Abstract
Aims
Research involving analgo-sedation is a priority for parents and professionals in paediatric intensive care, and current guidelines are based on low-quality evidence. Future research will require an understanding of current practice and research priorities of healthcare professionals. This survey aimed to identify perceived barriers to research, describe the current UK analgo-sedation practice and assess outcome priorities for future research.
Methods
A 26-question web-based survey was emailed to all Paediatric Critical Care Society members (n=1000) in April/May 2021. Responses were analysed either by ‘unit’ or at the individual respondent level. Questions related to four patient categories: ‘infant (< 3 months of age) ‘paediatric’ > 3 months of age, ‘cardiac’ and ‘non-cardiac’.
Results
Two hundred sixteen healthcare professionals responded and responses were available from 100% of the UK paediatric intensive care units (n=29) for all questions. Most units (96%, 28/29) routinely use scoring systems for sedation adequacy but few routinely screen for delirium (24%, 7/29). The most highly prioritised outcome measure was the duration of mechanical ventilation. Respondents were most likely to agree to randomise paediatric general intensive care patients to trials comparing two different alpha agonists and least likely to randomise neonatal cardiac patients to trials comparing benzodiazepines with alpha agonists. The most common perceived barrier to research was unit familiarity with a particular regimen, followed by the perception that parents would not provide consent.
Conclusions
This study provides a snapshot of the UK analgo-sedation practice and highlights the importance of public involvement in planning future trials, as well as consultation work across the spectrum of stakeholder clinicians to maximise the acceptability of study design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research involving analgo-sedation is a priority for both parents of patients and healthcare professionals (HCPs) in paediatric intensive care units (PICUs) in the UK. Analgo-sedation-related research questions featured twice in the top ten during a recent UK Delphi exercise [1]. The 2022 Society of Critical Care Medicine Guidelines on Prevention and Management of Pain, Agitation, Neuromuscular Blockade and Delirium in Critically Ill Paediatric Patients with Consideration of the ICU Environment and Early Mobility (SCCM-PANDEM guideline) features 50 recommendations, only 3 of which are based on high-quality evidence [2], highlighting the need for further research.
Paediatric analgo-sedation research has, however, been hindered by poor patient recruitment, with two large randomised controlled trials (RCTs) comparing benzodiazepines with clonidine closing prior to reaching recruitment targets [3, 4]. Future research will require an understanding of current practice, analgo-sedation research priorities of HCPs and acceptability to parents and professionals.
Recent published survey data from the European Society of Paediatric Intensive Care Medicine (ESPNIC) describes the use of benzodiazepine and opioid combination therapy as continuing to make up usual care in the majority of PICUs. This survey only included responses from 9 UK PICUs. This is similar to a survey of mainly North American PICUs from 2014. These surveys examine reported ‘usual care’ and also some aspects of monitoring (pain and sedation scores) but with limited coverage of UK patients and did not assess respondents’ attitudes to sedation-related research [5, 6]. Concerns exist relating to the utilisation of benzodiazepines in PICU patients because of a dose-response relationship between exposure and incidence of delirium [7, 8]. Dexmedetomidine and clonidine do not interfere with natural sleep patterns in paediatric patients, and it is plausible that this sleep-like sedation may be less deliriogenic that that achieved with benzodiazepines [9]. However, there is a complete lack of prospective evidence to determine if primary sedation with alpha agonists can modifiy delirium incidence.
The aims of this survey were to collect responses from the UK PICU professional population to inform the design of future analgosedation research trials:
Firstly, to gain insight into the views of UK professionals regarding the conduct of future research to enable researchers to optimise the design of future studies to maximise recruitment. Specifically, to assess the acceptability of randomising different patient groups to classes of the sedative agent and to document perceived barriers.
Secondly, to explore current practice, including the most frequent analgo-sedative combinations and the availability of alpha agonists to document ‘usual care’.
Finally, to assess the feasibility of delirium incidence as an outcome measure in future trials by recording whether delirium is recognised and recorded in a structured fashion and to ask healthcare professionals how they would prioritise available outcome measures.
