Abstract
Acute coronary syndrome (ACS) is a heart disease with a high mortality rate. Recently, more and more evidence illustrated that microRNAs (miRNA) participated in regulating the occurrence of heart disease. This study aimed to detect the level of serum miR-320a-3p in patients with ACS, predict its possibility as a candidate gene for diagnosis, and explore its potential mechanism in the regulation of ACS. 139 ACS patients and 126 controls were recruited in this study. The expression level of miR-320a-3p was determined by qRT-PCR. The predictive value in ACS was assessed by receiver operating characteristic (ROC) curve. Enzyme-linked immunosorbent assay (ELISA) was used to measure the protein expression levels of inflammatory factors. The downstream targets of miR-320a-3p were verified by luciferase reporter gene assay. In ACS patients and rat models, the expression level of serum miR-320a-3p was significantly increased. ROC curve revealed that abnormal expression of miR-320a-3p was of diagnostic value for ACS. In an in vivo rat model, down-regulation of miR-320a-3p inhibited the production of von Willebrand factor (vWF), Heart fatty acid-binding protein (H-FABP), interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α). In other words, down-regulation of miR-320a-3p reduced rat vascular endothelial injury and inflammation. X-linked inhibitor of apoptosis protein (XIAP) was determined to be a direct target of miR-320a-3p. miR-320a-3p is useful for the diagnosis of ACS. Animal experiments confirmed that up-regulated miR-320a-3p promoted vascular endothelial injury and inflammatory response by targeting XIAP, thus promoting the development of ACS. MiR-320a-3p may be a new breakthrough in the diagnosis and treatment of ACS.
Similar content being viewed by others
1 Introduction
Ischemic heart disease (IHD) is a relatively common acquired heart disease in middle-aged and elderly people, and its incidence ranks first among all types of heart disease [1]. Acute coronary syndrome (ACS) is the main manifestation of IHD, and its clinical manifestations mainly include acute myocardial infarction [2] and unstable angina pectoris (UAP) [3]. The main cause of ACS is rupture of atherosclerotic plaque, which has a high morbidity and mortality rate, and is a very serious medical emergency [4]. At present, the clinical diagnosis of ACS is primarily dependent on clinical symptoms, vascular endoscopy, or invasive angiography [5, 6]. Some of these detection methods are susceptible to clinical subjectivity, some are expensive and time-consuming, and some may cause certain harm to the patient [7]. Therefore, the most important goal at this stage is to clarify the pathogenesis of ACS and explore diagnostic methods that are highly sensitive, easy to operate, and less harmful to the body.
MicroRNA (miRNA) is a type of non-coding RNA molecules with about 22 nucleotides in length [8]. By binding to the 3'-UTR region on the mRNA of target genes, miRNA inhibits mRNA translation or leads to mRNA degradation, which is of great significance in the growth, development, metabolism, and disease regulation of organisms [9, 10]. A great number of experimental studies at home and abroad are devoted to exploring the correlation between miRNA and the occurrence of ACS and the possibility of miRNA as diagnostic markers of ACS. For example, Wu et al. revealed that the expression of miR-145 in the serum of ACS patients was down-regulated, and with the decrease of miR-145 expression, the levels of vWF and IL-6 showed an upward trend, suggesting that the abnormal expression of miR-145 was related to ACS [11]. In addition, the study of Li et al. confirmed that the expression of miR-186-5p in the serum of ACS patients was increased, and after treatment with corresponding measures, its expression decreased, which indicates that the development of ACS is related to the abnormal expression of miR-186-5p [12]. A lot of literatures have shown that miR-320a-3p plays an important role in heart or cardiovascular diseases. A study showed that in the serum of patients with diabetic cardiomyopathy, miR-320a-3p was significantly up-regulated [13]. Another study on myocardial ischemia/reperfusion injury pointed out that overexpression of miR-320a-3p significantly promoted cardiomyocyte apoptosis [14]. Nevertheless, the expression and role of miR-320a-3p in ACS are unknown. X-linked inhibitor of apoptosis protein (XIAP) is a member of the IAP family with the strongest ability to inhibit apoptosis. Due to its powerful anti-apoptotic properties, XIAP is often used as a target molecule for cancer therapy drugs. Studies have shown that XIAP was found in endothelial cells and exhibited anti-atherosclerosis effects [15, 16]. It is interesting to study whether this target is involved in the regulation of ACS by miR-320a-3p and solving this problem can provide a useful basis for us to further understand the influence of miR-320a-3p on the pathogenesis of ACS. In the present study, we assessed the diagnostic value of miR-320a-3p for ACS by measuring the level of miR-320a-3p in ACS patients, and the influence of abnormally expressed miR-320a-3p on the ACS model was evaluated by establishing an ACS rat model.
