Abstract
Background
This study aimed at describing the epidemiology of (neuro)cysticercosis as well as its clinical and radiological characteristics in a Taenia solium endemic district of Zambia.
Methods
This was part of a cross-sectional community-based study conducted in Sinda district to evaluate an antibody-detecting T. solium point-of-care (TS POC) test for taeniosis and (neuro)cysticercosis. All TS POC cysticercosis positive (CC+) participants and a subset of the TS POC cysticercosis negative (CC-) received a clinical evaluation and cerebral computed tomography (CT) examination for neurocysticercosis (NCC) diagnosis and staging.
Results
Of the 1249 participants with a valid TS POC test result, 177 (14%) were TS POC CC+ . Cysticercosis sero-prevalence was estimated to be 20.1% (95% confidence intervals [CI] 14.6–27.0%). In total, 233 participants received a CT examination (151 TS POC CC+ , 82 TS POC CC-). Typical NCC lesions were present in 35/151 (23%) TS POC CC+ , and in 10/82 (12%) TS POC CC- participants. NCC prevalence was 13.5% (95% CI 8.4–21.1%) in the study population and 38.0% (95% CI 5.2–87.4%) among people reporting epileptic seizures. Participants with NCC were more likely to experience epileptic seizures (OR = 3.98, 95% CI 1.34–11.78, p = 0.01) than those without NCC, although only 7/45 (16%) people with NCC ever experienced epileptic seizures. The number of lesions did not differ by TS POC CC status (median: 3 [IQR 1–6] versus 2.5 [IQR 1–5.3], p = 0.64). Eight (23%) of the 35 TS POC CC+ participants with NCC had active stage lesions; in contrast none of the TS POC CC- participants was diagnosed with active NCC.
Conclusion
NCC is common in communities in the Eastern province of Zambia, but a large proportion of people remain asymptomatic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neurocysticercosis (NCC) is the most common parasitic disease of the central nervous system globally [1]. The disease may occur after accidental ingestion of Taenia solium eggs excreted by persons infected with an intestinal T. solium tapeworm (taeniosis), which is acquired by consumption of undercooked pork containing T. solium cysticerci. NCC is common in low-income and middle-income countries (LMICs) and primarily related to poor sanitation and hygiene, free-range pig husbandry, and improper slaughterhouse practices [2, 3]. Globalization and immigration, however, have led to NCC being also increasingly reported outside LMICs [4, 5]. NCC accounts for about 30% of all acquired epilepsy cases in endemic areas [6]. It presents as two main forms, parenchymal and extra-parenchymal. The clinical presentation of patients is, amongst other factors, primarily determined by cyst localization and the inflammatory response [7, 8]. Cysts within the brain parenchyma or in the cortical sulci in the subarachnoid space often cause epileptic seizures that generally respond well to anti-seizure medication [9]. In contrast, extra-parenchymal NCC in the ventricles or basal cisterns often has a severe course with poor outcome, requiring prolonged treatment with high rates of mortality and disability [8, 10].
Several studies have assessed the high burden and economic impact of porcine and human cysticercosis (CC) in different parts of Zambia [11, 12]. Studies carried out in pigs in the Eastern, Southern and Western provinces indicated high prevalence proportions of porcine cysticercosis, ranging from 15 to 34% based on detection of circulating antigen (Ag-ELISA) [13, 14]. Based on full carcass dissection, prevalence proportions of 46% and 68% were detected in slaughter age pigs in two districts of the Eastern province [15].
Endemicity of T. solium infections in humans was confirmed in different studies in the Eastern province of Zambia with taeniosis prevalence proportions ranging from 6.3 to 12% based on copro-Ag-ELISA, and with cysticercosis sero-prevalence ranging from 5.8% to 13% (based on serum Ag-ELISA) and from 34 to 39% (based on serum antibody detection) [2, 12, 16, 17]. Prevalence of NCC in people with epilepsy was also estimated to be over 50% within the same province [18].
Whilst data on human cysticercosis sero-prevalence are available, data on epidemiology and clinical presentation of NCC are largely lacking in sub-Saharan Africa [19], as neuroimaging, the cornerstone of NCC diagnosis, is virtually non-available in rural Africa, including Zambia. In addition, with the wide variation of radiological features for NCC between regions, there is very little information from within Africa, although such data are essential to accurately estimate the burden of NCC in different communities [12, 20,21,22].
This study aimed to calculate the CC sero-prevalence and NCC prevalence in Sinda district of the Eastern province of Zambia, to compare the clinical features of people with and without NCC, and to describe neuroradiological features of people with NCC. Additionally, we aimed to assess the prevalence of NCC among people with epileptic seizures (PWE) and to describe the differences in clinical and neuroradiological characteristics between PWE with NCC and those without NCC.
