Abstract
Background
This Bayesian network analysis was performed to assess the effects of different intravenous sedatives on outcomes in adult critically ill patients.
Methods
We searched for and gathered data from MEDLINE, Cochrane Central Register of Controlled Trials, Elsevier and Web of Science databases. Bayesian network analysis was performed to evaluate the effect of different intravenous sedatives on outcome in adult critically ill patients. Random errors were evaluated by trial sequential analysis (TSA).
Results
Twenty-seven studies including 8,599 critically ill adult patients were enrolled in the analysis. Comparisons among lorazepam, midazolam, propofol, dexmedetomidine, haloperidol and placebo or usual care were presented in a network plot. No significant differences were found for longest mortality in critically ill patients. However, when compared with midazolam, dexmedetomidine had a shorter ICU length of stay and a lower incidence of delirium. Meanwhile, midazolam had a longer ICU length of stay when compared with placebo, propofol and usual care. Subgroup analyses were performed respectively in sepsis, invasive ventilated patients and postoperative patients, as well as patients with higher severity of disease. Lower mortality was found in dexmedetomidine group when compared with placebo in postoperative patients. No differences were found for mortality, ICU length of stay and incidence of delirium in other subgroups. When compared with other sedatives, dexmedetomidine shortened ICU length of stay significantly in ventilated patients. TSA indicated lack of firm evidence for a beneficial effect.
Conclusions
No differences were found for longest mortality of different sedatives in adult critically ill patients. However, when compared with midazolam, dexmedetomidine had a shorter ICU length of stay and a lower incidence of delirium. TSA indicated lack of firm evidence for the results. More powered, randomized, controlled trials are needed to determine the effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Sedation is used to avoid agitation-related harm, mitigate stress and improve patient comfort [1]. With various sedation goals, sedative regimens are administrated selectively in different patients. The 2013 PAD guidelines suggest using nonbenzodiazepines (such as propofol and dexmedetomidine) over benzodiazepines (such as midazolam and lorazepam) for sedation in adult critically ill patients, for their advantages in shortening ICU length of stay, and decreasing the incidence of delirium [2].
With their own distinct pharmacological properties and limitations, sedation regimens used intravenously including midazolam, dexmedetomidine, propofol and lorazepam, haloperidol are available for common clinical use in ICU. Many studies comprehensively analyze the role of each sedative for critically ill patients [3,4,5,6,7,8]. However, the latest randomized, controlled study [9] showed that in ventilated patients, using dexmedetomidine for sedation did not result in lower 90-day mortality than usual care, which showed difference with previous studies.
Bayesian network analysis provides evidence from direct and indirect comparisons. In this study, we aim to perform a Bayesian network analysis for the effect of sedation regimens on outcome in adult critically ill patients.
2 Material and Methods
The protocol was registered in the International prospective register of systematic reviews (CRD42019137705).
2.1 Search Strategy
We searched the following databases: Elsevier, Cochrane Central Register of Controlled Trials, Medline and Web of Science databases. We used keywords as searching terms: ‘sedative’ or ‘sedation’ or ‘dexmedetomidine’ or ‘propofol’ or ‘benzodiazepine’ or ‘midazolam’ or ‘lorazepam’ and ‘critical ill’ or ‘critical illness’ or ‘intensive care units’ or ‘intensive care’ or ‘ICU’ or ‘critical care’ or ‘ventilated’ or ‘mechanical ventilation’. Studies published from inception to June 11, 2019 were searched. Additional files or supplemental materials of the studies were reviewed if available. Additional file 1—Search strategy showed search strategies.
2.2 Study Selection
Potential eligible studies were screened, and relevant full-text manuscripts were acquired. Two reviewers assessed studies for the inclusion and exclusion criteria, independently. If there were disagreements, we discussed with another reviewer.
2.3 Inclusion and Exclusion Criteria
Features of the included studies were as follows:
-
1.
Type of studies were prospective and randomized trials;
-
2.
Population were adult patients who admitted to ICU;
-
3.
