Abstract
Introduction
The ongoing visceral leishmaniasis (VL) elimination programme in India is targeting the elimination of the disease VL but not the pathogen. The persistence of hidden parasite pool may initiate a resurgence in suitable conditions. This study dealt with a novel approach to unearth such pathogen pool and their proper management to prevent the resurgence of VL.
Materials and Methods
We deployed a new approach for detection of pathogen pool by following up the VL and post kala-azar dermal leishmaniasis patients treated during the last 10 years along with mass sero-surveillance within a radius of 500 m of recently treated individuals.
Results
We followed up 72.6% (3026/4168) previously treated VL and post kala-azar dermal leishmaniasis patients and diagnosed 42 (1.4%) new and 38 (1.3%) recurrent post kala-azar dermal leishmaniasis. We detected 93 asymptomatic leishmanial infection, 8 VL and 1 post kala-azar dermal leishmaniasis by mass sero-surveillance.
Conclusion
Our three-step process including mapping and follow-up of previously treated cases, mass surveillance within 500 m of radius of known cases, and 6 monthly follow-on clinical and serological screening of asymptomatic cases, enabled detection of previously undetected cases of post kala-azar dermal leishmaniasis and VL. Recurrent post kala-azar dermal leishmaniasis deserves special attention regarding their treatment guideline. Early diagnosis and effective treatment of all leishmaniasis cases will hasten pathogen elimination and prevent resurgence of VL. This may help the policymakers to develop appropriate strategy for elimination of pathogen to prevent resurgence of VL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Visceral leishmaniasis (VL), also known as kala-azar, is a vector borne neglected tropical disease caused by a protozoa belonging to the genus Leishmania and is transmitted by female sandfly of the genus Phlebotomous [1]. More than 80 endemic countries in tropical and subtropical regions reported an estimated 50,000–90,000 new VL cases annually. Ten countries i.e., Brazil, China, Ethiopia, India, Iraq, Kenya, Nepal, Somalia, South Sudan, and Sudan reported 95% of the global VL cases [2]. Three adjoining countries of WHO South East Asia region - India, Bangladesh and Nepal account for more than 50% of the global burden, to which India contributes the major share [3]. In India, about 165.4 million population of 54 endemic districts of Bihar, Jharkhand, West Bengal, and Uttar Pradesh are at risk of infection [4]. India reported 818 VL cases in 2022, of which 52 were from West Bengal [4].
India, Bangladesh, and Nepal jointly launched a VL elimination programme in 2005 supported by the World Health Organization (WHO). The initiative was aimed to eliminate VL as a public health problem by 2015 by reducing the annual incidence to less than 1/10,000 population at the sub-district or district level [4, 5]. The deadline was subsequently reset to 2017 and then to 2023 [6]. Nepal and Bangladesh have reached the target in 2013 and 2016 respectively [7, 8]. India is close to the goal except in few hot spot areas with ongoing transmission [9]. Historically, VL epidemics in India occur cyclically at an interval of 10–15 years [10]. In the Indian subcontinent Leishmania donovani is the only parasite, Phlebotomus argentipes is the sole vector, and man is the only vertebrate host. The average life span of female sandfly is about 12 days [11]. So, the hidden parasite pools persist in humans during inter-epidemic period and serve as the source of pathogens for resurgence of VL. Two forms of leishmaniasis - post kala-azar dermal leishmaniasis and asymptomatic leishmanial infection play a crucial role as such reservoir. Post kala-azar dermal leishmaniasis, a dermal morbidity characterised by macular, maculo-papular, and nodular lesions, usually developed among 15–20% of apparently cured VL patients following months to years of treatment [12]. Individuals, without prior VL, may also develop the condition [12,13,14,15,16,17]. It has long been considered to act as the only inter-epidemic reservoir of anthroponotic VL, and the existence of a few cases is sufficient to trigger a new epidemic in a given community [12, 15, 18]. Recently xenodiagnosis studies proved that all forms of post kala-azar dermal leishmaniasis are infectious to sandflies [19]. There is no standard definition of asymptomatic leishmanial infection, but is usually diagnosed by a positive serological test, polymerase chain reaction (PCR), or leishmanin skin test [20, 21]. Mathematical modelling suggests that asymptomatic leishmanial infection may serve as a reservoir of parasites which may cause resurgence of VL, although their infectiousness to sandflies is not well established [22]. The rural poor people, commonly affected with leishmaniasis, are not much concerned about the skin lesions of post kala-azar dermal leishmaniasis. Usually, they do not consult the health service provider during early stage and thus maintain the pathogen in the community. Searching of such parasite pool in the community poses a great challenge to the health system.