2 Methods
2.1 Study Design
This study was part of the SOLID project “Evaluation of an antibody detecting point-of-care test for the diagnosis of Taenia solium taeniosis and (neuro)cysticercosis in Zambia”, of which detailed procedures, and objectives are published elsewhere [23]. In short, the project aimed at evaluating a novel T. solium point-of-care (TS POC) antibody-detecting prototype test for the diagnosis of T. solium taeniosis (T), cysticercosis (CC) and NCC. The test is an in-house produced standard lateral flow assay (LFA) based on two recombinant proteins, rES33 and rT24H [24]. This TS POC test was also assessed in patients with neurological signs/symptoms compatible with NCC (epileptic seizures and/or severe progressive headache) in hospital settings in Tanzania, where it was found to have a sensitivity and specificity of 49% (95% CI 41–58%) and 91% (95% CI 89–94%), respectively, for diagnosis of NCC [25]. In the main study in Zambia in which this one is embedded, the sensitivity was estimated to be 26% (95% CI 14–44%) and the specificity 88% (95% CI 85–90%) for NCC diagnosis [26].
The study was conducted between December 2017 and November 2021 in Sinda district, Eastern province of Zambia and followed a cross-sectional and community-based design [24]. In short, four (Butao, Chinzure, Mtore, and Ndaula) Neighbourhood Health Communities (NHC; hereafter named “communities”) comprising 40 villages were included. Within each village, households were randomly selected from a list of households established in a census one year prior to the study, resulting in a study population of 2775 people, who were visited to offer TS POC testing. The inclusion criteria were living in the area and being at least 10 years old. Those who were pregnant or reported to be severely ill were excluded from the study. All eligible consenting household members were included in the study and were tested with the TS POC test. Every participant who tested positive for CC on the TS POC test (i.e. TS POC T + CC+ or TS POC T-CC+) and every tenth who tested negative for CC (i.e. TS POC T + CC- or TS POC T-CC-) was requested to give a blood sample and invited for a clinical work-up and cerebral computed tomography (CT) examination (Fig. 1). The blood sample was used for serological testing for CC using the rT24-EITB and the B158/B60 Ag-ELISA as reference tests.
2.2 Clinical Characteristics
The clinical work-up of the selected participants comprised an in-depth medical history, a general physical and neurological examination as well as CT examination with and without contrast. The medical history included collecting demographic data as well as information regarding seizure history and semiology. Everyone coming for clinical work-up was screened for epileptic seizures using a validated questionnaire (A1 Table) [21]. Headache assessment started with the screening question whether people regularly experienced headache; among all people who regularly experience headache, information on location, intensity, duration, frequency, and quality of the headache was also collected. Headache intensity was assessed using a pictorial headache intensity score chart. The neurological examination involved assessment of the patients’ cranial nerves, muscle strength, muscle tone, reflexes, extrapyramidal system, coordination and gait, sensory system, and mental state.
2.3 Radiological Assessment
CT examinations were performed at the Chipata central hospital. We used the Neusoft helical multi-slice scanner (Neusoft Medical Systems, Shenyang, China), which is a 16-slice machine with a slice thickness of 1.5 mm. The evaluation of CT scans and diagnosis of NCC followed the same procedure as described by Stelzle et al., 2022 [19]. In short, CT scans were evaluated by two independent reviewers (CR: neuroradiologist; AF: NCC specialist and neurologist) who were blinded to the TS POC test results. A third reviewer (ASW: neurologist) adjudicated in case of disagreement. Diagnosis of NCC was made following the principles of the revised Del Brutto criteria [27] and the stage of NCC lesions was categorized as active (viable cysts: vesicular stage; degenerating cysts: colloidal or granular nodular stage) or inactive (calcified stage) [28, 29]. Depending on their location the lesions were described as parenchymal (frontal lobe, temporal lobe, parietal lobe, occipital lobe, cerebellum, brainstem) or extra-parenchymal (intraventricular, subarachnoid in basal cisterns, and subarachnoid in cortical sulci). The radiological as well as the clinical assessment followed the same procedures as the SOLID project in Tanzania [19, 25].
2.4 Statistical Analyses
The first part of this study was an epidemiological assessment of CC and NCC prevalence in the study district. Cysticercosis sero-prevalence was calculated based on the cysticercosis reference test results taking into account the study design (i.e. the TS POC CC result). The reference tests were the rT24H-EITB and the B158/B60 Ag-ELISA. Details on the test procedures have been published elsewhere [24]. All prevalence proportions were determined using survey-weighted generalized linear models [30, 31]. Weights were determined using the two-phase function [31] including the TS POC test result combination as stratum identifier in phase two and including households as cluster variable. This approach is used to account for the two-phase design and assumes that the reference standard is missing at random given the TS POC test result combination of both test strips.