Regimens were used intravenously for sedation;
-
4.
Studies reported all-cause mortality, including ICU or hospital mortality, 28-day or 90-day mortality, or mortality at other time points.
Studies with theses features were excluded:
-
1.
Who were not published as original articles;
-
2.
Studies compared sedation regimens with other sedation methods, eg. music therapy;
-
3.
Studies with no data of mortality;
-
4.
Full-text articles were unachievable.
Primay outcome was as follows:
Longest mortality, if there are different mortality data published in one study, for example, 28-day and 90-day mortality were both recorded, the longest complete followup was preferentially used.
Secondary outcome were grouped as follows:
-
1.
ICU length of stay
-
2.
Incidence of delirium
-
3.
Incidence of bradycardia and hypotension
2.4 Quality Assessment
Two reviewers assessed study quality independently. If there were disagreements, a discussion was performed with another reviewer. Six aspects were performed for assessing the risk of bias, including allocation concealment, random sequence generation, blinding, incomplete outcome data, selective reporting and other bias. The risk of bias was assesseed as low risk, unclear risk, or high risk.
2.5 Data Extraction
Data including mortality were extracted by two reviewers. If there were disagreements, a discussion was performed with another reviewer. If there were different morality values, the longest mortality was used. An e-mail contact to the authors was perfomed if information was insufficient.
2.6 Statistical Analysis
Data were analyzed using Stata version 14. For dichotomous data, the odd ratio (OR) was reported. For continuous data, mean difference (MD) a with 95% confidence intervals (CIs) were reported. Most continuous data were displayed by mean ± standard deviation (SD). If continuous data were reported in median and interquartile ranges, they were converted to mean ± SD by the following equation: median instead of mean, and SD was equal to interquartile range divided by 1.35.
We performed pair-wise meta-analyses firstly, and used forest plots to investigate the statistical heterogeneity and the I2 statistic. Bayesian network using Markov chain Monte Carlo methods in WinBUGS was used to assess dichotomous data in all arms of each study. We used p value < 0.05 and 95% CIs to assess significance.
To assess consistency, when the inconsistency factors were close to 0, and the 95% CI contained the neutral value, as well as the random effects variance and the inconsistency variance were roughly equal, the data were seemed as consistent. The consistency model was used.
The probability of best sedatives, the second and the third best was assessed by calculation of the OR for each sedation regimen when compared with another.
In the cumulative meta-analysis, considering type-I errors always resulting from an increased risk of error, trial sequential analysis (TSA; TSA software version 0.9 Beta; Copenhagen Trial Unit, Copenhagen, Denmark) was used to combine size estimation with an adjusted threshold for total statistical significance. Calculated as diversity-adjusted information size, information size was suggested by the relative risk reduction of the intervention.
3 Results
3.1 Study Location and Selection
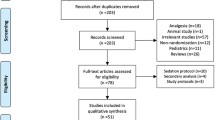
A number of 8,635 records were shown by initial search, and 2,016 records as duplicates. Then the rest 6,619 records were screened, and 67 eligible studies were included. Among them, 35 studies were excluded for no data on mortality; 3 studies were excluded for the study type; 2 studies were excluded for the improper control group and administration route. Finally, 27 studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] including 8,599 critically ill adult patients were gathered in the meta-analysis. The flow diagram is shown in Fig. 1.
Additional file 2—Excluded articles showed the detailed excluded articles.
3.2 Patient and Public Involvement
No patient involved.
3.3 Characteristics of the Studies
Characteristics of the studies are presented in Table 1. Half of the studies were carried out in the United States and Europe; five studies were performed in more than one country; and half of the studies were multi-center studies. Overall, five sedation regimens, usual care and placebo were analyzed. Eight studies had more than 100 participants per group, 23 studies had two-arms and 4 studies had three-arms involving two different sedatives compared with placebo or usual care. Level of sedation are shown in Additional file 3. Detailed longest mortality data and other outcome are shown in Additional file 3 and 4.