Materials and methods
A 26-item web-based survey (SurveyMonkey Inc., CA, USA) was designed by a group of Paediatric Critical Care Society Study Group (PCCS-SG) members based on a literature review and piloted in a single tertiary paediatric intensive care unit (PICU) amongst 30 relevant HCPs, following which 4 questions were modified for clarity. Following approval by the wider UK PCCS-SG the survey link was distributed by email to all PCCS (Paediatric Critical Care Society) members (n=1000). Recipients were informed of the planned use of data, and no personally identifiable information was collected; therefore, consent was assumed by completion. UK requirements do not require formal ethical approval for staff surveys; therefore, no formal ethical approval was sought. Supplementary figure S1 shows the survey tool. The 26-item questions were a mixture of multiple choice, ranked items, and free text answers.
Questions in the survey sought responses for four distinct groups of patients around sedative/analgesic use and acceptability of future studies: ‘cardiac’ patients were defined as patients for whom the primary reason for intensive care admission was recovery from cardiac surgery, or receiving medical treatment for a cardiac disorder, and ‘general’ patients were patients admitted for all other reasons. Patients were divided into ‘cardiac’ and ‘non-cardiac’ as there may be differing risks and requirements for agents between these groups, as well as different professional stakeholders. ‘Infant’ patients were defined as patients < 3 months of age and paediatric >3 months of age. The infant age group was separated as this age group may have different sedative requirements and is the group with the strongest recommendations against benzodiazepine use [10].
Analysis
Data were imported directly from SurveyMonkey (Momentive Inc., San Mateo, CA, USA) into Microsoft Excel for analysis (Microsoft Corporation, 2018). All surveys with at least one question answered were analysed. Data were analysed descriptively using percentages. Questions 6–12 and 25 (See Supplementary figure S1) were analysed at a unit level with accuracy checked if less than 80% concordance was observed (via email directly to the clinical lead of the unit). All other questions were analysed at the respondent level. The free text answers were categorised by one author according to the most common themes identified. A random sample of answers was then categorised by a second author to ensure consistency. The number of answers on each theme was then quantified.
Results
Two hundred sixteen HCPs answered at least one question. Fifty-five percent (119/216) of respondents were nurses and 37% (80/216) doctors. All questions analysed at a unit level had representation from 100% of UK PICUs (29/29). This represents a response rate of approximately 20% of UK PCCS members. Seventeen (59%) of the units had responses from both doctors and nurses 6 units had responses from nurses only and 6 from doctors only (21% and 21%). All questions within the survey were optional, meaning that the completion rate was variable between questions. The number of respondents for each question is reported with the relevant result. Table 1 shows the distribution of responses across UK PICUs.
Table 2 is a summary of answers to questions analysed at the respondent level. When considering respondents who answered the question, in paediatric cardiac patients, 64% (72/112) respondents would be prepared to randomise patients to a trial comparing dexmedetomidine and midazolam, and 71% (81/112) comparing clonidine and dexmedetomidine. In general patients, this increased to 70% (94/139) and 77% (107/139). In neonatal cardiac patients, less than half (46%, 52/113) would randomise patients to a trial comparing dexmedetomidine and midazolam, and 64% (72/113) to compare the 2 alpha agonists, rising to 51% and 71% (70/137 and 97/137) in general neonatal patients.
One hundred and two respondents placed a free text suggestion stating their perceived barriers to analgo-sedation-related research. The most common perceived barrier to conducting analgo-sedation-related research expressed by 25% (26/102) respondents was a unit preference or familiarity with a particular regimen, followed by the perception that parents would not provide consent for their child to participate (25%, 25/102).
