theobroma cacao, L) in Ghana: spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop Prot. 2015;72:66–75.
Opoku IY, Assuah MK, Aneani F. Management of black pod disease of cocoa with reduced number of fungicide application and crop sanitation. Afr J Agric Res. 2007;2:601–4.
Opoku, JY, Akrofi, A.Y., Alex Appiah, A. (2007). The spread of phytophthora megakarya on cocoa in Ghana. J Ghana Sci Assoc 2(3).
Akrofi AY, Appiah AA, Opoku IY. Management of Phytophthora pod rot disease on cocoa farms in Ghana. Crop Prot. 2003;22:469–77.
Jørgensen LN, Heick TM. Azole use in agriculture, horticulture, and wood preservation: is it indispensable? Front Cell Infect Microbiol. 2021;11:7302. https://doi.org/10.3389/fcimb.2021.730297.
Miotto A, Ceretta CA, Brunetto G, Nicoloso FT, Girotto E, Farias JG, Tiecher TL, De Conti L, Trentin G. Copper uptake, accumulation and physiological changes in adult grapevines in response to excess Copper in soil. Plant Soil. 2014;374:593–610.
Savithri P, Biju J, Poongothai S (2003) Effect of copper fungicide sprays on the status of micronutrient in soils of hot semi-arid region of India. Tamil Nadu Agricultural University, Coimbatore 641 003.
Opoku IY, Gyasi EK, Onyinah GK, Opoku E, Fofie T (2006) The National Cocoa Diseases and Pests Control Programme (CODAPEC): achievements and challenges. In 15th international cocoa conference, section 9, San Jose’, Costa Rica.
Kumi E. Farmers’ perceptions of the effectiveness of the cocoa disease and pest control programme (CODAPEC) in Ghana and its effects on poverty reduction. Am J Exp Agric. 2015;7:257–74.
Adjinah KO, Opoku IY (2010) The National Cocoa Diseases and Pest Control (CODAPEC): achievements and challenges. from http://news.myjoyonline.com/features/201004/45375.asp.
Georgieva SS, McGrath SP, Hooper DJ, Chambers BS. Nematode communities under stress; the long-term effects of heavy metals in soil treated with sewage sludge. Appl Soil Ecol. 2002;20:27–42.
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L. Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci. 2019;10:1068. https://doi.org/10.3389/fpls.2019.01068.
USEPA (2002) National recommended water quality criteria.
USEPA. Soil Sampling, laboratory services and applied service division. Athens: Georgia; 2020.
Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29–38.
Manoj KM, Narayan CJ. Effects of Sonajhuri (Acacia auriculiformis) plantation on soil health in Purulia district, West Bengal, India. For Resour Resil Confl. 2021;27:343–58.
Buckman ES, Oduro I, Plahar WA, Tortoe C. Determination of the chemical and functional properties of yam bean (Pachyrhizus erosus (L.) Urban) flour for food systems. Food Sci Nutr. 2017;6(2):457–63.
Motsara MR, Roy RN (2008) Guide to laboratory establishment for plant nutrient analysis (FAO) Fertilizer and plant nutrition bulletin (19).
Rosen G, Rivera-Duarte I, Johnston R, Podegracz J (2010) Sinclair and dyes inlets toxicity study: an assessment copper bioavailability and toxicity in surface waters adjacent to the Puget sound naval Shipyard and Puget Sound Naval Shipyard and Intermediate Maintenance Facility, SSC Pacific.
Logah Y, EY Safo , Quansah C, Danso I (2010) Soil microbial biomass carbon, nitrogen and phosphorus dynamics under different amendments and cropping systems in the semi – deciduous forest zone of Ghana West African. J Appl Ecol 17.
Laaksonen TJ, Roos YH, Labuza TP. Comparisons of the use of desiccators with or without vacuum for water sorption and glass transition studies. Int J Food Prop. 2006;4:545–63.
