Abstract
Background
Tamil Nadu state reported the second highest number of confirmed COVID-19 cases in India. In this study, we aimed to describe and determine the risk factors for early death among the first 10,000 COVID-19 deaths in the state.
Methods
We conducted a cross sectional analysis of state government administrative data to describe deaths, examine the differences between early deaths and non-early deaths, and calculate the risks of early death for several independent variables. All p-values < 0.05 were considered statistically significant.
Results
In total, 4147 early deaths (41.5%) were recorded; the median age of patients who suffered from early death was significantly lower [64 years; interquartile range (IQR): 55–72] when compared with patients who did not suffer from early death (65 years; IQR: 56–73). After adjusting for comorbidities, age, and the time elapsed from the onset of symptoms to hospitalization; we found that the risk of early death was significantly lower for males [adjusted odds ratio (aOR): 0.82; 95% confidence interval (CI): 0.72, 0.93; p = 0.002], among rich individuals (aOR: 0.76; 95% CI: 0.63, 0.92; p = 0.004), in the richest districts (aOR: 0.70; 95% CI: 0.59, 0.84; p < 0.001) and for those who received treatment in private facilities (aOR: 0.45; 95% CI: 0.40, 0.51; p < 0.001.
Conclusions
The risk of early deaths among the first 10,000 reported COVID deaths in the Tamil Nadu state of India was higher in patients treated in government hospitals especially in the poorest districts probably indicating a lack of infrastructure in government facilities or the overburdening of government facilities at least in the early phase of the pandemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a pandemic that affected 618 million people and caused 6.54 million deaths globally as of 3 October 2022 [1]. India was one of the most affected countries with approximately 44 million reported cases and 500,000 deaths as of 3 Jan 2023 [2].
India’s first COVID-19 case was reported on 30 January 2020 in a medical student who returned from China. The burden of cases and mortality rate varied across different states in India. Maharashtra reported the highest number of confirmed COVID-19 cases, followed by Tamil Nadu, while Gujarat, Andhra Pradesh and West Bengal reported the highest case fatality rate (CFR) during the early days of the pandemic [3]. Tamil Nadu reported its first confirmed case on 5 March 2020. Though not the wealthiest Indian state, Tamil Nadu has an effective public health and health care delivery system [4]. The state reported a CFR of 1.54% in October 2020 [5].
Previous studies from India have reported an increased rate of COVID-19 mortality among males and those with comorbidities [6,7,8,9]. Male sex has been shown to be associated with increased expression levels of angiotensin-converting enzyme 2 (ACE2) receptors; these receptors are important for the SARS-CoV-2 to bind and enter host cells. In a previous study, Pinto et al. found that a higher expression level of ACE2 was associated with an increased severity of COVID-19 and that high expression levels of the ACE2 receptor may be one of the reasons underlying the higher mortality rate in male patients with COVID-19 than female patients [10]. A systematic review and meta-analyses of 42 studies showed that older age was associated with an increased risk of mortality [11]. These analyses also revealed a high risk of mortality among hospitalized COVID-19 patients with chronic obstructive pulmonary disease (COPD), cardiovascular disease, diabetes, hypertension, obesity, cancer, acute kidney injury and increased levels of D-dimer. A study from Kerala reported a median time of 4 days from COVID-19 positivity to hospital admission and 2 days from hospital admission to death [7]. Another study reported a duration of 11 days between illness onset and death [8]. A study by Laxminarayan et al. identified older age and male sex as the key predictors for the time to death [4]. Furthermore, the timing of death in COVID-19 patients was shown to differ with comorbidities and the severity of illness.
Although there have been some studies from different parts of India that investigated COVID-19 mortality, most of these were limited by small sample sizes. Almost all of these studies showed that death occurred predominantly in elderly males and that the level of comorbidity increased the risk of death. However, the existing literature does not describe the risk factors associated with early death in COVID-19 patients. Understanding the factors associated with mortality with respect to the different timings of death could guide physicians in the clinical management of COVID-19 patients, so that physicians could prioritize patients by risk scoring. Recognizing these risk factors can help develop effective and timely interventions that would help to reduce mortality. Therefore, this study was undertaken to identify the risk factors for early mortality among COVID-19 patients who died during the early days of the pandemic in the Tamil Nadu state of India.
1.1 Research Objectives
-
1.
To describe the characteristics of early deaths among the first 10,000 COVID-19 fatalities in the Tamil Nadu state of India.
