Abstract
Objectives
To evaluate literature from a 12-year period (2010–2021) on the antimicrobial resistance profile of Pseudomonas aeruginosa from the Arabian Gulf countries (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates).
Methods
An electronic literature search was conducted for articles on antimicrobial resistance in P. aeruginosa and associated phenotypes, covering the period of 1st January 2010 to 1st December 2021.
Results
Antimicrobial resistance in the Arabian Gulf was highest to meropenem (10.3–45.7%) and lowest to colistin (0.0–0.8%), among the agents tested. Annual data showed that ceftazidime resistance (Kuwait), piperacillin-tazobactam non-susceptibility (Qatar), and aztreonam, imipenem, and meropenem resistance (Saudi Arabia) increased by 12–17%. Multiple mechanisms of carbapenem resistance were identified and multiple clones were detected, including high-risk clones such as ST235. The most common carbapenemases detected were the VIM-type metallo-β-lactamases.
Conclusions
Among P. aeruginosa in the Arabian Gulf countries, resistance to meropenem was higher than to the other agents tested, and meropenem resistance increased in Saudi Arabia during the study period. Resistance to colistin, a classic antibiotic used to treat Pseudomonas spp. infections, remained low. The VIM-type β-lactamase genes were dominant. We recommend local and regional antimicrobial resistance surveillance programs to detect the emergence of resistance genes and to monitor antimicrobial resistance trends in P. aeruginosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, Pseudomonas aeruginosa is a leading cause of nosocomial infections [1], and in 2019, was estimated to have caused more than 500,000 deaths across 11 infection types, and more than 250,000 deaths associated with antimicrobial resistance [2, 3]. P. aeruginosa is an opportunistic pathogen exhibiting multiple mechanisms of resistance to multiple classes of antibiotics, including efflux pumps, enzymatic degradation, and target site modification [4]. P. aeruginosa is intrinsically-resistant to many antimicrobials, including members of the cephalosporins and fluoroquinolones [4]. As a result, multidrug-resistant (MDR; defined as non-susceptible to ≥1 agent in ≥3 antimicrobial classes [5]) P. aeruginosa has been listed as a ‘serious threat’ [6] and carbapenem-resistant (CR) P. aeruginosa has been designated a ‘Priority 1/critical’ pathogen [7]. Some CR strains produce carbapenemases, with VIM being the most common carbapenemase in P. aeruginosa, globally [8, 9].
P. aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa; defined as non-susceptible to piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin and levofloxacin [10]) has also been highlighted in treatment guidance from the Infectious Diseases Society of America (IDSA) [11]. Treatment choices for antimicrobial-resistant P. aeruginosa should be dependent on local antimicrobial susceptibility patterns; however, the polymyxins, carbapenems, and aminoglycosides are available antipseudomonal options [4, 11]. For infections caused by DTR-P. aeruginosa, novel agents such as ceftolozane-tazobactam or ceftazidime-avibactam may be prescribed [4, 11].
The Arabian Gulf region is burdened by the same challenges of treating antimicrobial-resistant P. aeruginosa infections as other geographical regions; however, fewer data from this region are available compared with others. Therefore, this review aims to present currently available literature describing the antimicrobial resistance profile of P. aeruginosa and to identify the gaps in data from selected countries of the Arabian Gulf region: Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates (UAE).
2 Material and Methods
2.1 Search Strategy and Selection Criteria
This review evaluated relevant studies addressing isolates of P. aeruginosa, published between 1st January 2010 and 1st December 2021 on the PubMed (Medline®), PubMed Central and Embase® databases. The search terms included keywords from MeSH and Emtree thesaurus. The primary search term was “Pseudomonas aeruginosa”, including synonyms or variations thereof. In addition, synonyms for the Arabian Gulf region were included, and the Arabian Gulf country names (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the UAE). The mid-search terms relating to the review topics were “resistance” and “infections”, including any synonyms or variations. A free text search with those terms in the title and abstract fields was also performed. Examples of the synonyms or variations for the search terms are shown in Supplementary Table 1.