2 Materials and Methods
2.1 Study Population and Sample Collection
This part of the research content is complied with the Declaration of Helsinki and has got the permission from the Ethics Committee of Weifang People’s Hospital. All individuals recruited for this study have signed written informed consent. A total of 265 subjects were selected in this study, including 139 patients diagnosed with ACS by this hospital and 126 patients with simple chest pain but excluded ACS as the control group. The diagnostic criteria of ACS referred to the 2013 ACCF/AHA ST-Elevation myocardial infarction management guidelines [2], the 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease [17], the 2014 AHA/ACC guideline for the management of patients with Non-ST-Elevation acute coronary syndromes [18]. Subjects with systemic inflammatory, malignant carcinoma, kidney infirmities, or left ventricular systolic lesions were excluded from the study. Venous blood was taken from all subjects, and the supernatant was retained after centrifugation and stored in a refrigerator at −80 C for later use. The demographic features and clinicopathological characteristics of subjects were collected and summarized.
2.2 RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
TRIzol LS Reagent (ThermoFisher, USA, Catalog No.: 10296010) was used to extract total RNAs from serum samples. RNA was reverse transcribed into cDNA using a one-step miRNA Reverse Transcription Kit (Haigene, Harbin, China, Catalog No.: D1801). The above cDNA sample and Seven 2 × SYBR Green qPCR MasterMix (Seven BIOTECH, Beijing, China, Catalog No.: 330502) were mixed to detect the relative expression of miR-320a-3p in an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems). The relative expression level of miR-320a-3p was normalized by U6 using 2−ΔΔCt method. The primer sequences for qRT-PCR analysis are as follows: the forward primers of miR-320a-3p and U6 were 5′-AAAAGCTGGGTTGAGAGGGCGA-3′ and 5′-CTCGCTTCGGCAGCACA-3′, respectively. And the reverse primers of both were the universal primers of the kit.
2.3 Establishment and Treatment of ACS Animal Model
All the animal-related protocols involved in this study were implemented under the standards for the care and use of laboratory animals. The Animal Care and Use Committee of Weifang People’s Hospital supported and approved the experimental programs. Sprague–Dawley rats were selected to establish an ACS model to simulate the state of ACS in human body and detect the expression of miR-320a-3p according to the previous literature [11]. Briefly, rats were intraperitoneally injected with 1% pentobarbital (Sigma, USA, Catalog No.: P-3761) for deep anesthesia and connected to a ventilator. Through ECG monitoring, the skin was cut at the upper intercostal area of the apex beat, and the heart was exposed after opening the chest cavity. The position of the anterior descending branch of the left coronary artery was confirmed and ligation with appropriate strength was performed with a medical 6/0 suture. After modeling, the chest cavity was sutured, and the animals were injected with penicillin to prevent infection. In the control group, thoracic cavity was opened only without vascular ligation. Rats in the model group were randomly split into four groups, which were respectively injected with 2 μg of miR-320a-3p mimic, miR-320a-3p inhibitor and miR-NC for 7 consecutive days for in vivo transfection via the tail vein. Finally, blood was collected from the tail vein of the rats, and the serum was separated and stored in the −80 C refrigerator for later use.
2.4 Enzyme-Linked Immunosorbent Assay (ELISA)
The concentration of von Willebrand factor (vWF) (Haematologic Technologies, USA, Catalog No.: HCVWF-0190) and heart-type fatty acid-binding protein (H-FABP) (Oxis research, UK, Catalog No.: 11230) and the levels of inflammatory factors, such as IL-6 (ThermoFisher, USA, Catalog No.: 88-7066-86), TNF-α (ThermoFisher, USA, Catalog No.: 88-7346-76), and IL-1β (ThermoFisher, USA, Catalog No.: 88-7120-77), were detected by ELISA according to the product instructions. In short, the reagent is balanced at room temperature for 30 min. At the same time, the samples to be tested were defrosted naturally at room temperature, and then co-incubated with antibodies for 1 h. Subsequently, the antibodies were removed, and the substrate solution was added and incubated at room temperature for 30 min in the dark. Finally, the stop solution was added to terminate the reaction, and the absorbance value was detected at 450 nm in the microplate reader (Bio-Tek Instruments, Germany) and the corresponding concentration was calculated. Each experiment was repeated in triplicate.