-
2.
To determine the risk factors associated with early death among these cases.
2 Materials and methods
We used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines to report this observational study [12]. We used a cross-sectional design to investigate the first 10,000 COVID deaths in Tamil Nadu. We used death data from the administrative bulletin released daily by the state health department for this analysis [13]. The data were available as pdf files; the data were extracted and entered in a pre-designed Microsoft Excel sheet. These data included all deaths, including patients who had died prior to reaching the hospital, as reported from government and private health facilities across the state. We recorded the following variables: age, sex, type of health facility (government or private), name of the district, the number of days from symptoms to admission and from admission to death, and the presence of comorbidities such as diabetes mellitus, hypertension, coronary artery disease (CAD), chronic kidney disease (CKD), asthma, cancer, tuberculosis (TB), and hypothyroidism. We grouped all patients into three age groups: children (< 17 years), adults (17–60 years) and older adults (> 60 years).
In addition, we used the multidimensional poverty index (MPI) [14] to classify districts based on poverty. MPI, the most widely used non-monetary poverty index in the world identifies overlapping deprivations at the household level across the three dimensions of living standards, health, and education by using ten indicators: nutrition, child mortality, years of schooling, school attendance, access to cooking fuel, sanitation, drinking water, electricity, and housing, and the ownership of assets. A higher MPI indicates a higher rate of household poverty. We categorized the districts into four groups based on MPI score: quartile 1 or the poorest (MPI score: 0.031–0.046), quartile 2 or poor (MPI score: 0.0216–0.030), quartile 3 or rich (MPI score: 0.013–0.0215), and quartile 4 or richest (MPI score: 0.004–0.012).
We defined multimorbidity as the presence of more than one comorbidity [15] and defined early death as death that occurred within 3 days of admission to hospital [17].
2.1 Data Analyses
First, we described the characteristics of all 10,000 COVID-19 deaths. Second, we investigated the differences in characteristics between patients who suffered early deaths and non-early deaths after excluding missing data relating to sex, facility type and the number of days from symptoms to admission and the number of days from admission to death. Pearson's chi-squared test and the Wilcoxon rank sum test were used to test for statistical differences. Finally, we performed multivariable logistic regression and calculated unadjusted and adjusted odds ratio for early deaths across independent variables. The model was adjusted for the following variables: age group, sex, facility, multimorbidity, the number of days from symptoms to admission, and district MPI quartiles. All p-values < 0.05 were considered significant. All analyses were performed in R software (R studio, version 4.2.2, 2022) [17].
2.2 Ethical Considerations
The data did not include any patient identifiers and did not involve any patient interviews. Therefore, there was no requirement for ethical approval or informed consent.
3 Results
The first death was reported on 7 March 2020 and the 10,000th death was reported on 8 October 2020. Table 1 summarizes the characteristics of the first 10,000 COVID-19 deaths reported from Tamil Nadu; 7371 were males (73.7%) and 6572 (65.72%) were older adults. Multimorbidity was present in 4420 (44.2%) of the subjects and 2359 (23.6%) subjects reported no comorbidities. The most common comorbidity was diabetes mellitus (57.7%), followed by hypertension (45.5%), CAD (15.3%) and CKD (11.1%). There were 1080 (10.8%) patients from districts from quartile 1 of the MPI (poorest districts), 1939 (19.4%) from quartile 2 (poor), 2432 (24.3%) from quartile 3 (rich), and 4549 (45.5%) patients from quartile 4 (richest districts). There were 6587 (65.9%) deaths in government health facilities. The median time from admission to death was 4 days (IQR: 1.9–8 days) and the median time from symptoms to death was seven days (IQR: 4–12 days). There were 4147 (41.5%) early deaths; 3008 of these were male (72.6%).
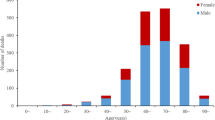
Figure 1 shows the distribution of comorbidities across sex. Diabetes mellitus, hypertension, hypothyroidism, bronchial asthma, and cancer were reported for a greater number of females while CAD, CKD, and TB were reported more often in males. The presence of any comorbidity was also reported more commonly in females.
Table 2 summarizes the differences between early deaths and non-early deaths. The median age of patients who suffered from early death was significantly lower [64 years (IQR: 55–72)] when compared with patients who did not suffer from an early death [65 years (IQR: 56–73); p = 0.002]. The majority of early deaths were reported from government hospitals, among the elderly, males, and from the richest districts. Among patients with multimorbidity, only 39.4% suffered an early death; this compared with 43.5% in patients who did not have multimorbidity.