Researchers conducted several studies to improve the detection of VL at community level by implementing various case search strategies [23, 24]. But no such effort has so far been made for detection of post kala-azar dermal leishmaniasis. This study was aimed to eliminate the hidden parasite pool using a novel approach of enhanced active case-search, along with the ongoing national programme to prevent the resurgence of VL in endemic districts of West Bengal, India.
2 Materials and Methods
2.1 Study Areas
West Bengal is one of the four provinces of India which is endemic for VL for long time. This study was undertaken in four VL endemic districts of West Bengal - Darjeeling, Uttar Dinajpur, Dakshin Dinajpur and Malda during January, 2022 - March, 2023. All these districts are situated on the northern side of the river Ganges with an international border with Bangladesh in the east and inter-state border with Bihar and Jharkhand (both endemic for VL) in the west. Darjeeling district has an international border with Nepal (in the west) (Fig. 1). All the neighbouring countries are endemic for VL. So, the study areas play an important role in trans-border transmission among three adjoining countries.
2.2 Study Design and Searching of Hidden Pathogen Pool
It was a community based longitudinal study for the detection of hidden parasite pools and their proper treatment for prevention of resurgence of VL. We searched for such pathogen pools using two different ways. First, we adopted a novel approach by clinical and serological follow-up of previously treated VL and post kala-azar dermal leishmaniasis patients as in Indian subcontinent most of the post kala-azar dermal leishmaniasis develop among treated VL subjects [25]. We collected the line-list of VL and post kala-azar dermal leishmaniasis patients treated during last 10 years from the State Health authority and verified with the records of respective districts and sub-districts. We also included additional records found at district/sub-district levels, if any, in the final line-list. We conducted sensitization meetings at districts and sub-districts levels with key stakeholders including Deputy Chief Medical Officer of Health-II, Block Medical Officer of Health, Vector Borne Disease Consultant, District Epidemiologist, Block Public Health Nurse, Auxiliary Nurse Midwife, Accredited Social Health Activist and Kala-azar Technical Supervisor. We created separate groups using social messaging application to networking all stakeholders at the sub-district level. Study teams accompanied by Accredited Social Health Activist visited door-to-door and examined all available enlisted individuals for any signs and symptoms of VL/post kala-azar dermal leishmaniasis and tested serologically by rK39 rapid diagnostic test (InBios INC, USA) for the detection of anti-leishmanial antibody. The clinical experts examined all rK39 positive cases with signs and symptoms of post kala-azar dermal leishmaniasis in medical camps for case confirmation. We examined all suspected recurrent post kala-azar dermal leishmaniasis patients parasitologically by microscopy and PCR.
Second, we conducted index case-based mass sero-surveillance around 500 m radius of an active VL/post kala-azar dermal leishmaniasis cases or those treated during previous three years to identify the cases of post kala-azar dermal leishmaniasis, VL and asymptomatic leishmanial infection. Expert clinicians examined all rK39 positive individuals clinically and/or parasitologically, for case confirmation. We followed up the individuals with asymptomatic leishmanial infection clinically and serologically at an interval of 6 months (Fig. 2).
2.3 Case Definition
New post kala-azar dermal leishmaniasis: Individuals positive for rK39 test with or without history of VL along with typical signs and symptoms of post kala-azar dermal leishmaniasis were diagnosed as ‘new’ post kala-azar dermal leishmaniasis.
Recurrent post kala-azar dermal leishmaniasis: It is defined as ‘an anti-leishmanial antibody positive case having documented history of diagnosis and treatment completion for post kala-azar dermal leishmaniasis with disappearance or significant reduction in dermal lesions and reappearance of new lesions and/ or increase in size of pre-existing residual hypo-pigmented lesions along with parasitological confirmation either by microscopy and/or PCR.