Figure 1A–D demonstrate patterns of reported sedative/analgesic use (108 respondents reported practice in cardiac patients and 153 in general patients).
a Patterns of reported use of sedative and analgesic agents by healthcare professionals in the UK in paediatric (> 3 months old) cardiac patients. This question was answered by 153 respondents for general patients and 108 respondents for cardiac patients (scale = ‘what percentage of patients treated within your unit would receive the following agent?’). b Patterns of reported use of sedative and analgesic agents by healthcare professionals in the UK in infant (< 3 months old) cardiac patients. This question was answered by 153 respondents for general patients and 108 respondents for cardiac patients. (Scale = ‘what percentage of patients treated within your unit would receive the following agent?’). c Patterns of reported the use of sedative and analgesic agents by healthcare professionals in the UK in paediatric (> 3 months old) non-cardiac patients. This question was answered by 153 respondents for general patients and 108 respondents for cardiac patients. (Scale = ‘what percentage of patients treated within your unit would receive the following agent?’). d Patterns of reported use of sedative and analgesic agents by healthcare professionals in the UK in infant (< 3 months old) non-cardiac patients. This question was answered by 153 respondents for general patients and 108 respondents for cardiac patients. (Scale = ‘what percentage of patients treated within your unit would receive the following agent?’)
Table 3 is a summary of the responses to the survey, which were analysed at the unit level. Dexmedetomidine was reportedly unavailable for any use in 41% (12/29 PICUs) and only used regularly in 14% (4/29). In units with a cardiac surgical programme, dexmedetomidine was unavailable at 33% (4/12) and used frequently at 16% (2/12) (Table 3). Most units (93%, 28/29) routinely used COMFORT-B scoring to assess sedation adequacy [11]. Seventy-two percent (21/29 units) used a scoring system to detect iatrogenic withdrawal syndrome (IWS), the most utilised tool being the withdrawal assessment tool version 1 [12]. Only 24% (7/29) of units reported that they used a scoring system to detect delirium, the most common score being the Cornell Assessment of Paediatric Delirium [13] (Table 3). Checking for lack of concordance was required in 3 cases regarding delirium and withdrawal scoring, and on each of these 3 occasions, the team was in the process of introducing the score, which may explain why some staff responded that they did use the score, whilst others reported that they did not.
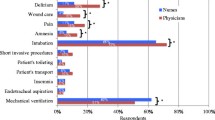
The most highly prioritised outcome measure for future trials was the duration of mechanical ventilation, followed by the frequency of occurrence of delirium and withdrawal. This question was answered by 136 respondents (Table 4).
Discussion
This survey demonstrates that current UK analgo-sedation practice continues to involve morphine and midazolam in the majority of cases; delirium screening at the time of this survey was infrequent and that trials of sedative agents would be acceptable to the majority of clinicians.
The strength of the study is that it is the first description of UK PICU analgo-sedation practice in over 15 years, and it includes responses from HCPs working in 100% of the 29 UK PICUs. This enables us to describe practice both according to unit of response, as well as to gauge the opinion of a wide range of professionals. A further strength is the multidisciplinary nature of the sample. The UK has previously been under-represented in published surveys, with responses from only 9 centres in a recent EPSNIC survey and Europe representing only 14% of responses in the North American equivalent [5, 6]. This may reflect both difficulties with survey dissemination to UK clinicians and an increasing survey burden to UK PICU clinicians.
The recent SCCM-PANDEM guidelines suggest that alpha agonists should be the primary sedative class for PICU sedation, with dexmedetomidine for cardiac surgical patients expected to extubate early [2]. However, the ESPNIC survey found that alpha agonists made up part of the first choice sedative regimen in just 18% of PICUs surveyed [5]. Between 10 and 17% of respondents in our survey (dependent on patient category) reported using clonidine in >75% of patients which appears to be consistent with the ESPNIC findings (Fig. 1) [5]. Dexmedetomidine use is not frequent within the UK according to our survey (Fig. 1), having been the first choice sedative in 11% of units in the ESPNIC survey. This survey reported that 51% of units use fentanyl as the first choice opiate which suggests a difference between the UK and mainland Europe, as between 7 and 14% of UK respondents replied that they used fentanyl in >75% of patients. This is in contrast with morphine, which was used as the first choice in 29% of units in the ESPNIC survey but used in >75% of patients by between 73 and 80% of respondents in this survey, reflecting that morphine is more likely to be the first choice opiate in the UK than in mainland Europe. Midazolam is the first choice sedative agent in 71% of units in the ESPNIC survey. In our survey, between 15 and 43% of respondents reported using midazolam >75% of the time perhaps suggesting midazolam use less frequently in the UK, although there is variability between different patient groups and the two surveys were designed differently, making direct comparisons difficult.