Table 3 shows the unadjusted and adjusted odd ratios for early deaths. In the unadjusted analyses, we found a lower risk of early death among males (OR: 0.9; 95% CI: 0.83, 0.99; p = 0.03). Patients in private hospitals had a 60% lower risk for early death (OR: 0.40; 95% CI: 0.37, 0.44; p < 0.001) when compared with patients in government hospitals. Patients from rich districts (OR: 0.71; 95% CI: 0.62, 0.82; p < 0.001) and the richest quartiles (OR: 0.65; 95% CI: 0.57, 0.74; p < 0.001) had a significantly lower risk for early death.
In the final adjusted model, we found that the risk for early death was significantly lower for males (aOR: 0.82; 95% CI: 0.72, 0.93; p = 0.002) among the rich (aOR: 0.76; 95% CI: 0.63, 0.92; p = 0.004), and the richest districts (aOR: 0.70; 95% CI: 0.59, 0.84; p < 0.001), and those who received treatment in private facilities (aOR: 0.45; 95% CI: 0.40, 0.51; p < 0.001), even after adjusting for other variables. There was no significant difference between people in the poorest and poor districts. Patients with CAD (aOR: 1.21; 95% CI:1.03–1.4; p = 0.02) had a higher risk of early death while there was no significant association between early death and diabetes mellitus (aOR: 0.89; 95% CI: 0.80, 1.00; p = 0.05), hypertension (aOR: 0.87; 95% CI:0.77,0.98; p = 0.02), CKD (aOR: 1.0; 95% CI:0.83,1.19; p > 0.9), bronchial asthma (aOR: 0.83; 95% CI: 0.59,1.15; p = 0.3), cancer (aOR: 1.08; 95% CI: 0.66,1.74; p = 0.8), TB (aOR: 0.83; 95% CI: 0.44,1.52; p = 0.6), or hypothyroidism (aOR: 0.81; 95% CI: 0.6,1.08; p = 0.2).
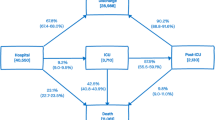
In our additional analysis (Fig. 2), we found that within the private sector, there was no difference in the risk of early death between districts for different levels of poverty. However, within the government sector, we found a significantly higher risk for early death in government hospitals located in the poorest and poor districts when compared with the government hospitals located in richest districts.
4 Discussion
This study used publicly available data to investigate predictors of early death among the first 10,000 COVID-19 deaths in the Indian state of Tamil Nadu. We found that 4147 of the first 10,000 COVID-19 deaths (41.5%) occurred within 3 days of hospitalization. Our research led to four key findings. First, patients residing in districts with a low MPI score (the rich and richest districts) reported a significantly lower risk for early death when compared with patients residing in the poorest districts. Second, private facilities reported a significantly lower risk for early death. Third, males had a significantly lower risk for early deaths, and finally, CAD was a significant predictor of early death.
Patients in the richest districts (quartile 4) had a 30% lower risk and patients in the rich districts (quartile 3) had a 24% lower risk for early death when compared with patients in the poorest districts (quartile 1), even after adjusting for age, sex, comorbidities, and the time elapsed from the onset of symptoms to admission. This may be linked to the shortage of health facilities available in the poorest districts of the state including human resources and intensive care facilities (ICU). This needs to be investigated further by examining differences in the availability of ICUs and specialist doctors between districts. Our findings corroborate the findings reported by Lewnard et al. [18] who reported 0·7% to 2·8% increase in pandemic-associated mortality per one standard deviation increase in each measure of community disadvantage in Chennai. Socioeconomic factors have been described as important determinants of COVID-19-related mortality in other countries. For example, a study by Hawkins et al. from the USA showed that counties with a lower socio-economic status (a higher Distressed Communities Index score) had higher COVID-19 death rates per 100,000 individuals when compared with non-distressed counties [19]. Similarly, the risk of dying from COVID-19 among confirmed cases in Columbia was higher in indigenous individuals and in those living in the very low socioeconomic strata [20]. However, very few studies investigated early deaths among hospitalized patients. Of these, a 2021 study by Gautam et al. from the Punjab state of India reported 15% patients dying within 48 h of admission [21], while a retrospective cohort study from New York reported 36% of African American patients dying within the first 3 days of hospitalization [22].