Asymptomatic leishmanial infection: It is defined as someone from an endemic area that exhibits an immune response (either humoral or T-cell mediated) against Leishmania or has parasites or parasitic DNA in the blood but remains healthy.
2.4 Collection of Slit Skin Scraping Samples from Post Kala-Azar Dermal Leishmaniasis Patients
We collected the slit skin scraping samples from suspected recurrent post kala-azar dermal leishmaniasis for detection of parasites by microscopy and leishmanial DNA by PCR following proper aseptic conditions. We used one part of tissue fluid and cells for preparation of 2 smears and another part collected in NET buffer [150 mM NaCl, 15 mM Tris–HCl (pH-8.30), 1 mM EDTA] for isolation of DNA.
2.5 Laboratory Investigations
2.5.1 Staining of Slit Skin Smear and Microscopy
We stained one smear with Giemsa and other with modified Ziehl–Neelsen stain. Two expert microscopists examined Giemsa-stained smear for LD bodies and Ziehl–Neelsen stained smear to rule out presence of acid-fast bacilli.
2.5.2 Isolation of DNA from Slit Skin Scraping Samples for the Detection of Leishmanial DNA by PCR Method
We extracted DNA from the slit skin scraping samples collected in NET buffer by using QIAamp DNA blood mini kit (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. We performed leishmania-specific nested PCR targeting the parasites’ SSU-rRNA region as described by Salam et al. [26]. For each set of PCR reaction, we used DNA from known VL cases as positive control while sterile sigma water as negative control.
2.6 Treatment of VL, New and Recurrent Post Kala-Azar Dermal Leishmaniasis
Local district/sub-district hospitals treated all diagnosed VL and post kala-azar dermal leishmaniasis cases according to the Indian national guidelines. After ruling out HIV co-infection, medical professionals administered a single intravenous infusion of liposomal amphotericin B (10 mg/Kg body weight) to treat VL cases. New and recurrent post kala-azar dermal leishmaniasis patients were treated with oral drug miltefosine for 12 weeks.
2.7 Ethics Statement
Before initiation of survey, study team explained the objectives and benefits to the enlisted patients, head of the family and other members. They were also informed about non-disclosure of their identity and the liberty to withdraw from the study at any time. We obtained written informed consent from suspected recurrent PKDL patients or from legal guardians (for minor) for collection of slit-skin scrapings and verbal consent from the head of the family during mass screening. The Clinical Research Ethics Committee of School of Tropical Medicine, Kolkata has approved the study protocol.
3 Results
3.1 Study Subjects
We obtained a line-list of 5145 previously treated VL and post kala-azar dermal leishmaniasis from state health authority and verified with the available data of respective districts and sub-districts. Of them, 2978 (57∙9%) were male and 2167 (42∙1%) were female. During the door-to-door follow-up, we could not identify and traced 379 cases, 598 had died before initiation of the study. Therefore, we targeted a total of 4168 individuals for follow-up.
3.2 Year Wise Incidence of Previously Treated VL and Post Kala-Azar Dermal Leishmaniasis Cases and their Treatment History as Recorded
Out of 5145, 858 VL cases were treated before 2010 and 4287 during 2010–2021. We could not ascertain the treatment history of 365 VL cases. Among 4780 patients, 2062 (43.1%), 1969 (41.2%) and 749 (15.7%) were treated with sodium stibo-gluconate, miltefosine and liposomal amphotericin B respectively (Fig. 3).
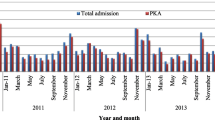
Among 5145 enlisted patients, 1048 had history of post kala-azar dermal leishmaniasis following VL. Of them 108 (10.3%), 684 (65.3%) and 256 (24.4%) received sodium stibo-gluconate, miltefosine and liposomal amphotericin B respectively (Fig. 4). Incidence of VL was highest in 2011 and that of post kala-azar dermal leishmaniasis in 2016. Thereafter, the annual incidence of both VL and post kala-azar dermal leishmaniasis declined gradually (Fig. 5).