The most recent North American-focussed study was in 2014 and showed a continuing predominance of opiate/benzodiazepine combination for the first choice analgo-sedation, alpha agonists being the first choice in 8% of units surveyed, although this may have changed in the subsequent years [6].
It is of interest that despite recent safety concerns regarding the use of chloral hydrate for sedation, only 12–26% of respondents reported never using chloral hydrate and 13–27% of respondents reported using it in almost all patients. In the recent European survey, Chloral hydrate was only used as the first choice in 1% of units, used for difficult sedation in 16% [5]. There are no Food and Drug Administration (FDA) approved products containing chloral hydrate available for use in North America, and its use there is highly restricted [14]. Recommendations within the UK are perhaps less restrictive. The UK government drug safety update recommends the use of chloral hydrate is restricted owing to carcinogenicity data in animals, and concerns regarding the immature metabolism of infants and neonates resulting in a prolonged half-life of metabolites in these groups, with an increased risk of undesirable effects [15]. However, the update does not go as far as to recommend complete cessation of chloral hydrate use for short-term sedation in the PICU setting, and our data demonstrates that the UK use continues to be widespread [15].
Our data suggest that overall, the UK was not currently meeting the SCCM-PANDEM recommendations for screening for delirium, and screening for delirium less frequently than North American PICUs [16]. However, this has changed within the last 6 months with a national drive to implement delirium scoring across PICUs. This is important as the frequency of delirium was prioritised by respondents as an outcome measure for future trials. The majority of respondents reported using midazolam ‘very often’ or ‘always’ in the paediatric age group, suggesting that, although it is recommended that benzodiazepine use is minimised to reduce incidence of delirium, the use remains widespread. We are closer to meeting the recommendations regarding screening for IWS, with over 70% of units having screening in place [2]. Delirium screening is, of course, not only important for recording outcome measures in research trials, but prevention and management are also recognised to be important for clinical patient care, with the presence of delirium having been associated with cognitive decline and increased length of stay in PICU patients [17].
There has been increasing research interest in the use of alpha-agonists as an alternative or adjunct to benzodiazepines for ICU sedation, with five RCTs, and one pilot RCT in children [4, 18,19,20,21,22]. Three of these studies compared dexmedetomidine with midazolam (including 110 children) with results demonstrating reductions in pain, frequency of IWS and opiate use [18,19,20]. One study compared clonidine with midazolam (124 children and found reduced frequency of IWS but increased use of vasoactive medications with clonidine [4]). A further study randomised patients to clonidine as an adjunct compared to a placebo (96 children) and demonstrated a reduction in benzodiazepine use in the clonidine group [21]. Many of these studies were, however, single-centre and underpowered making interpretation difficult. A pilot ‘BABY-SPICE’ trial assessed the feasibility of a larger paediatric trial using dexmedetomidine as the primary sedative in the PICU and found it to be safe and feasible [22]. In the absence of prospective trial evidence, observational data, such as Sperotto et al. from the PROSDEX study, which demonstrated improvement in patient comfort with dexmedetomidine infusion, may be considered although with some incidence of haemodynamic side effects [23]. The RESTORE trial was a cluster randomised RCT that tested the effect of a paediatric sedation management protocol on clinical outcomes. In 2016, Grant et al. carried out a secondary analysis of trial data which highlighted that dexmedetomidine use was already prevalent in the USA by 2013 and found some evidence that it may be of benefit for primary sedation in children without very severe critical illness and useful in facilitation of difficult extubation in some patients [24].