Quality assessments are shown in Fig. 2. Risk of bias graph is shown in Additional file 5.
Network graph for the longest mortality is shown in Fig. 3. In a network plot, direct comparisons between sedatives including lorazepam, midazolam, propofol, dexmedetomidine, haloperidol and placebo or usual care are represented by edges, and the nodes represent a sedation strategy. Each two-arm study contributes to one comparison. The edge thickness is proportional to inverse standard error of random effects model comparing different sedation strategy, nodes are weighted according to the number of studies.
Network graph for the longest mortality. Direct comparisons between five sedatives including lorazepam, midazolam, propofol, dexmedetomidine, haloperidol and placebo or usual care are represented by edges in a network plot, and the nodes represent a sedation strategy. Each two-arm study contributes to one comparison. The thickness of the edge is proportional to inverse standard error of random effects model comparing different sedation strategy, nodes are weighted according to the number of studies. Usual care defined as midazolam combined with propofol were used for sedation
3.4 The Impact of Different Sedatives on Longest Mortality and ICU Length of Stay
The effects of different sedation regimens on longest mortality are summarized by Fig. 4. For longest mortality, no significant differences were found among different sedatives. However, when compared with dexmedetomidine, placebo, propofol and usual care, midazolam group had significantly longer ICU length of stay.
Longest mortality and ICU length of stay of sedation regimens. Sedation regimens are shown in alphabetical order. Results are the ORs in the column-defining treatment compared with the ORs in the row-defining treatment. For longest mortality, ORs higher than 1 favor the column-defining treatment. For ICU length of stay, ORs lower than 1 favor the first drug in alphabetical order. Significant results are shown in bold and underscored
TSA was performed with α = 0.05 and β = 0.20 (power 80%) to correct for random error and repetitive testing of sparse data. Based on the intervention effect, diversity-adjusted information size was suggested using a random effects model (with a relative risk reduction of 12% regarding mortality and 5141 patients). TSA indicated lack of reliable evidence for a beneficial effect of dexmedetomidine for mortality (Additional file 6), considering the monitoring boundary not surpassed and the required information size not reached.
3.5 The Impact of Different Sedation Regimens on Incidence of Delirium in Adult Critically ill Patients
The effect of different sedatives on delirium are summarized by Fig. 5. When compared with midazolam, dexmedetomidine group had a significantly lower incidence of delirium.
3.6 The Impact of Different Sedation Regimens on Hypotension and Bradycardia
The effects of different sedation regimens on the incidence of hypotension and bradycardia in adult critically ill patients are reported by Additional file 7. When compared with usual care, dexmedetomidine group and propofol group had higher incidence of hypotension. When compared with midazolam and placebo, dexmedetomidine group had higher incidence of bradycardia.
3.7 The Impact of Different Sedation Regimens on Outcome in Sepsis patients
Detailed characteristics of the studies which included sepsis patients are summarized by Additional file 8 (Table S8-1). For longest mortality (Figs. S8-1, S8-2), ICU length of stay, no significant differences were found among sedatives in sepsis patients.
No significant differences were found for longest mortality (Fig. S8-3), ICU length of stay (Fig. S8-4), incidence of delirium (Fig. S8-5) among sepsis patients who used dexmedetomidine compared with other sedatives.
3.8 The impact of Different Sedation Regimens on Outcome in Invasive Ventilated Patients
Detailed characteristics of the studies which included invasive ventilated patients are summarized by Additional file 9 (Table S9-1). For longest mortality, no statistically significant differences were found among sedatives in invasive ventilated patients by pair-wise meta-analyses (Fig. S9-1).
No statistically significant differences were found for longest mortality (Fig. S9-2) and incidence of delirium (Fig. S9-4) among invasive ventilated patients who used dexmedetomidine compared with other sedatives. However, compared with sedatives other than dexmedetomidine, dexmedetomidine for sedatives shortened ICU length of stay (Fig. S9-3) and duration of mechanical ventilation significantly (Fig. S9-5).