3.3 History of Recurrent VL and Post Kala-Azar Dermal Leishmaniasis as Recorded Among Previously Treated Cases
It was evident from the records that certain number of VL and post kala-azar dermal leishmaniasis cases received treatment more than once. So, they were considered as recurrent VL or post kala-azar dermal leishmaniasis respectively. We recorded 25 recurrent VL, among them 9 (0.44%, 95% CI 0.20–0.83), 13 (0.66%, 95% CI 0.35–1.13) and 3 (0.40%, 95% CI 0.08–1.17) had history of treatment with sodium stibo-gluconate, miltefosine and liposomal amphotericin B respectively for their initial episode of VL (Table 1). Fifty cases had history of recurrent post kala-azar dermal leishmaniasis. Among them, 15 (13.89%, 95% CI 7.99–21.87) were treated with sodium stibo-gluconate, 26 (3.80%, 95% CI 2.50–5.52) with miltefosine and 9 (3.52%, 95% CI 1.62–6.57) with liposomal amphotericin B for their initial episode of post kala-azar dermal leishmaniasis (Table 2).
3.4 Incidence of New and Recurrent Post Kala-Azar Dermal Leishmaniasis Among Previously Treated Individuals
Out of 4168 previously treated cases, 3192 (76.6%) had history of VL only and 976 (23.4%) had both VL and post kala-azar dermal leishmaniasis history. We diagnosed 42 (1.9%, 95% CI 1∙4–2.5) as new post kala-azar dermal leishmaniasis among 2248 (70.4%) previously treated VL cases followed up. Out of 42 new post kala-azar dermal leishmaniasis cases, 30 (71.4%) had hypo-pigmented lesions and 12 (28.6%) had mix of hypo-pigmented and papulo-nodular lesions. Out of these 42 diagnosed ‘new’ post kala-azar dermal leishmaniasis, 23 (3.1%, 95% CI 1.9–4.7), 10 (0.9%, 95% CI 0.5–1.8) and 9 (1∙8%, 95% CI 0.8–3.4) had history of sodium stibo-gluconate, miltefosine and liposomal amphotericin B treatment respectively for their initial VL. We observed a significant difference between the incidence of new post kala-azar dermal leishmaniasis cases among sodium stibo-gluconate and miltefosine treated VL cases (p = 0.0021) but no such difference was observed between the incidence of new post kala-azar dermal leishmaniasis cases among miltefosine and liposomal amphotericin B (p = 0.2237) and sodium stibo-gluconate and liposomal amphotericin B (p = 0.2011) treated VL cases. The average interval between initial VL and diagnosis of post kala-azar dermal leishmaniasis was 4∙1 years with a range of 3–17 years.
We confirmed 38 (4.9%, 95% CI 3.5–6.6) recurrent post kala-azar dermal leishmaniasis among 778 (79.7%) cases with history of both VL and post kala-azar dermal leishmaniasis. Among them, parasite was demonstrated in 35 (92.1%) whereas parasitic DNA was detected in 36 (94.7%) cases by PCR (Fig. 6). Eight (21.1%) of them had hypo-pigmented lesions and 30 (78.9%) had mix of hypo-pigmented and papulo-nodular lesions. The treatment history for their initial episode of the disease was 3 (4%, 95% CI 0.8–11.3) with sodium stibo-gluconate, 26 (5.1%, 95% CI 3.4–7.4) with miltefosine and 9 (4.7%, 95% CI 2.2–8.7) with liposomal amphotericin B. There was no significant difference between the incidence of recurrent post kala-azar dermal leishmaniasis across all three previous treatment regimens (p = 1.00) (Table 3). The average interval between initial episode and diagnosis of recurrent post kala-azar dermal leishmaniasis was 1.9 years with a range of 1–9 years. Interestingly 7 cases had more than one episode of recurrent post kala-azar dermal leishmaniasis with a highest of 4 episodes of recurrence in one patient.
3.5 Results of Mass Sero-Surveillance Surrounding 500 m of Active or Treated VL/Post Kala-Azar Dermal Leishmaniasis During Preceding Three Years
We targeted 197 foci for active mass sero-survey with a population of 20,297, of them 9542 (47.01%) were examined. By this way, we diagnosed 8 (0.08%, 95% CI 0.04–0.17) VL and 1 (0.01%, 95% CI 0–0.06) post kala-azar dermal leishmaniasis (without history of VL) and 93 (0.97%, 95% CI 0.79–1.19) asymptomatic leishmanial infection. Individuals with asymptomatic leishmanial infection are being followed up for development of VL or post kala-azar dermal leishmaniasis (Table 4).