Research in the critically ill paediatric population is challenging, with a small, heterogeneous population and concerns regarding long-term neurodevelopmental side effects of sedative/analgesic agents [25]. However, since the closure of the CLOnidine compared with midazolam for SEDation of paediatric patients (CLOSED) and Safety profile, and the Efficacy and Equivalence in Paediatric Intensive Care Sedation (SLEEPS) trials [3, 4], the adoption of research without prior consent has greatly enhanced feasibility of recruitment to trials in the UK [26, 27]. Of course, not all trials are eligible for research without prior consent, and Patient and Public Involvement and Engagement (PPIE) work used in the design of a study would have to carefully examine the acceptability of differing models of consent for any study being considered. We have demonstrated that, generally, HCPs find trials comparing sedative agents acceptable, but that the patient groups that they would be most concerned about recruiting into trials would be patients under 3 months of age, and those with cardiac conditions. It is therefore important to consider the needs of these groups carefully in study design in order to maximise safety and acceptability, to ensure that the patient groups with the highest need for safety data are not excluded from the research that may provide it. PPIE may increase the willingness of clinicians to recruit patients if acceptability to parents was demonstrated.
It is the opinion of the study team that research in paediatric analgo-sedation is feasible, for the following reasons: usual care is still predominantly made up of opiate-midazolam combinations, in spite of a building but a limited body of evidence that an alternative strategy may be beneficial. In general, the majority of clinicians would be prepared to randomise their patients into trials. In order for a prospective trial to succeed, a strategic approach is required, with detailed PPIE work to establish the acceptability of research without prior consent for a sedation trial. National work is required to engage with healthcare professionals in all groups, perhaps with a particular focus on cardiac intensive care staff, surgeons and anaesthetists to optimise the design of any trial to ensure acceptability. The widespread adoption of delirium scoring is required.
This study is limited because it is a self-reported survey of practice and, as such, the accuracy of responses as a representation of clinical practice cannot be verified. A national survey provides only preliminary evidence and should be followed by prospective audit data in the planning of future trials, although it is useful in documenting the perceptions of clinicians.
It is further limited by some incompletely answered questions, although representation was available for all questions from 100% of units. The distribution of respondents between units is not even with some units over-represented. The categorisation of free text answers was by authors and therefore there is a degree of subjectivity to this approach. Lastly, the respondents to the survey are all HCPs, and parents’ views were not sought.
Conclusion
Morphine and midazolam remain the most frequently used agents for PICU analgo-sedation. Dexmedetomidine is not yet universally available, even in cardiac centres. If future research is to consider priority outcome measures, it is necessary to extend the rollout of delirium screening nationally. There is a need for PPIE in planning any future trials, as well as the engagement of all of the professional stakeholder groups.
Availability of data and materials
All data is available from the corresponding author on request.
Abbreviations
- CLOSED:
-
CLOnidine compared with midazolam for SEDation of paediatric patients
- HCP:
-
Healthcare professional
- ICU:
-
Intensive care unit
- IWS:
-
Iatrogenic withdrawal syndrome
- PICU:
-
Paediatric Intensive Care Unit
- PCCS:
-
Paediatric Critical Care Society
- PCCS-SG:
-
Paediatric Critical Care Society Study Group
- PPI:
-
Patient and public involvement
- RCT:
-