In our present study, private facilities reported a significantly lower risk for early death. Furthermore, the risk of early death was significantly higher in government facilities located in the richest districts even when compared with private facilities located in the poorest districts. Moreover, we found significant differences in the risk of early death across government facilities located in districts with different levels of poverty. These differences between the government and private sector persisted even after adjusting for age, the time elapsed from the onset of symptoms to admission, and comorbidities. These findings may indicate significant differences in the availability of specialist care facilities within government institutions, at least in the initial phase of the pandemic. Districts that are in proximity to the state capital and districts which have a good presence of industries attract more government resources, including hospitals. This may explain the differences between government facilities across these districts. However, private sector hospitals may exhibit certain uniformity in the availability of facilities, including specialists and ICUs. This may explain the absence of differences between private facilities across districts at different levels of poverty. Alternatively, government facilities may have been overburdened due to the closure of most private clinics and hospitals; this may have compromised the care of most of the severe cases in government facilities, at least in the initial phase of the pandemic [23]. We do not have information relating to each patient’s intensive care admission status; this may have limited our analyses. However, we adjusted for most of the serious comorbidities which can be attributed to early deaths.
Next, we showed that males had a significantly lower risk for early deaths when compared with females, although more than 73% of the first 10,000 deaths were males. This finding was similar to a previous analysis reported by Joe et al., who showed that although males shared a higher burden of death in India, the case fatality ratio for females was relatively higher than for males [24]. In general, the COVID-19-related mortality is higher among males, as shown by a previous meta-analysis [25], and is considered to reflect a general sex-disparity in terms of excess mortality [26]. However, other studies have demonstrated heterogeneity in sex disparities relating to COVID mortality; for example, in their analysis of a longitudinal dataset of sex-disaggregated COVID-19 data in the USA, Danielsen et al. showed that sex-disparities in COVID mortality varied in both magnitude and direction across states and over time [27]. Similarly, a study from Ethiopia, which investigated data from June 2020 to October 2021, showed that early deaths (within 3 days of hospitalization) were relatively higher among females when compared with males although the total number of deaths was higher for males [17].
Finally, our analysis showed that CAD was a significant risk factor for early death, as reported previously in a study from Ethiopia which specifically investigated risk factors for early death [17]. In general, systematic reviews, meta-analyses and large cohort studies have demonstrated significant associations between CAD and COVID mortality, including early studies from Pakistan and China [28,29,30,31,32]. Similar to the Ethiopian study, we did not identify a significant association between early death and diabetes mellitus, hypertension, cancer, or CKD. However, a previous systematic review has reported higher COVID mortality with diabetes and hypertension [30]. In contrast to the Ethiopian study, we did not find a significant effect for bronchial asthma in early deaths [17].
4.1 Strengths and Limitations
We used a relatively large volume of data pertaining to 10,000 COVID-19 deaths across various districts and health facilities in the Tamil Nadu state. This is the first study to report factors associated with early and non-early deaths from India. Very few studies have investigated early deaths on a global basis. Our study adjusted for the time from symptom onset to admission; this was not performed in previous studies. However, our study is based on reported administrative data and therefore has several limitations linked to the nature of the data. Our data included only deaths and not all COVID-19 cases, thus limiting our analysis. Furthermore, the data for the time from symptom onset to admission was missing for 39% of the patients. Moreover, as a general limitation of our secondary data, we were unable to include several important variables such as ICU admission status in our analysis; this may have affected the generalizability of our findings.
5 Conclusion
Using data relating to the first 10,000 covid deaths from an Indian state, we found that even after adjusting for comorbidities, age, and the time of symptom onset to hospitalization, the risk for early deaths was higher in government hospitals especially in the poorest districts in the early phase of the pandemic, thus indicating a lack of infrastructure in government facilities or the overburdening of government facilities. Thus, the government should invest more healthcare resources in districts that are economically poorer, which are, in most cases, distant from the state capital. As the pandemic is ongoing and new strains are appearing, and also considering the threat of the emergence of new pathogens, governments should continuously take stock of existing infrastructure in public facilities. More studies on the risk factors associated with early death need to be conducted to obtain information related to the changing trends in the predictors of early death. Cohort studies on the risk factors for early death should be undertaken in other parts of India to inform efforts needed to enhance mitigation as well as to develop health policies.