4 Discussion
Our efforts through a three-step process of mapping and follow-up of previously treated cases, mass surveillance around known cases, and regular clinical and serological screening of asymptomatic cases enabled detection of 42 new and 38 recurrent post kala-azar dermal leishmaniasis. A significant number of recurrent post kala-azar dermal leishmaniasis is a matter of concern for VL elimination programme and attention should be paid to their treatment modalities.
The ongoing VL elimination programme in Indian subcontinent is targeting the elimination of the disease VL but not the pathogen [6]. The main components of the programme are: (a) early case detection and complete treatment, (b) integrated vector management, (c) effective disease surveillance, (d) social mobilization and behavioural changes, and (e) operational research [4,5,6]. Case detection is performed by three different ways; house-to-house survey, camp-based approach and index case-based mass screening depending upon the epidemiological situation [27]. By adopting these approaches, the annual incidence of VL has come down below the elimination target in Indian sub-continent. The persistence of a significant number of undiagnosed cases of post kala-azar dermal leishmaniasis in the community raises the question whether an annual incidence target for this condition should be incorporated into the National VL elimination program.
Previous studies showed that active door-to-door case search for VL, in addition to passive case detection, resulted in reducing the time to diagnosis and risk of transmission [23]. But no such effort has so far been given for the detection of post kala-azar dermal leishmaniasis. Active search for post kala-azar dermal leishmaniasis is a major constrain as they are apparently healthy and do not seek treatment [28, 29]. It is also difficult to reach all individuals living in endemic areas. In India, majority of post kala-azar dermal leishmaniasis developed among treated VL cases [25]. Considering this, the strategy for case searching need to be modified and focused among the individuals having history of VL and/or post kala-azar dermal leishmaniasis. By this approach we diagnosed and treated 42 new post kala-azar dermal leishmaniasis.
Detection of a significant number of recurrent post kala-azar dermal leishmaniasis (38/778, 4.9%) is an important finding of this study which remained overlooked in terms of its magnitude and treatment modalities. Analysis of the secondary programme data also revealed comparable number of recurrent post kala-azar dermal leishmaniasis (50/1048, 4.8%).
Case confirmation of recurrent post kala-azar dermal leishmaniasis is challenging as it requires demonstration of pathogen by microscopy or by PCR or by both along with clinical signs. We confirmed this by both the methods. Collection and examination (microscopy and PCR) of slit skin samples need some technical expertise and instrumental facilities which are not readily available at the field level. Two to three block primary health centres per district may be identified and existing clinical and laboratory staff can to be trained for this purpose.
We did not find any significant association between recurrences of post kala-azar dermal leishmaniasis with any specific anti-leishmanial drug. Presently, liposomal amphotericin B is used for the treatment of VL and miltefosine for post kala-azar dermal leishmaniasis. Miltefosine is provided to the patients on weekly basis for 12 weeks. Since supervised administration for each dose is not feasible, the adherence to miltefosine cannot be ascertained accurately. But recurrence of post kala-azar dermal leishmaniasis following treatment with liposomal amphotericin B is a matter of concern, as it is administered following hospitalization. So, the doses and duration of liposomal amphotericin B for the treatment of post kala-azar dermal leishmaniasis need a relook. There is no guideline for the treatment of recurrent post kala-azar dermal leishmaniasis both at national and international level. So, clinical trials are necessary to explore various dosages and durations of liposomal amphotericin B in combination with miltefosine or other anti-leishmanial drugs for formulation of appropriate guideline. Researchers and policymakers must prioritise this issue.
Majority of treated post kala-azar dermal leishmaniasis patients ignore the reappearance of skin lesions that delayed diagnosis and initiation of treatment. This results in persistence of parasites in the community. Regular awareness among the residents of endemic areas is an important aspect of ongoing programme through information, education, communication and behavioural, change, communication activities. Since the knowledge about the disease is moderate to poor among 75.2% population [30], enhancement of health education in endemic areas will improve the treatment seeking behaviour.