For the second part of the study, total number and proportions were used to describe the demographical, clinical, and radiological characteristics of NCC, disaggregated by TS POC CC result. Differences in these factors between people with and without NCC were compared using logistic regression models adjusted for the TS POC CC result. Differences in the number of NCC lesions by TS POC CC result were analysed using Wilcoxon rank sum tests. Clinical characteristics between PWE with and without NCC were only described and no statistical test was performed because of the small numbers. For categorical variables, numbers and proportions were presented, whereas the median and interquartile range (IQR) were presented for continuous variables. A p-value of < 0.05 was considered as statistically significant. R version 4.1.1 was used to perform all analyses.
3 Results
3.1 Prevalence of Cysticercosis and Neurocysticercosis
Across the four communities, a total number of 1249 of participants had a valid TS POC test result with 224 (18%) from Butao, 293 (23%) from Chinzure, 438 (35%) from Mtore, and 294 (24%) from Ndaula (Table 1). The median age for participants was 28 years (interquartile range 18 to 44 years). Twenty percent were children under the age of 15 years. More females than males were recruited (57% versus 43%). Of all people included, 1072 (86%) participants were negative for CC on the TS POC test, and 177 (14%) participants were CC+ with variation across communities (p < 0.001). In all villages at least one person was CC+ apart from two villages in which only five and eight people were included. Applying these results with the results of the reference tests, as published previously by Mubanga et al.[24], yielded a cysticercosis sero-prevalence of 20.1% (95% CI 14.6–27.0%) for at least one of the two cysticercosis reference tests being positive; Cysticercosis sero-prevalence based on antigen ELISA alone was 13.3% (95% CI 8.9–19.5%) and 8.8% (95% CI 5.4–13.9%) based on the rT24H-EITB (Table 2).
Of the 177 TS POC CC+ participants, 151 (85%) had a CT examination (Fig. 1) and of those 35 had NCC (23%), among which eight (5%) were in the active stage. Of the 1072 TS POC CC- participants, 82 had a CT examination and of those, 10 had NCC (12%) with no lesion in active stage. Participants who did not receive a CT examination did not differ demographically from those with a CT examination (A2 Table). Accounting for the study design, a prevalence of 13.5% (95% CI 8.4–21.1%) for any type of NCC and 1.1% (95% CI 0.6–2.0%) for active stage NCC was estimated. The estimated lifetime prevalence of epileptic seizures in the study population was 4.1% (95% CI 1.8–8.9%) and the NCC prevalence among PWE was 38.0% (95% CI 5.2–87.4%) (Table 2).
3.2 Characteristics of Participants with Neurocysticercosis
Overall, 233 participants underwent CT examination. Of those, 45 (19%) had NCC-typical lesions. Males and females were equally affected (OR = 1.68, 95% CI 0.86–3.39, p = 0.13). Participants with NCC were on average 5.9 years (95% CI – 0.4 to 12.2 years) older than those without NCC (p = 0.07, Table 3). One of the 27 (4%) children (between 10 and 14 years) who received a CT examination, had NCC. Small-scale farmers more commonly had NCC than participants of other occupations (OR = 5.28, 95% CI 1.56–17.86, p = 0.007). Sero-positivity in the reference tests was statistically significantly associated with NCC, but nonetheless more than 10% of people with negative serology had NCC. Overall, 15/231 participants irrespective of the TS POC test result (6%) screened positive for epileptic seizures, and participants who reported epileptic seizures more commonly had NCC than those who did not report epileptic seizures (OR = 3.98, 95% CI 1.34–11.78, p = 0.01) (Table 3). Yet, there were no marked differences in terms of seizure semiology, frequency, or anti-seizure medication (A3 Table). Of all participants with NCC, 16% (7/45) had ever experienced an epileptic seizure in their life. Among those with active NCC, 36% (4/11) had ever experienced an epileptic seizure in their life while only 9% (3/34) among those with inactive NCC, experienced an epileptic seizure. Thirty-three percent (15/45) of participants with NCC reported experiencing headaches regularly and this was of varying intensities, quality, duration and frequency (Table 3, A4 Table). Overall, among the participants who reported experiencing headaches regularly, there were more without NCC compared to those with NCC, though the difference was not significant (OR = 1.74, 95% CI 0.85–3.59, p = 0.13; Table 3). The majority of the participants with NCC reportedly neither experienced an epileptic seizure (84%; 38/45) nor regular headaches (67%; 30/45).
From the clinical examination of the 233 participants, only one exhibited a neurological deficit (facial palsy; A3 Table). The rest of the participants with NCC had normal neurological examination findings.