Abbreviations
- aOR:
-
Adjusted odds ratio
- BA:
-
Bronchial asthma
- CAD:
-
Coronary artery disease
- CFR:
-
Case fatality rate
- CI:
-
Confidence Interval
- CKD:
-
Chronic kidney disease
- COVID-19:
-
Coronavirus disease 2019
- ICU:
-
Intensive care facilities
- MP:
-
Multidimensional poverty index
- OR:
-
Odds ratio
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TB:
-
Tuberculosis
References
COVID-19 Map. Johns Hopkins Coronavirus Resource Center. Accessed January 4, 2023. https://coronavirus.jhu.edu/map.html
India: WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. Accessed January 17, 2023. https://covid19.who.int
Ansari ZAA, Desai HD, Sharma K, et al. Prevalence and cross states comparison of case fatality rate and recovery rate of COVID 19/SARS-COV-2 in India. J Fam Med Prim Care. 2021;10(1):475–80. https://doi.org/10.4103/jfmpc.jfmpc_1088_20.
Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370(6517):691–7. https://doi.org/10.1126/science.abd7672.
Vijay Anand V, Arunkumar Yogaraj G, Priya S, Priya Raj P, Brinda Priyadharshini C, Sridevi PN. A cross-sectional study on COVID19 mortality among people below 30 years of age in Tamilnadu-2020. Clin Epidemiol Glob Health. 2021;12:100827. https://doi.org/10.1016/j.cegh.2021.100827.
Baruah TD, Kannauje PK, Ray R, et al. Hospital mortality among COVID-19 patients – Experience of a multi-disciplinary tertiary care teaching hospital of Chhattisgarh in Central India. J Fam Med Prim Care. 2022;11(10):6499. https://doi.org/10.4103/jfmpc.jfmpc_584_22.
Krishnan RA, Ravindran RM, Vincy VS, et al. Analysis of daily COVID-19 death bulletin data during the first two waves of the COVID-19 pandemic in Thiruvananthapuram district, Kerala, India. J Fam Med Prim Care. 2022;11(10):6190. https://doi.org/10.4103/jfmpc.jfmpc_382_22.
Rohini C, Kuriakose S, Philip S, Sreedevi S. Analyzing COVID-19 mortality in Ernakulam district of Kerala: A retrospective review of death reports. J Fam Med Prim Care. 2022;11(10):6209. https://doi.org/10.4103/jfmpc.jfmpc_648_22.
Jain P, Agarwal N, Saxena V, Srivastav S, Solanki H. Mortality in patients with Coronavirus disease 2019 (COVID- 19) and its clinicoradiological and laboratory correlates: A retrospective study. J Fam Med Prim Care. 2022;11(10):6197. https://doi.org/10.4103/jfmpc.jfmpc_364_22.
Pinto BGG, Oliveira AER, Singh Y, et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J Infect Dis. 2020;222(4):556–63. https://doi.org/10.1093/infdis/jiaa332.
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. https://doi.org/10.1186/s12879-021-06536-3.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Koya SF, Ebrahim SH, Bhat LD, et al. COVID-19 and Comorbidities: Audit of 2,000 COVID-19 Deaths in India. J Epidemiol Glob Health. 2021;11(2):230. https://doi.org/10.2991/jegh.k.210303.001.
Government of India. National Multidimensional Poverty Index Baseline Report.; Niti Ayog. 2021. https://www.niti.gov.in/sites/default/files/2021-11/National_MPI_India-11242021.pdf
Agrawal U, Azcoaga-Lorenzo A, Fagbamigbe AF, et al. Association between multimorbidity and mortality in a cohort of patients admitted to hospital with COVID-19 in Scotland. J R Soc Med. 2022;115(1):22–30. https://doi.org/10.1177/0141076821105171514.
Mezgebu TA, Sibhat MM, Getnet MT, et al. Risk factors of early mortality among COVID-19 deceased patients in Addis Ababa COVID-19 care centers, Ethiopia. PLoS One. 2022;17(9):e0275131. doi:https://doi.org/10.1371/journal.pone.0275131
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Lewnard JA, Mahmud A, Narayan T, et al. All-cause mortality during the COVID-19 pandemic in Chennai, India: an observational study. Lancet Infect Dis. 2022;22(4):463–72. https://doi.org/10.1016/s1473-3099(21)00746-5.