Recently, the asymptomatic leishmanial infection has drawn attention regarding its role in maintaining parasite in the community. Several studies have documented significant number of asymptomatic leishmanial infection in endemic areas including West Bengal [31,32,33]. Identification of asymptomatic leishmanial infection is only possible by mass sero-surveillance of the entire population by rK39 test which is labour and resource-intensive. Serological screening of population surrounding 500 m of active and recently treated cases is a feasible cost-effective alternative and included in the national policy. We have diagnosed 93 asymptomatic leishmanial infection, 8 VL and 1 post kala-azar dermal leishmaniasis. Among 93 asymptomatic leishmanial infection, disease conversion to VL was recorded in one individual during one year follow-up. The main challenge faced during follow-up was the unavailability of a significant number of previously treated individuals due to migration. Additional measures should be strategized to bring migrant workers under clinic-parasitological surveillance.
Vector control is one of the most important aspects for prevention of resurgence of VL. In India, indoor residual spray of DDT was in practice till 2016. It has been replaced by a synthetic pyrethroid - alpha cypermethrin due to emergence of DDT resistance. The used insecticide is effective but its application need close monitoring [34].
5 Conclusion
For elimination of pathogen pool, additional thrust should be given for detection of new and recurrent post kala-azar dermal leishmaniasis through follow-up of all previously treated VL and post kala-azar dermal leishmaniasis cases as it was done in the present study along with existing case search methods. It should be continued for long period as post kala-azar dermal leishmaniasis can develop after many years. Similarly, previously treated post kala-azar dermal leishmaniasis cases should also be observed for recurrence of skin lesions. Similar studies in other VL endemic areas are suggested to ascertain the feasibility and effectiveness of the case finding strategy deployed in this study. A clinical trial with different combination of anti-leishmanial drugs for formulation of treatment policy for recurrent post kala-azar dermal leishmaniasis is also warranted. This may help the policymakers at national and international level to develop appropriate strategy for eliminating pathogen to prevent resurgence of VL in future.
Availability of Data and Materials
All relevant data are within the manuscript and its supporting information files i.e., Tables and Figures.
Abbreviations
- VL:
-
Visceral leishmaniasis
- PCR:
-
Polymerase Chain Reaction
- WHO:
-
World Health Organization
- rK-39:
-
Recombinant product of K39
- DNA:
-
Deoxyribo nucleic acid
- HIV:
-
Human immunodeficiency virus
- p value:
-
Probability value
References
Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–70.
WHO Leishmaniais factsheet. 2023. https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis.
Rijal S, Sundar S, Mondal D, Das P, Alvar J, Boelaert M. Eliminating visceral leishmaniasis in South Asia: the road ahead. BMJ. 2019;364: k5224.
National Vector Borne Disease Control Programme. Accelerated plan for kala-azar elimination 2017. Directorate National Vector Borne Disease Control Programme. 2017. Available from: https://nvbdcp.gov.in/WriteReadData/l892s/Accelerated-Plan-Kala-azar1-Feb2017.pdf
Thakur CP, Meenakshi Thakur AK, Thakur S. Newer strategies for the kala-azar elimination programme in India. Indian J Med Res. 2009;29:102–4 (PMID: 19287067).
Sundar S, Singh OP, Chakravarty J. Visceral Leishmaniasis elimination targets in India, strategies for preventing resurgence. Expert Rev Anti Infect Ther. 2018;16(11):805–12. https://doi.org/10.1080/14787210.2018.1532790.
Rijal S, Sundar S, Mondal D, Das P, Alvar J, Boelaert M. Eliminating visceral leishmaniasis in South Asia: the road ahead. BMJ. 2019;2019(364): k5224. https://doi.org/10.1136/bmj.k5224.
Bhattacharya SK, Dash AP. Elimination of kala-azar from the Southeast Asia Region. Am J Trop Med Hyg. 2017;96(4):802–4. https://doi.org/10.4269/ajtmh.16-0279.
World Health Organization. Independent assessment of kala-azar elimination programme India. 2020. Available at:https://iris.who.int/bitstream/handle/10665/350947/9789290227960-eng.pdf?sequence=1
Napier LE. The principles and practice of tropical medicine. London: W. Thacker & Co.; 1943.
Hurwitz I, Hillesland H, Fieck A, Das P, Durvasula R. The paratransgenic sand fly: a platform for control of Leishmania transmission. Parasites Vectors. 2011;4:82. https://doi.org/10.1186/1756-3305-4-82.