3.9 The Impact of Different Sedation Regimens on Outcome in Postoperative Patients
Detailed characteristics of the studies which included postoperative patients are summarized by Additional file 10 (Table S10-1). For longest mortality, dexmedetomidine group reduced longest mortality significantly when compared with placebo, however, no other significant differences were found among sedatives in postoperative patients (Fig. S10-1).
No significant differences were found for longest mortality (Fig. S10-2) and ICU length of stay (Fig. S10-3) among postoperative patients who used dexmedetomidine or other sedatives. However, there was a trend toward lower incidence of delirium (Fig. S10-4) for postoperative patients who used dexmedetomidine (p = 0.05), when compared with sedatives other than dexmedetomidine.
3.10 The Impact of Different Sedation Regimens on Outcome in Patients with a Higher Severity of Disease
The effects of different sedation regimens on outcome in severe critically ill patients are summarized by Additional file 11. In 19 studies, the severity of disease was reported, 17 studies by the acute physiology and chronic health evaluation II score (APACHE II score). The distinction between higher and lower severity was differentiated by the means or medians of APACHE II scores. For higher severity of disease (APACHE II score ≥ 20), the detailed characteristics of the studies are presented by Table S10-1. No significant differences were found among sedatives for mortality in more severe patients (Fig. S11-1).
No significant differences were found for longest mortality (Fig. S11-2), ICU length of stay (Figure S11-3), incidence of delirium (Figure S11-4) among patients with a higher severity of disease who used dexmedetomidine compared with other sedatives.
4 Discussion
This is an integrate analysis for mortality, ICU length of stay, incidence of delirium, common adverse events including hypotension and bradycardia when compare different intravenous sedatives in adult critically ill patients and common type of ICU patients (including all critically ill patients, sepsis patients, mechanical ventilated patients, postoperative patients and patients with higher severity of disease). In this study, no significant differences were found for mortality of different sedatives. However, when compared with midazolam, dexmedetomidine group had a shorter ICU length of stay and a lower incidence of delirium significantly. Meanwhile, midazolam group have a longer ICU length of stay when compared with placebo, propofol and usual care. When compared with usual care, dexmedetomidine group and propofol group had a significantly higher incidence of hypotension. When compared with midazolam and placebo, dexmedetomidine group had a significantly higher incidence of bradycardia.
There are many network meta-analyses exploring the effectiveness of sedatives in the critically ill [7, 35,36,37]. However, this is the first network analysis to evaluate the effect of sedatives on mortality, ICU length of stay and incidence of delirium in critically ill patients, moreover, subgroup analyses were performed in sepsis, ventilated and postoperative patients, which covering most kinds of patients admitted to ICU. Second, other drugs which not commonly used for sedation were excluded for comparison, as management of pain and sedation should use for different objectives. In this meta-analysis, TSA indicated lack of firm evidence due to considerable heterogeneity between groups. More randomized, controlled trials are needed to determine the effects.
In subgroup analyses, comparisons of different sedatives were performed in sepsis, ventilated and postoperative patients, as well as patients with a higher severity of disease, respectively. In sepsis patients, no statistically significant differences were found among sedatives in sepsis patients for longest mortality, ICU length of stay and the incidence of delirium. In invasive ventilated patients, no significant differences were found for longest mortality and incidence of delirium among who used dexmedetomidine or other sedatives. However, compared with sedatives other than dexmedetomidine, dexmedetomidine for sedatives shortened ICU length of stay significantly.
In postoperative patients, dexmedetomidine reduced longest mortality significantly when compared with placebo, however, no other statistically significant differences were found among sedatives. However, there was a trend toward lower incidence of delirium for postoperative patients who used dexmedetomidine, when compared with sedatives other than dexmedetomidine, which were consistent with another network analysis [38].
The latest guideline recommends early goal-directed sedation, which targeted effective analgesia and light sedation. So it is difficult to compare the drugs used for sedation without considering the level of sedation. Most of the ongoing research in this field does take this into account. Although included studies used different method to assess the sedation level, only few studes targeted deep sedation, most studies targeted light sedation, as a result, we still need more studies to observe the relationship between sedation level and outcome.