3.3 Radiological Characteristics of Neurocysticercosis
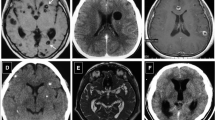
Forty-five participants had NCC, 35 TS POC CC+ and 10 TS POC CC-. Six of the 35 TS POC CC+ participants with NCC (17%) had epileptic seizures, and one of the ten TS POC CC- with NCC (10%). The total number of NCC lesions was 263 for TS POC CC+ participants and 46 for TS POC CC- (Table 4). Median number of lesions did not differ by TS POC CC status (TS POC CC+ : 3 (IQR 1–6) versus TS POC CC-: 2.5 (IQR 1–5.3), p = 0.64). TS POC CC+ participants with epileptic seizures did not have more lesions compared with TS POC CC+ participants who had never experienced epileptic seizures (median: 3 (IQR 2–7) versus 3 (IQR 1–6), p = 0.63). There was also no association between the number of lesions and the likelihood of epileptic seizures (Table A5) though more patients with NCC and epileptic seizures had parenchymal lesions (Table 4). Only the TS POC CC+ participants had active stage lesions – all in vesicular stage. Eight of the eleven (73%) patients with active lesions, also had calcified lesions (i.e. mixed lesions). Individuals with TS POC CC- NCC all had calcified lesions only. All 45 NCC participants had parenchymal lesions, 10 had further extra-parenchymal lesions which were all located in the cortical sulci in the subarachnoid space. There was no association between the likelihood of extra-parenchymal lesions and TS POC CC status.
4 Discussion
In this study we assessed the sero-prevalence of CC and the prevalence of NCC in Sinda district of the Eastern province in Zambia. Although our study used a design based around a TS POC CC test with imperfect accuracy, the modelling of the results made our findings reliable and independent from the accuracy of the TS POC CC test. The TS POC CC test has been evaluated for NCC in a clinical setting, among people with epileptic seizures where it highlighted a limited sensitivity. The sensitivity was, however, comparable to the sensitivity of the current reference tests. Sensitivity of the TS POC CC test was even excellent for the detection of active-stage NCC lesions irrespective of the presence or absence of neurological signs and symptoms [25, 26].
In our study, every seventh participant was cysticercosis positive on the TS POC CC test, but positivity differed considerably between and within health communities with the highest burden being observed in Chinzure and the lowest in Mtore. Cysticercosis prevalence assessed by the current reference tests was even higher with every fifth person of the community having the infection. It is difficult to pinpoint exactly the factors present in the study area or population that can explain the observed higher CC positivity in Chinzure compared to the other three communities.
A sizeable proportion (13.5%) of people had NCC lesions – however, only a small fraction (not even every tenth) of these had lesions in active stage which may indicate a reduction in infection pressure over the last years [32, 33]. Community-based studies of NCC are scarce, but in a study conducted in a T. solium endemic area in Peru, 18% of residents had NCC [34].
We also observed that only a small proportion of people with NCC reported having ever had epileptic seizures, and that among people with epileptic seizures, more than every third patient had NCC lesions. As comparison, among people without epileptic seizures, around every eighth person had NCC. This is important, as many epidemiological studies on NCC only include people with epileptic seizures or serologically positive people for further neuroimaging [18, 35]. As a comparison, in a previous study in an adjacent district to our study area, more than 50% of people with epilepsy had NCC [18]. In that study, however, the prevalence of NCC was higher than in our study population probably because only people with active epilepsy were recruited for neuroimaging whereas our study included all people who ever had an epileptic seizure. These findings are consistent with observations from other Latin American countries and globally [6, 36]. The differences between the health communities re-emphasize the varying burden of T. solium on a very granular level which has been demonstrated before [20, 37].
We also found that participants with NCC were older than participants without NCC (although not significant) which is consistent with previously published studies [38,39,40]. Older age not only predisposes to more frequent infection but also to changes in the immune system which over some time protects the host against inflammatory reaction towards the parasite [41].
All NCC patients with epileptic seizures had calcifications on neuroimaging, some had additional active stage lesions. Recurrent seizures are still common in patients with calcified parenchymal brain cysticerci, although their frequency is often lower than in case of active/degenerating stage lesions [42,43,44]. However, in our study, a good proportion (82%) of participants with calcifications reportedly had never experienced an epileptic seizure. This phenomenon could be explained by the fact that there were relatively few NCC lesions per patient, that epileptic seizures may only occur when lesions are located near the grey matter-white matter junction [45] or by the hypothesis that there may be a genetic predisposition to low seizure threshold in people with NCC who develop epilepsy [46, 47].
Moreover, we found that a substantial proportion of people with NCC, especially those with calcified NCC, were negative in both reference tests. This inaccuracy of the reference tests in detecting calcified lesions has previously been described [25, 40].