Zijlstra EE, Musa AM, Khalil EAG, El-Hassan IM, El-Hassan AM. Post kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;2003(3):87–97. https://doi.org/10.1016/s1473-3099(03)00517-6.
Zijlstra EE, El-Hassa AM. Leishmaniasis in Sudan. Post kala-azar dermal leishmaniasis. Trans Roy Soc Trop Med Hyg. 2001;95:S164–76. https://doi.org/10.1016/s0035-9203(01)90218-4.
Thakur CP, Kumar A, Mitra G, Thakur S, Sinha PK, Das P, et al. Impact of amphotericin-B in the treatment of kala-azar on the incidence of PKDL in Bihar. India Indian J Med Res. 2008;128:38–44 (PMID: 18820357).
Rahman KM, Islam S, Rahman MW, Kenah E, Ghalib CM, Zahid MM, et al. Increasing incidence of post-kala-azar dermal leishmaniasis in a population based study in Bangladesh. Clin Infect Dis. 2010;50:73–6. https://doi.org/10.1086/648727.
Garg VK, Agrawal S, Rani S, Joshi A, Agarwalla A, Das ML, et al. Post-kala-azar dermal leishmaniasis in Nepal. Int J Dermatol. 2001;40:179–84. https://doi.org/10.1046/j.1365-4362.2001.01198.x.
Kordofani YM, Nour YT, El-Hassan AM, Shalayel MH. Post kala-azar dermal leishmaniasis in Sudan. East Mediter Health J. 2001;7:1061–4 (PMID: 15332749).
Addy M, Nandy A. Ten years of kala-zar in West Bengal, Part I. Did post kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ. 1992;70:341–6 (PMID: 1638662).
Mondal D, Bern C, Ghosh D, Rashid M, Molina R, Chowdhury R, Nath R, et al. Quantifying the infectiousness of post-kala-azar dermal leishmaniasis toward sand flies. Clin Infect Dis. 2019;69(2):251–8. https://doi.org/10.1093/cid/ciy891.
Hasker E, Kansal S, Malaviya P, Gidwani K, Picado A, Singh RP, Chourasia A, et al. Latent infection with Leishmania donovani in highly endemic villages in Bihar. India PLoS Negl Trop Dis. 2013;7: e2053. https://doi.org/10.1371/journal.pntd.0002053.
Srivastava P, Gidwani K, Picado A, Van der Auwera G, Tiwary P, Ostyn B, et al. Molecular and serological markers of Leishmania donovani infection in healthy individuals from endemic areas of Bihar. India Trop Med Int Health. 2013;18:548–54. https://doi.org/10.1111/tmi.12085.
Stauch A, Sarkar RR, Picado A, Ostyn B, Sundar S, Rijal S, et al. Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis. 2011;5: e1405. https://doi.org/10.1371/journal.pntd.0001405.
Bindroo J, Priyamvada K, Chapman LAC, Mahapatra T, Sinha B, Banerjee I, et al. Optimizing village-level targeting of active case detection to support visceral leishmaniasis elimination in India. Front Cell Infect Microbiol. 2021;11: 648847. https://doi.org/10.3389/fcimb.2021.648847.
Dubey P, Das A, Priyamvada K, Bindroo J, Mahapatra T, Mishra PK, et al. Development and evaluation of active case detection methods to support visceral leishmaniasis elimination in India. Front Cell Infect Microbiol. 2021;11: 648903. https://doi.org/10.3389/fcimb.2021.648903.
Kumar R, Das VN, Topno RK, Pal B, Imam A, Agrawal K, et al. Para-kala-azar dermal leishmaniasis cases in Indian subcontinent—a case series. Pathog Glob Health. 2016;110(7–8):326–9. https://doi.org/10.1080/20477724.2016.1258163.
Salam MA, Mondal D, Kabir M, Ekram ARMS, Haque R. PCR for diagnosis and assessment of cure in kala-azar patients in Bangladesh. Acta Trop. 2010;113:52–5. https://doi.org/10.1016/j.actatropica.2009.09.005.