Accepted publication types included journal articles, systematic reviews, meta-analyses, clinical studies, randomised clinical trials, and global/regional/national reports. Case reports, letters, editorials, notes, conference abstracts, and non-English publications were excluded, as were study abstracts with no mention of P. aeruginosa or <10 isolates tested, unclear methodology, or environmental samples. Extracted study information from each article are presented in Table 1 (study findings) and Supplementary Table 2 (study methodologies). Study data are presented in Table 1, 2, Figs. 2, 3, and Supplementary Tables 3, 4.
2.2 Studies Included in This Review
Following initial identification and screening of publication titles, 34 full-text articles included data on the Middle East region [8, 9, 12,13,14,15, 50] or the Arabian Gulf countries (Bahrain, 2; Kuwait, 2; Oman, 3; Qatar, 6; Saudi Arabia, 12; and the UAE, 2) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34, 36,37,38,39,40,41,42] (Fig. 1). For the Arabian Gulf countries, there were 22 prospective studies (Bahrain: [16, 17]; Kuwait: [18]; Qatar: [23,24,25,26,27, 38]; Saudi Arabia: [28,29,30,31,32, 34, 35, 39,40,41,42]; and UAE: [36, 37]) and 5 retrospective studies (Kuwait: [19]; Oman: [20,21,22]; and Saudi Arabia: [33]). Several studies included data on other species and were presented separately from the P. aeruginosa subset (Kuwait: [18, 19]; Oman: [20,21,22]; and UAE: [37]).
Across the 26 Arabian Gulf studies with documented isolate numbers, a total of 101,839 P. aeruginosa isolates contributing data on antimicrobial resistance were distributed as follows: Bahrain, 100 isolates (0.1%) [16, 17]; Kuwait, 150 isolates (0.1%) [18, 19]; Oman, 2620 isolates (2.6%) [20,21,22]; Qatar, 18,267 isolates (17.9%) [23,24,25,26,27, 38]; Saudi Arabia, 78,702 isolates (77.3%) [28,29,30,31,32,33,34, 39,40,41,42]; and the UAE, 2000 isolates (2.0%) [36, 37]. These studies were most frequently set in hospital wards (50.0%), followed by a mixture of hospital wards, community settings and ICUs (34.6%). Most studies collected isolates from a variety of culture sources associated with infection (76.9%; including blood, lower respiratory tract, and skin and soft tissue), with only three studies (11.5%) including a proportion of isolates associated with no infection (from colonized patients). Automated antimicrobial susceptibility testing was most frequently used (57.7%), followed by disk diffusion (23.1%), and broth microdilution methodology or E-test (3.8% each). Almost all of these studies used CLSI breakpoints to interpret MIC results (92.3%).
2.3 Antimicrobial Surveillance Data from the ATLAS Program
In addition to the published studies that met the eligibility criteria, this review included global antimicrobial surveillance data from the Antimicrobial Testing Leadership and Surveillance (ATLAS) program (accessible through a publicly available database [53]). The ATLAS program is industry-sponsored and aims to monitor and assess the in vitro activities of selected antimicrobial agents against clinical bacterial isolates collected from hospitalized patients worldwide. The ATLAS program annually collects a prerequisite number of nonduplicate bacterial isolates of clinically significant species (one isolate per species per patient) from documented infection types (intra-abdominal, urinary tract, skin and soft tissue, lower respiratory tract, and bloodstream) [8, 9, 12].
This review presented ATLAS data from other countries and regions, in addition to a total of 1474 isolates from the Arabian Gulf countries (Kuwait, 1101 isolates; Qatar, 116 isolates; and Saudi Arabia, 257 isolates) presented in Table 2 and Fig. 2 [53].