Concerning typical neurological signs/symptoms of NCC, we focused on evaluation and analyses of participants with epileptic seizures. Yet, headache is also a typical symptom and the second most encountered manifestation of NCC after seizures [5, 48, 49]. However, although we investigated headache during our clinical assessment, we realized by time of analyses that answers were too inconsistent and unspecific to be evaluated for NCC. Nevertheless, our assessment showed that there is a high burden of headache in our study population (irrespective of NCC status), with about one third of participants reporting regular headache. This is in line with the findings of the Global Burden of Disease study which found a considerable burden of headache that is often not optimally recognized and treated [50]. Headache within the sub-Saharan region are a common finding [51, 52]. An association between calcified NCC and headache was also recently established in a community case–control study conducted in Ecuador [53]. This study also found that the burden of infection had no significant effect on the outcome of the headache among NCC patients. Consequently, for future NCC studies, headache should be evaluated in far greater detail.
5 Strengths and Limitations
Our study was community-based and had a relatively large sample size. As we included the TS POC result as a stratification factor for further clinical work-up, we were able to infer the true prevalence of NCC in the total study population, irrespective of inaccuracies of serological tests. Nonetheless, selection bias may have been present as only some households were visited and not everybody living in the households was present during the recruitment and TS POC testing. However, as households were selected at random, we do not think that this has impacted prevalence estimates considerably.
Our study also had some limitations. We are well aware that the serological tests used in our study are not perfect to diagnose cysticercosis [24], but since they are still very often used, the tests were utilized only to allow comparison with previously published studies. In addition, we used a CT scanner and not a magnetic resonance imaging (MRI) for the detection of NCC lesions because MRI is not available in the province. Generally, CT scanners are good for detecting calcified lesions, but may miss some active stage lesions which more likely are captured by MRI. Hence, we may have underestimated the active NCC prevalence; the prevalence of any type of NCC will likely not have been affected considerably. Especially also, because all participants had a CT scan with and without contrast which may have increased likelihood of detection of lesions. For some participants, there was quite a long time between TS POC test and the CT examination. This is because the CT scanner was often dysfunctional which resulted in a postponing of appointments. Furthermore, the CT examinations were done about 120 km away from the study site and participants had to be taken in small groups, which meant they had to spend a whole day to undergo CT examination. These delays may have affected our results. However, in a hospital-based setting among people with epileptic seizures [25], an assessment on the effect of this delay was done and the results were negligible. We thus believe this effect would not be considerably different in our study.
Furthermore, in this study people were asked if they had ever experienced an epileptic seizure. Although a validated questionnaire was used, recall bias and stigma will likely have affected the assessment—however, we do not think that there would be a difference between people with and without NCC.
6 Conclusion
NCC is common in Sinda district in Eastern province of Zambia. Active stage lesions, however, are relatively infrequent. We demonstrated that a large proportion of people with NCC reportedly never experienced epileptic seizures and that many people with NCC (especially those with only calcified lesions) are serologically negative for T. solium cysticercosis antibodies. The prevalence of NCC in Sinda district communities calls for closer collaboration between human and veterinary public and environmental health services in order to break the T. solium lifecycle in humans, pigs and potentially also the environment—in an equitable, interdisciplinary One Health approach with affected communities at its core.
Availability of Data and Materials
All data used are within the manuscript and its supporting information files.
References
Flisser A. Where are the tapeworms? Parasitol Int. 2006. https://doi.org/10.1016/j.parint.2005.11.018.
Mwape KE, Phiri IK, Praet N, Speybroeck N, Muma JB, Dorny P, et al. The Incidence of Human Cysticercosis in a Rural Community of Eastern Zambia. PLoS Negl Trop Dis. 2013;7: e2142. https://doi.org/10.1371/journal.pntd.0002142.
Winkler AS. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob Health. 2012;106:261–74. https://doi.org/10.1179/2047773212Y.0000000047.
Pradhan S, Kumar R, Gupta RK. Intermittent symptoms in neurocysticercosis: Could they be epileptic? Acta Neurol Scand. 2003;107:260–6. https://doi.org/10.1034/j.1600-0404.2003.01380.x.
Stelzle D, Abraham A, Kaminski M, Schmidt V, De Meijere R, Bustos J, et al. Clinical characteristics and management of neurocysticercosis patients: a retrospective assessment of case reports from Europe. J Travel Med. 2022. https://doi.org/10.1093/jtm/taac102.
Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4: e870. https://doi.org/10.1371/journal.pntd.0000870.
Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5: e1152. https://doi.org/10.1371/journal.pntd.0001152.
Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202–15. https://doi.org/10.1016/S1474-4422(14)70094-8.
Blocher J, Schmutzhard E, Wilkins PP, Gupton PN, Schaffert M, Auer H, et al. A cross-sectional study of people with epilepsy and neurocysticercosis in Tanzania: clinical characteristics and diagnostic approaches. PLoS Negl Trop Dis. 2011;5: e1185. https://doi.org/10.1371/journal.pntd.0001185.
Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011;9:123–33. https://doi.org/10.1586/eri.10.150.
Gabriël S, Mwape KE, Phiri IK, Devleesschauwer B, Dorny P. Taenia solium control in Zambia: the potholed road to success. Parasite Epidemiol Control. 2019. https://doi.org/10.1016/j.parepi.2018.e00082.
Zulu G, Stelzle D, Mwape KE, Welte TM, Strømme H, Mubanga C, et al. The epidemiology of human Taenia solium infections: A systematic review of the distribution in Eastern and Southern Africa. PLoS Negl Trop Dis. 2023;17: e0011042. https://doi.org/10.1371/JOURNAL.PNTD.0011042.
Phiri IK, Dorny P, Gabriel S, Willingham AL, Speybroeck N, Vercruysse J. The prevalence of porcine cysticercosis in Eastern and Southern provinces of Zambia. Vet Parasitol. 2002;108:31–9. https://doi.org/10.1016/S0304-4017(02)00165-6.
Sikasunge CS, Phiri IK, Phiri AM, Siziya S, Dorny P, Willingham AL. Prevalence of Taenia solium porcine cysticercosis in the Eastern, Southern and Western provinces of Zambia. Vet J. 2008;176:240–4. https://doi.org/10.1016/j.tvjl.2007.02.030.
Chembensofu M, Mwape KE, Van Damme I, Hobbs E, Phiri IK, Masuku M, et al. Re-visiting the detection of porcine cysticercosis based on full carcass dissections of naturally Taenia solium infected pigs. Parasit Vectors. 2017. https://doi.org/10.1186/s13071-017-2520-y.
Mwape KE, Phiri IK, Praet N, Muma JB, Zulu G, van den Bossche P, et al. Taenia solium infections in a rural area of Eastern Zambia—a community based study. PLoS Negl Trop Dis. 2012;6:1–9. https://doi.org/10.1371/journal.pntd.0001594.
Zulu G, Sikasunge CS, Welte TM, Simuunza MC, Stelzle D, Schmidt V, et al. Epidemiology of intestinal helminthiasis with an emphasis on taeniasis in Chipata district of the Eastern province of Zambia. PLoS Negl Trop Dis. 2023;17: e0011561. https://doi.org/10.1371/JOURNAL.PNTD.0011561.
Mwape KE, Blocher J, Wiefek J, Schmidt K, Dorny P, Praet N, et al. Prevalence of neurocysticercosis in people with epilepsy in the Eastern province of Zambia. PLoS Negl Trop Dis. 2015;9: e0003972. https://doi.org/10.1371/journal.pntd.0003972.
Stelzle D, Makasi C, Schmidt V, Trevisan C, van Damme I, Welte TM, et al. Epidemiological, clinical and radiological characteristics of people with neurocysticercosis in Tanzania—a cross-sectional study. PLoS Negl Trop Dis. 2022;16: e0010911. https://doi.org/10.1371/journal.pntd.0010911.
Keller L, Stelzle D, Schmidt V, Carabin H, Reinhold AK, Keller C, et al. Community-level prevalence of epilepsy and of neurocysticercosis among people with epilepsy in the Balaka district of Malawi: a cross-sectional study. PLoS Negl Trop Dis. 2022;16: e0010675. https://doi.org/10.1371/journal.pntd.0010675.
Stelzle D, Schmidt V, Ngowi BJ, Matuja W, Schmutzhard E, Winkler AS. Lifetime prevalence of epilepsy in urban Tanzania—a door-to-door random cluster survey. ENeurologicalSci. 2021;24: 100352. https://doi.org/10.1016/j.ensci.2021.100352.
Stelzle D, Kaducu J, Schmidt V, Welte TM, Ngowi BJ, Matuja W, et al. Characteristics of people with epilepsy in three Eastern African countries—a pooled analysis. BMC Neurol. 2022. https://doi.org/10.1186/s12883-022-02813-z.
Van Damme I, Trevisan C, Mwape KE, Schmidt V, Magnussen P, Zulu G, et al. Trial design for a diagnostic accuracy study of a point-of-care test for the detection of Taenia solium taeniosis and (Neuro)cysticercosis in community settings of highly endemic, resource-poor areas in Zambia: challenges and rationale. Diagnostics. 2021. https://doi.org/10.3390/diagnostics11071138.
Mubanga C, Van Damme I, Trevisan C, Schmidt V, Phiri IK, Zulu G, et al. Evaluation of an antibody detecting point of care test for diagnosis of Taenia solium cysticercosis in a Zambian rural community: a prospective diagnostic accuracy study. Diagnostics. 2021. https://doi.org/10.3390/DIAGNOSTICS11112121/S1.