2.5 Luciferase Reporter Gene Assay
Through the prediction of target genes, we found that miR-320a-3p had a complementary binding site with X-linked inhibitor of apoptosis protein (XIAP), and it was preliminarily speculated that XIAP was the target gene of miR-320a-3p, and luciferase reporter gene assay was used to verify this prediction. Specific steps were as follows: the human embryonic kidney cell line HEK-239T cells were obtained from American Type Culture Collection (ATCC, Manassas, Virginia, USA). HEK-239 T cells were cultured in DMEM supplemented with 10% FBS and 1% double antibiotics in a cell incubator at 37 C. The 3′-UTR sequence fragment of XIAP was cloned into pGL3 vector to construct wild-type (wild-type) reporter vector XIAP 3′-UTR-WT and mutant-type (mutant-type) reporter vector XIAP 3′-UTR-MUT. The HEK-239T cells were seeded into 24-well plate and cultured overnight until the cell density reached 60% or more. Subsequently, according to the product specification, the cells were co-transfected with the above-mentioned vector and miR-NC, miR-320a-3p mimic or miR-320a-3p inhibitor using Lipofectamine 3000, respectively. After 48 h of transfection, cells were collected, and the luciferase activity of each group was measured using the dual-luciferase reporter system (Promega). Renilla luciferase was chosen as a control gene.
2.6 Statistical Analysis
Data were presented as (mean ± SD). ROC curve was constructed to estimate the diagnostic value of miR-320a-3p in ACS. Student t test was used for comparison between two groups. One-way ANOVA was selected for multiple groups’ comparison, and chi-square test was selected for comparison between categorical variables. Multiple linear regression analysis was used to determine the independent effects of variables related to miR-320a-3p. Logistic regression analysis was used to evaluate the relationship between different variables and the occurrence of ACS. A P value less than 0.05 was considered to be significantly different.
3 Results
3.1 General Clinical Data and Pathological Characteristics
The general clinical data and pathological characteristics of all individuals were shown in Table 1. The subjects were between 40 and 65 years old, and there was no statistically significant difference between the two groups in indicators such as age, sex composition, total cholesterol [19], triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) (P > 0.05). Besides, the levels of vWF, H-FABP, TNF-α, IL-1β and IL-6 in ACS patients were significantly higher than those in the control group (P < 0.001).
3.2 Increased Expression Level of miR-320a-3p in ACS Patients
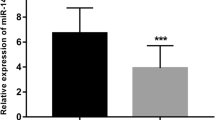
The qRT-PCR results revealed that the expression level of serum miR-320a-3p in ACS patients exhibited an increased trend in comparison with the control group (Fig. 1, P < 0.001), suggesting that miR-320a-3p may play an important role in the course of ACS.
3.3 miR-320a-3p has Diagnostic Value for ACS
ROC analysis is a statistical method widely used in clinical diagnosis and screening. In this study, ROC curve was built to evaluate the diagnostic significance of miR-320a-3p for ACS. The results displayed that the AUC value of this curve was 0.910. The sensitivity and specificity of miR-320a-3p were 80.6% and 87.3% at the cut-off value of 1.336, indicating that miR-320a-3p had the ability to recognize ACS patients (Fig. 2). The above result suggested that miR-320-3p had a certain diagnostic value for ACS.
3.4 Multivariate Linear Regression Analysis and Logistic Regression Analysis
Multivariate linear regression analysis was used to evaluate the correlation between miR-320a-3p level and relevant clinical indicators. As shown in Table 2, it showed that LDL, vWF, H-FABP, TNF-α, IL-1β and IL-6 were main factors affecting the serum miR-320a-3p level (all P < 0.05). Meanwhile, logistic regression analysis was used to evaluate the relationship between different variables and the occurrence of ACS. In Table 3, it was found that the level of vWF (OR = 0.014, 95% CI = 0.004–0.043, P < 0.001) and miR-320a-3p (OR = 0.027, 95% CI = 0.009–0.082, P < 0.001) were the independent influencing factors for the occurrence of ACS among variables.