Randomised controlled trial
- SCCM-PANDEM:
-
Society of Critical Care Medicine Guidelines on Prevention and Management of Pain, Agitation, Neuromuscular blockade and Delirium in Critically Ill Paediatric Patients with Consideration of the ICU Environment and Early Mobility
- SLEEPS:
-
Safety Profile, Efficacy and Equivalence in Paediatric Intensive Care Sedation
- SPICE:
-
Sedation Practice in Intensive Care Evaluation
- UK:
-
United Kingdom
References
Tume LN, Menzies JC, Ray S et al (2021) UK paediatric intensive care society study group. Research Priorities for U.K. Paediatric critical care in 2019: healthcare professionals’ and parents’ perspectives. Paediatr. Crit Care Med 22(5):e294–e301. https://doi.org/10.1097/PCC.0000000000002647
Smith HAB, Besunder JB, Betters KA et al (2022) 2022 Society of critical care medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill paediatric patients with consideration of the ICU environment and early mobility. Paediatr Crit Care Med 23(2):e74–e110. https://doi.org/10.1097/PCC.0000000000002873
Neubert A, Baarslag MA, Dijk MV et al (2017) CLOSED consortium. The CLOSED trial; CLOnidine compared with midazolam for SEDation of paediatric patients in the intensive care unit: study protocol for a multicentre randomised controlled trial. BMJ Open 7(6):e016031. https://doi.org/10.1136/bmjopen-2017-016031
Wolf A, McKay A, Spowart C et al (2014) Prospective multicentre randomised, double-blind, equivalence study comparing clonidine and midazolam as intravenous sedative agents in critically ill children: the SLEEPS (Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation) study. Health Technol Assess 18(71):1–212. https://doi.org/10.3310/hta18710
Daverio M, von Borell F, Ramelet AS, Sperotto F, Pokorna P, Brenner S et al (2022) Pain and sedation management and monitoring in pediatric intensive care units across Europe: an ESPNIC survey. Crit Care 26(1):88
Kudchadkar SR, Yaster M, Punjabi NM (2014) Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake-up call for the pediatric critical care community*. Crit Care Med 42(7):1592–1600
Smith HAB, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Thompson JL, Chandrasekhar R, Williams SR, Griffith K, Ely EW, Fuchs DC, Pandharipande PP (2017) Delirium and benzodiazepines associated with prolonged ICU stay in critically ill infants and young children. Crit Care Med 45(9):1427–1435. https://doi.org/10.1097/CCM.0000000000002515
Mody K, Kaur S, Mauer EA, Gerber LM, Greenwald BM, Silver G, Traube C (2018) Benzodiazepines and development of delirium in critically ill children: estimating the causal effect. Crit Care Med 46(9):1486–1491. https://doi.org/10.1097/CCM.0000000000003194
Eberl S, Ahne G, Toni I, Standing J, Neubert A (2021) Safety of clonidine used for long-term sedation in paediatric intensive care: a systematic review. Br J Clin Pharmacol 87(3):785–805
Harris J, Ramelet AS, van Dijk M, Pokorna P, Wielenga J, Tume L, Tibboel D, Ista E (2016) Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med 42(6):972–986. https://doi.org/10.1007/s00134-016-4344-1
Ista E, van Dijk M, Tibboel D et al. Assessment of sedation levels in paediatric intensive care patients can be improved by using the COMFORT “behavior” scale. Paediatr Crit Care Med 2005 6(1):58-63
Franck LS, Harris SK, Soetenga DJ et al (2008) The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in paediatric patients. Paediatr Crit Care Med 9(6):573–580. https://doi.org/10.1097/PCC.0b013e31818c8328
Traube C, Silver G, Kearney J et al (2014) Cornell assessment of paediatric delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med 42(3):656–663. https://doi.org/10.1097/CCM.0b013e3182a66b76
Grissinger M (2019) Chloral hydrate: is it still being used? Are there safer alternatives? Pharm Therapeut 44(8):444–459
UK Medicines and Healthcare Products Regulatory Authority. Chloral hydrate, cloral betaine (Welldorm): restriction of paediatric indication. Downloaded from https://www.gov.uk/drug-safety-update/chloral-hydrate-cloral-betaine-welldorm-restriction-of-paediatric-indication 16th December 2022.