Stelzle D, Makasi CE, Schmidt V, Van Damme I, Trevisan C, Ruether C, et al. Evaluation of a point-of-care test for the diagnosis of Taenia solium neurocysticercosis in rural southern Tanzania: a diagnostic accuracy study. Lancet Infect Dis. 2024;24:98–106. https://doi.org/10.1016/S1473-3099(23)00378-X.
Zulu G, Stelzle D, Mwape KE, Van Damme I, Trevisan C, Mubanga C, et al. The performance of a point-of-care test for the diagnosis of neurocysticercosis in a resource-poor community setting in Zambia—a diagnostic accuracy study. 2024. https://doi.org/10.2139/SSRN.4746924.
Del Brutto OH, Nash TE, White AC, Rajshekhar V, Wilkins PP, Singh G, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202–10. https://doi.org/10.1016/J.JNS.2016.11.045.
Zhao J-L, Lerner A, Shu Z, Gao X-J, Zee C-S. Imaging spectrum of neurocysticercosis. Radiol Infect Dis. 2015;1:94–102. https://doi.org/10.1016/j.jrid.2014.12.001.
Escobar A. The pathology of neurocysticercosis. In: Rodriguez-Carbajal J, Taveras J, Taveras J, editors. Cysticercosis Cent. Nerv. Syst. Charles C. Thomas; 1983. p. 27–57.
Coughlin SS, Trock B, Criqui MH, Pickle LW, Browner D, Tefft MC. The logistic modeling of sensitivity, specificity, and predictive value of a diagnostic test. J Clin Epidemiol. 1992;45:1–7. https://doi.org/10.1016/0895-4356(92)90180-U.
Lumley T. Survey: analysis of complex survey samples. R Packag Version. 40 2020;9:1–19. https://doi.org/10.18637/jss.v009.i08.
Gilman RH, Del Brutto OH, García HH, Martínez M. Prevalence of taeniosis among patients with neurocysticercosis is related to severity of infection. Neurology. 2000;55:1062. https://doi.org/10.1212/WNL.55.7.1062.
Hamamoto Filho PT, Singh G, Winkler AS, Carpio A, Fleury A. Could differences in infection pressure be involved in cysticercosis heterogeneity? Trends Parasitol. 2020;36:826–34. https://doi.org/10.1016/j.pt.2020.07.003.
Moyano LM, O’Neal SE, Ayvar V, Gonzalvez G, Gamboa R, Vilchez P, et al. high prevalence of asymptomatic neurocysticercosis in an endemic rural community in Peru. PLoS Negl Trop Dis. 2016. https://doi.org/10.1371/journal.pntd.0005130.
Guzman C, Garcia HH. Current diagnostic criteria for neurocysticercosis. Res Rep Trop Med. 2021;12:197–203. https://doi.org/10.2147/rrtm.s285393.
Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis. 1999;29:1203–9. https://doi.org/10.1086/313470.
Carabin H, Millogo A, Cissé A, Gabriël S, Sahlu I, Dorny P, et al. Prevalence of and factors associated with human cysticercosis in 60 villages in three provinces of Burkina Faso. PLoS Negl Trop Dis. 2015;9: e0004248. https://doi.org/10.1371/journal.pntd.0004248.
Fleury A, Dessein A, Preux P, Dumas M, Tapia G, Larralde C, et al. Symptomatic human neurocysticercosis. J Neurol. 2004;251:830–7. https://doi.org/10.1007/s00415-004-0437-9.
Cavellani CL, Faleiros ACG, de Lino RS, dos Reis MA, de Teixeira VPA. Cysticercosis in the elderly. Ann Diagn Pathol. 2007;11:330–3. https://doi.org/10.1016/j.anndiagpath.2006.12.008.
Stelzle D, Schmidt V, Keller L, Ngowi BJ, Matuja W, Escheu G, et al. Characteristics of people with epilepsy and Neurocysticercosis in three eastern African countries—a pooled analysis. PLoS Negl Trop Dis. 2022;16: e0010870. https://doi.org/10.1371/journal.pntd.0010870.
Praet N, Speybroeck N, Rodriguez-Hidalgo R, Benitez-Ortiz W, Berkvens D, Brandt J, et al. Age-related infection and transmission patterns of human cysticercosis. Int J Parasitol. 2010;40:85–90. https://doi.org/10.1016/j.ijpara.2009.07.007.
Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8. https://doi.org/10.1212/01.WNL.0000129481.12067.06.
Carpio A, Romo ML. The relationship between neurocysticercosis and epilepsy: an endless debate. Arq Neuropsiquiatr. 2014;72:383–90. https://doi.org/10.1590/0004-282X20140024.
Stelzle D, Makasi C, Schmidt V, Trevisan C, Van Damme I, Ruether C, et al. Efficacy and safety of antiparasitic therapy for neurocysticercosis in rural Tanzania: a prospective cohort study. Infection. 2023;51:1127–39. https://doi.org/10.1007/s15010-023-02021-y.