3.5 Overexpression of miR-320a-3p Increased the Levels of vWF, h-FABP and Inflammatory Cytokines in ACS Rat Model
In comparison with the control group, the expression of serum miR-320a-3p in ACS rats was significantly increased, and miR-320a-3p was upregulated or downregulated after transfection with miR-320a-3p mimic or miR-320a-3p inhibitor, indicating that transfection had been successfully carried out in vivo (Fig. 3A, P < 0.05). In the serum of ACS rat model, the levels of VWF and h-FABP were significantly increased, and down-regulation of miR-320a-3p could reverse this result (Fig. 3B, C, P < 0.05). Moreover, the results of inflammatory factors were shown in Fig. 3D. In the ACS rat model, the levels of TNF-α, IL-1β and IL-6 were all increased significantly, while down-regulation of miR-320a-3p expression could significantly inhibit the generation of inflammatory factors, and significantly decreased the relative expressions of TNF-α, IL-1β and IL-6 (P < 0.001).
A The relative expression of miR-320a-3p in ACS rat model before and after transfection in vivo (**P < 0.01 vs. control, &P < 0.05 or &&P < 0.01 vs. miR-NC). B The relative expression of vWF in ACS rat model before and after transfection in vivo (***P < 0.001 vs. control, &P < 0.05 or &&P < 0.01 vs. miR-NC). C The relative expression of H-FABP in ACS rat model before and after transfection in vivo (**P < 0.01 vs. control, &P < 0.05 or &&P < 0.01 vs. miR-NC). D The relative expression of TNF-α, IL-1β and IL-6 in ACS rat model before and after transfection in vivo (***P < 0.001 vs. control, &&&P < 0.001 vs. miR-NC)
3.6 XIAP is a Target Gene of miR-320a-3p
By means of target gene prediction, we speculated that XIAP was the target gene of miR-320a-3p, and their complementary sequences were shown in Fig. 4A. The luciferase reporter gene results showed that the luciferase activity of XIAP 3’-UTR-WT was significantly decreased after transfection with miR-320a-3p mimic, while the luciferase activity of XIAP 3’-UTR-WT was significantly increased after transfection with miR-320a-3p inhibitor (Fig. 4B, P < 0.001). In addition, neither miR-320a-3p mimic nor inhibitor transfection had any effect on the luciferase activity of XIAP 3’-UTR-MUT. Figure 4C confirmed the results of luciferase reporter gene assay, indicating that XIAP is a target gene of miR-320a-3p and was negatively regulated by miR-320a-3p (P < 0.05).
XIAP is a target gene of miR-320a-3p. A Complementary sequences of miR-320a-3p and XIAP. B Luciferase reporter gene assay was used to assess the relationship of miR-320a-3p and XIAP (***P < 0.001). C The expression of XIAP was measured by qRT-PCR (***P < 0.001 vs. control, &&P < 0.01 or &P < 0.05 vs. miR-NC)
4 Discussion
With the changes in living standards and dietary structure, the incidence of heart disease gradually tends to be younger. ACS is one of the most dangerous types of all types of heart disease, with an acute onset and high mortality rate. Therefore, the early diagnosis and treatment of ACS is particularly important. As a candidate for biomarkers, miRNA has multiple advantages, such as easy availability and stable properties. If a biomarker with high sensitivity and good specificity is found, it is extremely important for the diagnosis, treatment, and prognosis of ACS.