Chen GC, He Z. Determination of soil microbial biomass phosphorus in acid red soils from southern China. Biol Fertil Soils. 2004;39:446–51. https://doi.org/10.1007/s00374-004-0734-6.
Barbieri M. The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J Geol Geophys. 2016;5:1.
Addo-Fordjour P, Gyimah H, Fei-Baffoe B, Akrofi AY. Impact of copper-based fungicide application on contamination of cocoa plants and soil in the Ahafo Ano North District, Ghana. Ecosyst Environ Conserv. 2013;19:303–10.
Sacristán D, Carbó E. Copper contamination in mediterranean agricultural soils: soil quality standards and adequate soil management practices for horticultural crops. In: Larramendy ML, Soloneski S, editors. Soil contamination: current consequences and further solutions. London: IntechOpen; 2016.
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci 1–9.
Jinzhao M, Min Z, Zhiguang L, Haining C, Yuncong CL, Yao S, Qiang M, Chenhao Z. Effects of foliar application of the mixture of copper and chelated iron on the yield, quality, photosynthesis, and microelement concentration of table grape (Vitis vinifera L.). Sci Hortic. 2019;254:106–15.
Ullah MR, Dijkstra FA. Fungicide and bactericide effects on carbon and nitrogen cycling in soils: a meta-analysis. Soil Syst. 2019;3:23.
Kumuduni NP, Sabry MS, Season SC, Daniel CWT, Yohey H, Deyi H, Nanthi SB, Jörg R, Yong SO. Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int. 2020;134:105046.
Palansooriya KN, Shaheen SM, Chen SS, Tsang DCW, Hashimoto Y, Hou D, Bolan NS, Rinklebe J, Ok YS. Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int. 2020;134:105046.
Yang S, Jansen B, Absalah S, Kalbitz K, Chunga Castro FO, Cammeraat ELH. Soil organic carbon content and mineralization controlled by the composition, origin and molecular diversity of organic matter: a study in tropical alpine grasslands. Soil Till Res. 2022;215:105203.
Herencia JF, Ruiz JC, Morillo E, Melero S, Villaverde J, Maqueda C. The effect of organic and mineral fertilization on micronutrient availability in soil. Soil Sci. 2008;173:69–80.
Upadhyay S, Raghubanshi AS (2020) Determinants of soil carbon dynamics in urban ecosystems, (Eds) Verma P, Singh P, Singh R, Raghubanshi AS Urban Ecology p. 299–314.
Ferrol N. Membrane transporters, an overview of the arbuscular mycorrhizal fungal transportome. In: Zaragoza Ó, Casadevall A, editors. Encyclopedia of mycology. London: Elsevier; 2021. p. 44–53.
Kabutey, K. (2017) Accumulation of copper from application of copper-based fungicides and its relationship with soil chemical properties and microbial biomass in Bibiani-Anhwiaso-Bekwai District of Ghana, thesis submitted to KNUST. http://dspace.knust.edu.gh/handle/123456789/10074.
Weil RR, NC Brady (2017) Soil phosphorus and potassium. In: The nature and properties of soils Ed. 15 Pearson, 15.
Akinnifesi TA, Asubiojo OI, Amusan AA. Effects of fungicide residues on the physico-chemical characteristics of soils of a major cocoa-producing area of Nigeria. Sci Total Environ. 2006;366:876–9.
Funding
There was no funding for this research work.
Author information
Authors and Affiliations
Contributions
KKVA, OA, LNAS: Conceptualization, methodology, investigation, formal analysis, data curation, validation, visualization, writing. OA, LNAS: Supervision, review. All authors reviewed the Manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent for participate
Since this study did not recruit human and/or animal subjects, this section does not apply.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kakutey, K., Sackey, L.N.A. & Akoto, O. Impact of accumulation of copper from application of copper-based fungicides on soil properties in Ghana. Discov Environ 1, 1 (2023). https://doi.org/10.1007/s44274-023-00004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-023-00004-2