Adamczyk B, Węgrzyn K, Wilczyński T, Maciarz J, Morawiec N, Adamczyk-Sowa M. The most common lesions detected by neuroimaging as causes of epilepsy. Med. 2021;57:294. https://doi.org/10.3390/medicina57030294.
Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–45. https://doi.org/10.1159/000068748.
Carpio A, Romo ML. Multifactorial basis of epilepsy in patients with neurocysticercosis. Epilepsia. 2015;56:973–4. https://doi.org/10.1111/epi.12978.
Fogang YF, Savadogo AA, Camara M, Toffa DH, Basse A, Sow AD, et al. Managing neurocysticercosis: challenges and solutions. Int J Gen Med. 2015;8:333–44. https://doi.org/10.2147/IJGM.S73249.
Pradhan S, Das A, Anand S, Deshmukh AR. Clinical characteristics of migraine in patients with calcified neurocysticercosis. Trans R Soc Trop Med Hyg. 2019;113:418–23. https://doi.org/10.1093/trstmh/trz018.
Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–76. https://doi.org/10.1016/S1474-4422(18)30322-3.
Winkler AS, Stelzhammer B, Kerschbaumsteiner K, Meindl M, Dent W, Kaaya J, et al. The prevalence of headache with emphasis on tension-type headache in rural Tanzania: a community-based study. Cephalalgia. 2009;29:1317–25. https://doi.org/10.1111/j.1468-2982.2009.01885.x.
Winkler AS, Dent W, Stelzhammer B, Kerschbaumsteiner K, Meindl M, Kaaya J, et al. Prevalence of migraine headache in a rural area of northern Tanzania: a community-based door-to-door survey. Cephalalgia. 2010;30:582–92. https://doi.org/10.1111/j.1468-2982.2009.01994.x.
Del Brutto OH, Robles AM, Mera RM, Costa AF, Darsan E, Milla L, et al. Calcified neurocysticercosis and headache in an endemic village: a case-control study nested to a population-based cohort. Am J Trop Med Hyg. 2018;99:729–34. https://doi.org/10.4269/ajtmh.18-0310.
Acknowledgements
We would like to acknowledge the study participants from Mtandaza community of Sinda district and the health staff from Mtandaza clinic for making this study possible. We acknowledge all members of the SOLID consortium who are not included in the author list of this paper: Benedict T. Ndawi, Charles E. Makasi, Mwelwa Chembensofu, Chishala Chabala, Famke Jansen, Helena Ngowi, Karen S. Møller, Maria V. Johansen, Maxwell Masuku, Richard Mambo.
Funding
This research was funded by the European & Developing Countries Clinical Trials Partnership (EDCTP) [grant number DRIA2014-308] and the German Federal Ministry of Education and Research (BMBF) [grant number: 01KA1617] within the research grant “Evaluation of an antibody detecting point-of-care test for the diagnosis of Taenia solium taeniosis, and (neuro)cysticercosis in communities and primary care settings of highly endemic, resource-poor areas in Tanzania and Zambia, including training of and technology transfer to the Regional Reference Laboratory and health centers (SOLID).”
Author information
Authors and Affiliations
Consortia
Contributions
GZ, DS, SG, ASW, CT, IVD, KEM, CM, IKP, PD, PM conceptualized the study. Data curation was done by GZ, DS, SG, ASW, CT, IVD and KEM. DS, and IVD performed the formal analysis. SG, ASW, PD, and KEM acquired the funding. The methodology was designed by GZ, DS, SG, ASW, CT, IVD, KEM and CM. Project administration was done by GZ, CM, SG, KEM and ASW. Validation was done by GZ, DS, SG, ASW, IVD and KEM. The project was supervised by KEM, SG and ASW. GZ and DS wrote the original draft and all authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics Approval and Consent to Participate
Ethical clearance for the SOLID project was obtained by all study partners: The University of Zambia Biomedical Research Ethics Committee (UNZABREC 005-07-17), the Technical University of Munich, Klinikum rechts der Isar, Ethical Committee (299/18S), Institute of Tropical Medicine, Belgium (IRB/AB/ac/112 Ref 1177/17) and the University of Antwerp, Belgium (EC UZA 17/31/352). The SOLID study was registered in the Pan African Trials Registry (PACTR201712002788898). All participants were informed about all parts of the study before inclusion, and all signed an informed consent. For illiterate participants and for underage participants (< 18 years) a guardian signed the informed consent, in presence of an impartial witness.
Consent for Publication
All authors have read and approved the manuscript for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zulu, G., Stelzle, D., Gabriël, S. et al. Neurocysticercosis Prevalence and Characteristics in Communities of Sinda District in Zambia: A Cross-Sectional Study. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00271-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00271-z