Rates of antimicrobial resistance (resistance data from the ATLAS database [53], except for the UAE [57]) or nonsusceptibility (non-susceptibility data from Oman presented to 1 decimal place but presented to the nearest whole number in the publication [20]) and difficult-to-treat resistance (DTR data from the ATLAS database [53]) among P. aeruginosa (2016–2021). Rates of antimicrobial resistance or non-susceptibility were presented as the percentage of resistant or non-susceptible P. aeruginosa (determined using CLSI breakpoints [64]) of the total isolates collected. DTR was defined as resistance to >1 antimicrobial in each of the following classes: cephalosporins (ceftazidime, cefepime or ceftriaxone), carbapenems (imipenem, meropenem, doripenem or ertapenem), and quinolones (ciprofloxacin or levofloxacin). Total isolate numbers [years of collection] were: KW, 757 (CZA, CST: 721) [2016–2021]; OM, 2362 [2016 and 2017]; QA, 116 [2019–2021]; SA, 160 (CZA, CST: 140) [2016, 2018–2021]; UAE, 9402 [2020]; IN, 1405 (CZA and CST: 1365) [2016, 2018–2021]; PK, 36 [2016 and 2017]; EMR incl Greece (Greece, Jordan, Israel, and Turkey), 2561 (CZA and CST: 2413) [2016–2021]; S. Europe excl Greece (Croatia, Italy, Portugal, Serbia and Spain), 6185 (CZA and CST: 5063) [2016–2021]; North America (Canada and United States), 5601 (CZA and CST: 4876) [2016–2021]; Latin America (Argentina, Brazil, Chile, Colombia, Costa Rica, Dominican Republic, Mexico, Panama and Venezuela), 6380 (CZA and CST: 5808) [2016–2021]; and Southeast Asia (Malaysia, Philippines, Singapore, Thailand and Vietnam), 2126 (CZA and CST: 2027) [2016–2021]). AMK, amikacin; CZA, ceftazidime-avibactam; CST, colistin; DTR, difficult-to-treat resistance; EMR, Eastern Mediterranean region; excl, excluding; IN, India; incl, including; KW, Kuwait; MEM, meropenem; OM, Oman; PK, Pakistan; QA, Qatar; S., Southern; SA, Saudi Arabia; TZP, piperacillin-tazobactam; and UAE, United Arab Emirates. -, no data collected for these antimicrobial agents in the selected countries
3 Antimicrobial Resistance in Pseudomonas aeruginosa
3.1 Rates of Antimicrobial Resistance and Phenotypes from the Middle East/Arabian Gulf Region
Figure 2 presents data on the rates of antimicrobial resistance and DTR phenotypes among isolates of P. aeruginosa from the Arabian Gulf countries, and other countries or regions [20, 43, 57]. The highest resistance rates observed in each Arabian Gulf country, and the others presented, were to meropenem, ranging from 10.3% (UAE) to >40.0% in Oman and Qatar (higher than the other countries and regions) (Fig. 2). The meropenem resistance rates in Oman and Qatar are reflective of single center data and not necessarily indicative of the resistance status in the country as a whole. Colistin resistance was low (<2.2%) globally (Fig. 2). Amikacin resistance was <5% in Qatar, Saudi Arabia, and the UAE (similar to Southern Europe, North America and Southeast Asia), but higher in Kuwait (10.7%) and Oman (20.0%). There were lower rates of DTR-P. aeruginosa (10.9–13.2%) in North America, Saudi Arabia, and Southeast Asia; rates of 16% in Southern Europe and EMR; and rates of 19.0–25.9% elsewhere (data not collected in Oman or the UAE; Fig. 2). Published rates of antimicrobial resistance or susceptibility from the Arabian Gulf countries, and other countries or regions are presented in Supplementary Table 3 [8,9,10, 12,13,14,15,16,17,18,19,20, 23, 24, 26,27,28,29,30, 32,33,34, 36, 37, 41,42,43,44].
The rates of antimicrobial susceptibility among a collection of P. aeruginosa isolates from Middle East countries (Israel, Jordan, Kuwait, and Saudi Arabia) ranged from 62.8% (levofloxacin) to >90% (amikacin and colistin) [12], according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2020 breakpoints [54]. However, there was no susceptibility breakpoint, and only intermediate or resistance breakpoints, for colistin when using Clinical and Laboratory Standards Institute (CLSI) 2020 breakpoints [55]. Susceptibility to ceftazidime-avibactam and ceftolozane-tazobactam was >90% in the Middle East and Middle East-Africa regions [12, 13].