Hawkins RB, Charles EJ, Mehaffey JH. Socio-economic status and COVID-19–related cases and fatalities. Public Health. 2020;189:129–34. https://doi.org/10.1016/j.puhe.2020.09.016.
Cifuentes MP, Rodriguez-Villamizar LA, Rojas-Botero ML, Alvarez-Moreno CA, Fernández-Niño JA. Socioeconomic inequalities associated with mortality for COVID-19 in Colombia: a cohort nationwide study. J Epidemiol Commun Health. 2021;75(7):610–5. https://doi.org/10.1136/jech-2020-216275.
Gautam P, Paul G, Sharma S, et al. Analysis of trimodal pattern of mortality among hospitalized COVID-19 patients- Lessons from tertiary care hospital. J Anaesthesiol Clin Pharmacol. 2022;38(5):107. https://doi.org/10.4103/joacp.joacp_58_22.
Gupta R, Agrawal R, Bukhari Z, et al. Higher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect Dis. 2021. https://doi.org/10.1186/s12879-021-05782-9.
Ganeshkumar MJP, Kaur P, et al. Epidemiology of COVID-19 and effect of public health interventions, Chennai, India, March–October 2020: an analysis of COVID-19 surveillance system. BMJ Open. 2022;12(3):e052067. https://doi.org/10.1136/bmjopen-2021-052067.
Joe W, Kumar A, Rajpal S, Mishra US, Subramanian SV. Equal risk, unequal burden? Gender differentials in COVID-19 mortality in India. J Glob Health Sci. 2020. https://doi.org/10.35500/jghs.2020.2.e17.
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021. https://doi.org/10.1186/s12879-021-06536-3.
Nielsen J, Nørgaard SK, Lanzieri G, Vestergaard LS, Moelbak K. Sex-differences in COVID-19 associated excess mortality is not exceptional for the COVID-19 pandemic. Sci Rep. 2021. https://doi.org/10.1038/s41598-021-00213-w.
Danielsen AC, Lee KM, Boulicault M, et al. Sex disparities in COVID-19 outcomes in the United States: Quantifying and contextualizing variation. Soc Sci Med. 2022;294:114716. https://doi.org/10.1016/j.socscimed.2022.114716.
Szarpak L, Mierzejewska M, Jurek J, et al. Effect of coronary artery disease on COVID-19—prognosis and risk assessment: a systematic review and meta-analysis. Biology. 2022;11(2):221. https://doi.org/10.3390/biology11020221.
Hessami A, Shamshirian A, Heydari K, et al. Cardiovascular diseases burden in COVID-19: systematic review and meta-analysis. Am J Emerg Med. 2021;46:382–91. https://doi.org/10.1016/j.ajem.2020.10.022.
Vasbinder A, Meloche C, Azam TU, et al. Relationship between preexisting cardiovascular disease and death and cardiovascular outcomes in critically ill patients with COVID-19. Circ Cardiovasc Qual Outcome. 2022. https://doi.org/10.1161/circoutcomes.122.008942.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/s0140-6736(20)30566-3.
Ayaz A, Arshad A, Malik H, Ali H, Hussain E, Jamil B. Risk factors for intensive care unit admission and mortality in hospitalized COVID-19 patients. Acute Crit Care. 2020;35(4):249–54. https://doi.org/10.4266/acc.2020.00381.
Acknowledgements
We thank the Tamil Nadu government and its Department of Health for making these data publicly available.
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
SFK, ZP, and SHE conceptualized the study, ZP, BV, SK, SDJ, PR, and JLA collected the data, ZP conducted the analysis under the supervision of JNW, SHE, SK, and SFK. ZP wrote the first draft, JMW, SK, SFK and SHE revised the draft, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethics approval and consent to participate
We did not collect primary data from participants. No person identifiers were included in the data used, and therefore no ethical approval was required.
Consent for publication
The study did not collect any primary data from participants.
Availability of data and material
The data that support the findings of this study are included in the paper as tables. The original pdf files used to extract the data are available freely from the Tamil Nadu government website, https://stopcorona.tn.gov.in/daily-bulletin/
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pilakkadavath, Z., Weinberg, J.M., Kuriakose, S. et al. Early Death Among COVID-19 Patients: A Cross-sectional Analysis of the First 10,000 COVID-19 Deaths from the Indian State of Tamil Nadu. Dr. Sulaiman Al Habib Med J 5, 151–158 (2023). https://doi.org/10.1007/s44229-023-00042-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44229-023-00042-1