Ista E, Redivo J, Kananur P, Choong K, Colleti J Jr, Needham DM, Awojoodu R, Kudchadkar SR, International PARK-PICU Investigators (2022) ABCDEF bundle practices for critically ill children: an international survey of 161 PICUs in 18 countries. Crit Care Med 50(1):114–125. https://doi.org/10.1097/CCM.0000000000005168
Dervan, Leslie A. MD, MS1,2; Di Gennaro, Jane L. MD, MS1,2; Farris, Reid W. D. MD, MS1,2; Watson, R. Scott MD, MPH1,3. Delirium in a tertiary PICU: risk factors and outcomes*. Pediatric Critical Care Medicine 21(1):p 21-32, January 2020. | DOI: 10.1097/PCC.0000000000002126
Aydogan MS, Korkmaz MF, Ozgül U et al (2013) Pain, fentanyl consumption, and delirium in adolescents after scoliosis surgery: dexmedetomidine vs midazolam. Paediatr Anaesth 23(5):446–452. https://doi.org/10.1111/pan.12128
Garisto C, Ricci Z, Tofani L et al (2018) Use of low-dose dexmedetomidine in combination with opioids and midazolam in paediatric cardiac surgical patients: randomized controlled trial. Minerva Anestesiol 84(9):1053–1062. https://doi.org/10.23736/S0375-9393.18.12213-9
Tobias JD, Berkenbosch JW (2004) Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J 97(5):451–455. https://doi.org/10.1097/00007611-200405000-00007
Salarian S, Khosravi R, Khanbabaei G et al (2019) Impact of oral clonidine on duration of opioid and benzodiazepine use in mechanically ventilated children: a randomized, double-blind, placebo-controlled study. Iran J Pharm Res 18(4):2157–2162. https://doi.org/10.22037/ijpr.2019.14862.12705
Erickson SJ, Millar J, Anderson B et al (2020) Baby SPICE Investigators and the Australian and New Zealand Intensive Care Society Paediatric Study Group (ANZICS-PSG). Dexmedetomidine sedation in mechanically ventilated critically ill children: a pilot randomized controlled trial. Paediatr. Crit Care Med 21(9):e731–e739. https://doi.org/10.1097/PCC.0000000000002483
Sperotto F, Mondardini MC, Dell'Oste C, Vitale F, Ferrario S, Lapi M, Ferrero F, Dusio MP, Rossetti E, Daverio M, Amigoni A (2020) Pediatric Neurological Protection and Drugs (PeNPAD) Study Group of the Italian Society of Neonatal and Pediatric Anesthesia and Intensive Care (SARNePI). Efficacy and safety of dexmedetomidine for prolonged sedation in the PICU: a prospective multicenter study (PROSDEX). Pediatr Crit Care Med 21(7):625–636. https://doi.org/10.1097/PCC.0000000000002350
Grant MJ, Schneider JB, Asaro LA, Dodson BL, Hall BA, Simone SL, Cowl AS, Munkwitz MM, Wypij D, Curley MA (2016) Randomized evaluation of sedation titration for respiratory failure study investigators. Dexmedetomidine Use in Critically Ill Children With Acute Respiratory Failure. Pediatr Crit Care Med 17(12):1131–1141. https://doi.org/10.1097/PCC.0000000000000941
Warner DO, Zaccariello MJ, Katusic SK et al (2018) Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the mayo anesthesia safety in kids (MASK) study. Anesthesiol 129(1):89–105. https://doi.org/10.1097/ALN.0000000000002232
Woolfall K, Frith L, Gamble C et al (2015) CONNECT advisory group. How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open 5(9):e008522. https://doi.org/10.1136/bmjopen-2015-008522
Maitland K, Molyneux S, Boga M et al (2011) Use of deferred consent for severely ill children in a multi-centre phase III trial. Trials 31(12):90. https://doi.org/10.1186/1745-6215-12-90
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Survey construction was performed by Dr. Rebecca Mitting and distribution by Dr. Padmanabhan Ramnarayan. The first draft of the manuscript was written by Dr. Rebecca Mitting, and all 3 authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
UK requirements do not require formal ethical approval for staff surveys; therefore, no formal ethical approval was sought.
All participants were informed of the purpose of the survey and therefore consent for participation was assumed upon completion.
Consent for publication
All participants were informed of the purpose of the survey and therefore consent for publication was assumed upon completion.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. S1.
PICS SG Sedation Survey.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitting, R., Tume, L.N., Ramnarayan, P. et al. A national survey of sedation practice and clinicians’ attitudes regarding sedation-related research in the UK paediatric intensive care units. Intensive Care Med. Paediatr. Neonatal 2, 4 (2024). https://doi.org/10.1007/s44253-024-00026-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00026-5