Interactions between sedation and ventilation are a topic of the highest importance when comparing the sedation regimens, however, few studies shown the detailed ventilatory settings in the study, so it is hard to find the relationship between them.
There are some limitations in the study. Firstly, critically ill patients who enrolled in the network analysis were heterogeneous. That’s why subgroup analysis was performed to seek the appropriate sedatives for the right patients. Secondly, the controlled sedatives were different, which may have an influence on the results. Last but not the least, this analysis included both direct and indirect comparisons, which lead to the reduced statistical power and uncertainty. Meanwhile, studies were included only if they published in English, which may introduce bias.
5 Conclusions
In this Bayesian network analysis, the results suggested clinically important differences exist between commonly prescribed sedation regimens for both ICU length of stay and incidence of delirium in favor of dexmedetomidine and usual care.
However, TSA indicated lack of firm evidence for the results, more powered, randomized, controlled trials are needed to determine the effects.
Data Availability
Not applicable.
Abbreviations
- APACHE II score:
-
Acute physiology and chronic health evaluation II score
- CI:
-
Confidence interval
- ICU:
-
Intensive care unit
- MD:
-
Mean difference
- OR:
-
Odd ratio
- SD:
-
Standard deviation
References
Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–73.
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306.
Slee A, Nazareth I, Bondaronek P, et al. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393(10173):768–77.
Flukiger J, Hollinger A, Speich B, et al. Dexmedetomidine in prevention and treatment of postoperative and intensive care unit delirium: a systematic review and meta-analysis. Ann Intensive Care. 2018;8(1):92.
Ng KT, Shubash CJ, Chong JS. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74(3):380–92.
Sun R, Wang S, Li S, et al. Effects of dexmedetomidine on delirium and mortality during sedation in ICU patients: a systematic review and meta-analysis protocol. BMJ Open. 2019;9(4): e025850.
Wang H, Wang C, Wang Y, et al. Sedative drugs used for mechanically ventilated patients in intensive care units: a systematic review and network meta-analysis. Curr Med Res Opin. 2019;35(3):435–46.
Zhang WQ, Xu P, Zhan XH, et al. Efficacy of dexmedetomidine for treatment of patients with sepsis: a meta-analysis of randomized controlled trials. Medicine. 2019;98(18): e15469.
Shehabi Y, Howe BD, Bellomo R, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380(26):2506–17.
Chamorro C, de Latorre FJ, Montero A, et al. Comparative study of propofol versus midazolam in the sedation of critically ill patients: results of a prospective, randomized, multicenter trial. Crit Care Med. 1996;24(6):932–9.
Barrientos-Vega R, Mar Sanchez-Soria M, Morales-Garcia C, et al. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997;25(1):33–40.
Sanchez-Izquierdo-Riera JA, Caballero-Cubedo RE, Perez-Vela JL, et al. Propofol versus midazolam: safety and efficacy for sedating the severe trauma patient. Anesth Analg. 1998;86(6):1219–24.
Swart EL, van Schijndel RJ, van Loenen AC, et al. Continuous infusion of lorazepam versus medazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27(8):1461–5.
Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34(5):1326–32.
Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–53.
Memis D, Kargi M, Sut N. Effects of propofol and dexmedetomidine on indocyanine green elimination assessed with LIMON to patients with early septic shock: a pilot study. J Crit Care. 2009;24(4):603–8.
Reade MC, O'Sullivan K, Bates S, et al. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open-label trial. Crit Care. 2009; 13(3):R75.
Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–99.
Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35(2):282–90.
Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21(6):394–400.
Huang Z, Chen YS, Yang ZL, Liu JY. Dexmedetomidine versus midazolam for the sedation of patients with non-invasive ventilation failure. Internal medicine (Tokyo, Japan). 2012;51(17):2299–305.
Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–60.