MDR rates among. P. aeruginosa were 30.6% and 38.1%, and the rate with a DTR phenotype was 7.4% for the Middle East region [12, 14]. Among MDR P. aeruginosa isolates, cefepime, meropenem and piperacillin-tazobactam susceptibility was 30–50%, which decreased to 0% among DTR isolates [12]. A 2016–2018 ATLAS surveillance study reported that no isolates of carbapenemase-producing P. aeruginosa collected in the Middle East-Africa region were resistant to colistin, whereas 32.3% were resistant to aztreonam, and ≥92.3% were resistant to the other tested agents, including amikacin, ceftazidime-avibactam, imipenem and meropenem [9]. These findings reflected the high proportions of MBL-positive isolates that were detected in all regions included in the study [9]. Rates of meropenem non-susceptibility were 25–35% among P. aeruginosa collected in the Middle East and Africa [12, 13, 15]. Among meropenem-non-susceptible respiratory P. aeruginosa isolates collected from adult intensive-care unit (ICU) patients in the Middle East-Africa region, susceptibility was 68.1% to ceftolozane-tazobactam, 34.9% to ceftazidime, and 28.2% to piperacillin-tazobactam [15].
In the above studies, the range of countries comprising the Middle East-Africa region (including Egypt, Israel, Jordan, Kenya, Kuwait, Lebanon, Morocco, Qatar, South Africa, and Tunisia) were from a wide range of social and economic settings. Antimicrobial susceptibility or resistance data and reports on P. aeruginosa isolates from the individual Arabian Gulf countries are described below. The main study findings are summarized in Table 1 and data presented in Supplementary Table 3.
3.1.1 Bahrain, Kuwait and Oman
More than 80% of ciprofloxacin-resistant P. aeruginosa isolates from Bahrain were also resistant to imipenem, meropenem and piperacillin-tazobactam; however, none were resistant to colistin [16, 17]. Similarly, high resistance rates to meropenem (≥87.5%) and low resistance rates to colistin (2.1%) were observed among MDR P. aeruginosa isolates from Kuwait (one of which was pandrug-resistant [PDR]; defined as resistant to all antimicrobial agents [5]) [18]. The rates of susceptibility to amikacin, ceftazidime, ciprofloxacin, imipenem and piperacillin-tazobactam were also notably lower in the study of MDR P. aeruginosa from Kuwait, compared with non-MDR isolates [18]. Conversely, P. aeruginosa from diabetic foot infections, collected in Kuwait, exhibited susceptibility rates of ≥65.7% to ceftazidime, meropenem, gentamicin, imipenem, and amikacin [19].
In Oman, extended-spectrum β-lactamase (ESBL) enzyme production was only detected in 2 of 2362 P. aeruginosa isolates collected [20]. The lowest rate of susceptibility among P. aeruginosa from Oman was to meropenem (58.0%), with ≥80.0% of isolates susceptible to amikacin, ceftazidime, ciprofloxacin, gentamicin and piperacillin-tazobactam. All isolates were susceptible to colistin. Another study in Oman found that 8.1% of all MDR isolates from a tertiary care teaching hospital were P. aeruginosa [21]. In the same hospital, the rate of CR P. aeruginosa in bacteremia was 22% between 2012 and 2016, with a rate of 43% reported in 2015 alone [22].
3.1.2 Qatar
Among P. aeruginosa from cystic fibrosis patients in Qatar, antimicrobial non-susceptibility ranged from 9.8% (piperacillin-tazobactam) to 41.0% (gentamicin); all isolates were susceptible to colistin [23]. Meropenem non-susceptibility was 11.5% and meropenem resistance was 58.3% among MDR isolates. Among the MDR subset, resistance rates ranged from 50.0% (piperacillin-tazobactam) to 100% (gentamicin, amikacin and cefepime), whereas none of the MDR isolates were resistant to colistin [23].