Recently, Mikko et al. confirmed the advantage and possibility of miRNAs as biomarkers for heart disease in a study on cardiogenic shock (CS). They found that the baseline levels of miR-21-5p, miR-122-5p and miR-320a-3p were significantly higher in the serum of non-surviving patients with CS than those of surviving patients, and that baseline levels above the median level of miR-320a-3p were independent predictors of 90-day all-cause mortality [20]. According to statistics, 80% of the cause of CS is usually ACS [21]. However, other cardiac emergencies, such as end-stage heart failure, acute severe mitral regurgitation, can also induce CS [22]. The expression of miR-320a-3p in ACS patients was detected in this study, on the basis of the above evidence. We found that the expression level of miR-320a-3p in ACS patients was higher than in non-ACS patients with chest pain, suggesting that the special role of miR-320a-3p in ACS. Meanwhile, in a study on ACS, Wang et al. confirmed that miR-208b and miR-499 have the potential to diagnose ACS [23]. Furthermore, Sun et al. substantiated that miR-335-5p was taking part in the formation of atherosclerotic plaques in ACS and was associated with inflammatory response [24]. ACS patients are often accompanied by vascular endothelial dysfunction and inflammatory injury, and vWF and H-FABP are useful biomarkers commonly used in the clinical evaluation of the extent and status of vascular endothelial injury [25, 26]. Similarly, the results of our research exhibited that miR-320a-3p had the ability to identify ACS patients and non-ACS patients, and the overexpression of miR-320a-3p was positively correlated with cytokines such as vWF, H-FABP, and TNF-α. The mentioned results offered a certain experimental basis for confirming the ability of miR-320a-3p as a diagnostic marker of ACS in clinical practice.
In the ACS rat model of the present study, overexpression of miR-320a-3p promoted the expression of vWF, H-FABP, TNF-α, IL-1β, and IL-6, and inhibiting the expression of miR-320a-3p could significantly down-regulate the expression levels of the above factors, which suggested that overexpression of miR-320a-3p may promote vascular endothelial dysfunction and inflammation in ACS patients. Buie et al. pointed out in a study on the risk factors of cardiovascular disease a few years ago that knocking down the expression of miR-320a ameliorated the abnormal apoptosis of vascular endothelium in mice, improved endothelial cell function and reduced cardiac abnormalities caused by doxorubicin [27]. In addition, Fasseu et al. confirmed that miR-320a was connected with inflammatory diseases, and in the colon biopsy of adult ulcerative colitis, the decrease of miR-320a was significantly associated with the decrease of inflammation [28]. Based on the above findings, the results of the animal model reconfirmed the results of clinical studies. We speculated that the overexpression of miR-320a-3p may play a promoting role in ACS by accelerating endothelial injury and inflammatory response.
Although miRNA is only about 20 nucleotides, it plays an irreplaceable role in gene expression by regulating a great number of target genes [29]. After target gene prediction, we preliminarily determined that the target gene of miR-320a-3p was XIAP. This result was proved by luciferase reporter gene assay and relative expression level of XIAP. XIAP is a member of the IAP family of apoptosis inhibitors and has been shown to be an important negative regulator involved in cell apoptosis [30]. Lu et al. found a significant decrease in XIAP expression in a mouse model of ischemia/reperfusion injury following acute myocardial infarction [31]. A study in a rabbit model of myocardial ischemia showed that in vivo transfection with XIAP protected against myocardial infarction and myocardial apoptosis caused by ischemia/reperfusion [32]. According to the results of this study and previous studies, we speculated that miR-320a-3p could regulate ACS by regulating cell apoptosis via targeting XIAP.
This study has some limitations. One is the mechanism of XIAP, which we need to verify at the cellular level and in vivo experiment level. Another is specific verification of vascular endothelial function, imaging manifestations may be used to evaluate whether vascular endothelial function is abnormal in the future in vivo experiments. In clinical studies, we demonstrated the high expression of miR-320a-3p in ACS patients, and the correlation between the expression level of miR-320a-3p and inflammation, as well as endothelial function status was again verified through the animal model of ACS. These results, in some certain extents, could provide theoretical support for the regulation of miR-320a-3p on the development of ACS.
Data availability
Data will be provided by the corresponding author upon reasonable request.
References
Townsend N, et al. Cardiovascular disease in Europe 2015: epidemiological update. Eur Heart J. 2015;36(40):2673–4.
O’Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78–140.
Li X, et al. Plasma miR-122 and miR-3149 potentially novel biomarkers for acute coronary syndrome. PLoS One. 2015;10(5): e0125430.
Kaur A, et al. Systematic review of microRNA biomarkers in acute coronary syndrome and stable coronary artery disease. Cardiovasc Res. 2020;116(6):1113–24.
Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36(1):1–12.
Mockel M. Biomarkers in the diagnosis of cardiovascular emergencies : acute coronary syndrome and differential diagnoses. Internist (Berl). 2019;60(6):564–70.