National Vector Borne Disease Control Programme. Standard Operating Procedure For Kala-Azar And Post Kala-Azar Dermal Leishmaniasis Case Search 2020. Available at: https://ncvbdc.mohfw.gov.in/Doc/SOP_Kala-azar_PKDL_Aug_2020.pdf
Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: a threat to the South-East Asia Region Kala-azar Elimination Programme. PLoS Negl Trop Dis. 2017;11(11): e0005877. https://doi.org/10.1371/journal.pntd.0005877.
Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S. Asymptomatic leishmania infection: a new challenge for leishmania control. Clin Infect Dis. 2014;58(10):1424–9. https://doi.org/10.1093/cid/ciu102.
Guha U, Chatterjee M, Sardar AA, Jana K, Saha P, Maji AK, et al. Assessment of knowledge, attitudes, and practices about visceral leishmaniasis in endemic areas of Malda District, West Bengal India. Am J Trop Med Hyg. 2021;104(2):646–52. https://doi.org/10.4269/ajtmh.20-0720.
Alvar J, Alves F, Bucheton B, Burrows L, Büscher P, Carrillo E, Felger I, et al. Implications of asymptomatic infection for the natural history of selected parasitic tropical diseases. Semin Immunopathol. 2020;42(3):231–46. https://doi.org/10.1007/s00281-020-00796-y.
Saha P, Ganguly S, Chatterjee M, Das SB, Kundu PK, Guha SK, et al. Asymptomatic leishmaniasis in kala-azar endemic areas of Malda district, West Bengal, India. PLoS Negl Trop Dis. 2017;11(2): e0005391. https://doi.org/10.1371/journal.pntd.0005391.
Guha SK, Sardar AA, Saha P, Chatterjee M, Jana K, Samanta A, et al. Challenges for maintaining post elimination phase of visceral leishmaniasis control programme in India: a field-based study. PLoS Negl Trop Dis. 2024;18(3): e0012028. https://doi.org/10.1371/journal.pntd.0012028.
Sardar AA, Saha P, Maji D, Guha U, Maji AK, Guha SK. Impact of Indoor Residual Spray (IRS) of synthetic pyrethroid (alphacypermethrin) on sand fly population in the Kala-azar endemic areas of Malda district, West Bengal. India Int J Trop Insect Sci. 2022;42:3293–302. https://doi.org/10.1007/s42690-022-00830-6.
Acknowledgements
We are thankful to the Health and Administrative Official of State (West Bengal), District (Malda, Dakshin Dinajpur, Uttar Dinajpur and Darjeeling) for their active support during the field study. We are also thankful to the study population for their active participation in the study. We are also thankful to Kaushik Bhattacharya and Kallol Saha (Kala-azar Technical Supervisor, Malda District) for their support during field study.
Funding
This work was financially supported by the National Health Mission, Government of West Bengal (Sanction No.: HFW-27011/146/2021-NHM SEC-Dept. of H&FW/2175 dated 28.07.2021).
Author information
Authors and Affiliations
Contributions
Conceptualization, SKG, AK Maji, and Pabitra Saha. Formal analysis, AK Maji, Pabitra Saha, AAS, AS, and SH. Funding acquisition, SKG, AK Maji, Pabitra Saha, and DM. Investigation, SKG, AAS, AK Misra, AS, Punita Saha, AM, KAA, DS, SK, SH, DM, AK Maji, and Pabitra Saha. Methodology, SKG, AAS, AK Misra, AS, Punita Saha, AM, KAA, DS, SK, SH, DM, AK Maji, and Pabitra Saha. Project Administration, SKG, AK Maji, Pabitra Saha, and DM. Supervision, SKG, AK Maji, Pabitra Saha, and AM. Visualization, Pabitra Saha, AS, and AAS. Writing original Draft, SKG, AK Maji, Pabitra Saha, AAS, and AS. Writing, review, editing, AK Misra, Punita Saha, AM, KAA, DS, SK, and DM.
Corresponding author
Ethics declarations
Conflict of Interest
We have no conflicts of interest concerning the work reported in this article.
Ethical Approval and Consent to Participate
The Clinical Research Ethics Committee of Calcutta School of Tropical Medicine has approved the study protocol. Patients indicated acceptance to answer questionnaires at the beginning of the survey.
Consent for Publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guha, S.K., Sardar, A.A., Misra, A.K. et al. Active Community-Based Case Finding of Endemic Leishmaniasis in West Bengal, India. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00260-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00260-2