Shehabi Y, Bellomo R, Reade MC, et al. Early goal-directed sedation versus standard sedation in mechanically ventilated critically ill patients: a pilot study. Crit Care Med. 2013;41(8):1983–91.
Devlin JW, Al-Qadheeb NS, Chi A, et al. Efficacy and safety of early dexmedetomidine during noninvasive ventilation for patients with acute respiratory failure: a randomized, double-blind, placebo-controlled pilot study. Chest. 2014;145(6):1204–12.
Zhou Y, Jin X, Kang Y, et al. Midazolam and propofol used alone or sequentially for long-term sedation in critically ill, mechanically ventilated patients: a prospective, randomized study. Crit Care. 2014;18(3):R122.
Cho JS, Shim JK, Soh S, et al. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016;89(3):693–700.
Conti G, Ranieri VM, Costa R, et al. Effects of dexmedetomidine and propofol on patient-ventilator interaction in difficult-to-wean, mechanically ventilated patients: a prospective, open-label, randomised, multicentre study. Crit Care. 2016;20(1):206.
Liu X, Zhang K, Wang W, et al. Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: a randomized controlled trial. Crit Care. 2016;20(1):298.
Reade MC, Eastwood GM, Bellomo R, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315(14):1460–8.
Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–902.
Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152(8): e171505.
Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA. 2017;317(13):1321–8.
Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-dose nocturnal dexmedetomidine prevents ICU delirium. a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018; 197(9):1147–1156.
Wang W, Liu Y, Liu Y, et al. Comparison of cognitive impairments after intensive care unit sedation using dexmedetomidine and propofol among older patients. J Clin Pharmacol. 2019;59(6):821–8.
Temesgen N, Chekol B, Tamirie T, et al. Adult sedation and analgesia in a resource limited intensive care unit - A Systematic Review and evidence based guideline. Ann Med Surg (Lond). 2021;66: 102356.
Zhang Z, Chen K, Ni H, et al. Sedation of mechanically ventilated adults in intensive care unit: a network meta-analysis. Sci Rep. 2017;7:44979.
Burry LD, Cheng W, Williamson DR, et al. Pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a systematic review and network meta-analysis. Intensive Care Med. 2021;47(9):943–60.
Cui Y, Li G, Cao R, et al. The effect of perioperative anesthetics for prevention of postoperative delirium on general anesthesia: a network meta-analysis. J Clin Anesth. 2019;59:89–98.
Acknowledgements
The authors thank Prof. Zhou for providing data. The authors thank Prof. Shehabi for kindly reply for information of the SPICE III study.
Funding
This work is partially supported by grants from the National Natural Science Foundations of China (81501705, 81671892, 81971888), grants from the Scientific Research Foundation of Graduate School of Southeast University (YBPY1604), grants from the Jiangsu Provincial Medical Youth Talent (QNRC2016808). This work is partially supported by grants from the Jiangsu Provincial Special Program of Medical Science (BL2013030, BE2018743, BE2019749), grants from Jiangsu Provincial Medical Priority Majors (ZDXKA2016025), Jiangsu Province's Key Provincial Talents Program (ZDRCA2016082).
Author information
Authors and Affiliations
Contributions
JYX carried out the analysis and interpretation of data and participated in drafting, editing and submitting the manuscript. All of the articles were reviewed by two reviewers (JYX and ZSW) independently in accordance with the inclusion criteria. Disagreements between the two reviewers were resolved by discussion with a third reviewer (WC). The quality of each article was assessed by JYX and JFX independently. Disagreements were resolved by consulting a third reviewer (XWZ). Using a data extraction table, ZHL and SSM independently extracted data. Disagreements were resolved by discussion with YZH and SQL until a consensus was achieved. YY was responsible for conception and design, and revising the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, JY., Wu, ZS., Chang, W. et al. Comparison of the Effects of Intravenous Sedatives on Outcome in Adult Critically ill Patients: a Bayesian Network Analysis. Intensive Care Res 2, 12–22 (2022). https://doi.org/10.1007/s44231-022-00002-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44231-022-00002-7