Yoo SM, et al. Computed tomography diagnosis of nonspecific acute chest pain in the emergency department: from typical acute coronary syndrome to various unusual mimics. J Thorac Imaging. 2017;32(1):26–35.
Parizadeh SM, et al. The diagnostic and prognostic value of circulating microRNAs in coronary artery disease: a novel approach to disease diagnosis of stable CAD and acute coronary syndrome. J Cell Physiol. 2018;233(9):6418–24.
Ahlin F, et al. MicroRNAs as circulating biomarkers in acute coronary syndromes: a review. Vascul Pharmacol. 2016;81:15–21.
O’Brien J, et al. Overview of microrna biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402.
Wu S, Sun H, Sun B. MicroRNA-145 is involved in endothelial cell dysfunction and acts as a promising biomarker of acute coronary syndrome. Eur J Med Res. 2020;25(1):2.
Li Z, et al. Association of Serum miR-186-5p with the prognosis of acute coronary syndrome patients after percutaneous coronary intervention. Front Physiol. 2019;10:686.
Li H, et al. Nuclear miR-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ Res. 2019;125(12):1106–20.
Tian ZQ, Jiang H, Lu ZB. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia-reperfusion injury by targeting AKIP1. Cell Mol Biol Lett. 2018;23:41.
Kim J, et al. X-linked inhibitor of apoptosis protein is an important regulator of vascular endothelial growth factor-dependent bovine aortic endothelial cell survival. Circ Res. 2008;102(8):896–904.
Deveraux QL, Reed JC. IAP family proteins–suppressors of apoptosis. Genes Dev. 1999;13(3):239–52.
Fihn SD, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130(19):1749–67.
Amsterdam EA, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Majumder S, et al. Dysregulated expression but redundant function of the long non-coding RNA HOTAIR in diabetic kidney disease. Diabetologia. 2019;62(11):2129–42.
Hanninen M, et al. Association of miR-21–5p, miR-122–5p, and miR-320a-3p with 90-day mortality in cardiogenic shock. Int J Mol Sci. 2020;21(21):7925.
Hochman JS, et al. Cardiogenic shock complicating acute myocardial infarction-etiologies, management and outcome a report from the SHOCK Trial Registry. Should we emergently revascularize Occluded Coronaries for cardiogenic shock? J Am Coll Cardiol. 2000;36(3):1063–70.
Harjola VP, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–9.
Wang W, et al. Diagnostic and prognostic impact of circulating microRNA-208b and microRNA-499 in patients with acute coronary syndrome. Biomark Med. 2020;14(2):87–95.
Sun D, et al. Overexpressed miR-335–5p reduces atherosclerotic vulnerable plaque formation in acute coronary syndrome. J Clin Lab Anal. 2021;35(2): e23608.
Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation. 2008;117(11):1449–59.
Swystun LL, et al. Genetic determinants of VWF clearance and FVIII binding modify FVIII pharmacokinetics in pediatric hemophilia A patients. Blood. 2019;134(11):880–91.
Jones Buie JN, et al. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. 2016;254:271–81.
Fasseu M, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5(10): e13160.
Agarwal V, et al. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4: e05005.
Vucic D. XIAP at the crossroads of cell death and inflammation. Oncotarget. 2018;9(44):27319–20.
Lu M, et al. MiR-134-5p targeting XIAP modulates oxidative stress and apoptosis in cardiomyocytes under hypoxia/reperfusion-induced injury. IUBMB Life. 2020;72(10):2154–66.
Kim SJ, Kuklov A, Crystal GJ. In vivo gene delivery of XIAP protects against myocardial apoptosis and infarction following ischemia/reperfusion in conscious rabbits. Life Sci. 2011;88(13–14):572–7.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
Y.Z. study conceptualization and writing (review & editing) the manuscript, Z.Z. Data curation, formal analysis and writing (original draft), A.Z. supervised the project and formal analysis and writing (original draft) the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Zhang, Z. & Zhang, A. MicroRNA-320a-3p Signatures as a Satisfactory Predictor of Acute Coronary Syndrome and Attenuates Inflammation by Targeting X-Linked Inhibitor of Apoptosis Protein. Artery Res 27, 143–150 (2021). https://doi.org/10.1007/s44200-021-00002-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44200-021-00002-w