In a multi-hospital study, the rate of MDR isolates collected in Qatar between 2014 and 2015 was 8.1% (2.4% of which were PDR) [24]. The rate of MDR P. aeruginosa at one of these hospitals before the implementation of an antimicrobial stewardship program was 9.0% and was 5.5% after implementation [25]. Most MDR P. aeruginosa isolates were resistant to cefepime, ciprofloxacin, piperacillin-tazobactam and meropenem (>90%); in addition, aminoglycoside resistance was 50–75%, and 3.4% were colistin-resistant [25]. Non-susceptibility to ceftazidime-avibactam and ceftolozane-tazobactam was 31.2% and 37.1%, respectively, among MDR P. aeruginosa [26]. The authors attributed ceftazidime-avibactam and ceftolozane-tazobactam non-susceptibility rates among the MDR isolates in their study to the production of ESBL and VIM enzymes [26]. Among a smaller subset of eight MDR isolates that produced metallo-β-lactamases (MBLs), but not ESBLs, all were susceptible to aztreonam but resistant to the other agents studied [27].
3.1.3 Saudi Arabia
Two studies of P. aeruginosa isolates reported low resistance to ceftazidime-clavulanic acid (<10%) and high resistance to gentamicin (41%) [28, 29]. All isolates were susceptible to colistin. In a single-center study of 156 P. aeruginosa isolates, 22.4% were ceftazidime-resistant, of which 71.4% were ESBL-producers and 42.9% were MBL-producers [28]. Thirty-nine (19.5%) of 200 clinical P. aeruginosa isolates collected at another center were ceftazidime-resistant, most of which were also resistant to other tested agents; however, all isolates were susceptible to colistin [29]. In a single-center study of 33 MDR P. aeruginosa isolates, the rates of resistance to colistin and the carbapenems were 6.1% (colistin), 39.4% (doripenem), 81.8% (meropenem), and 90.9% (imipenem) [30]. In a multi-hospital study, the percentage of carbapenemase production among 39 ceftazidime- and carbapenem-resistant isolates of P. aeruginosa collected was 28.2% [31].
Another multicenter study statistically compared the overall resistance rates to tested agents among 121 P. aeruginosa isolates [32]. The rate of resistance to piperacillin-tazobactam (4.9%) was significantly lower than to ceftazidime, levofloxacin, aztreonam, ciprofloxacin, piperacillin, imipenem, ticarcillin and meropenem (P < 0.05). In contrast, the rate of resistance to meropenem (30.6%) was significantly higher than to piperacillin-tazobactam, amikacin, cefepime, gentamicin, ceftazidime, levofloxacin, aztreonam, ciprofloxacin, piperacillin and imipenem (P < 0.05). The overall MDR rate was 10.7% [32]. A higher rate of meropenem resistance was observed among respiratory isolates than among other infection types (41.5%; P < 0.05).
P. aeruginosa isolates from three different ICUs in one hospital showed the lowest rates of resistance to the class of aminoglycosides (amikacin [18.8%], tobramycin [20.0%] and gentamicin [31.7%]), while 30.0% of isolates were resistant to colistin [33]. The study recorded a multidrug resistance rate of 60.9% among P. aeruginosa [33]. Similarly, the resistance rates among P. aeruginosa isolates from seven ICUs ranged from 8.9% (cefepime) to 41.1% (imipenem). The rate of meropenem resistance was 27.8% [34]. In that ICU study, 36.7% of 90 P. aeruginosa isolates collected were MDR [34]. A higher rate of 93.1% MDR P. aeruginosa isolates were associated with ventilator-associated pneumonia (VAP) in another ICU study in Saudi Arabia [35].
3.1.4 United Arab Emirates
A total of 1969 P. aeruginosa were collected in a cross-sectional multicenter study, of which 23.9% of isolates were identified as carbapenem-non-susceptible [36]. Among a subset of 37 carbapenem-non-susceptible isolates that underwent molecular characterization, 10.8% of isolates were MDR and 37.8% were XDR. No study isolates were characterized as PDR because all were susceptible to colistin [36]. A single-center study of 31 P. aeruginosa isolates reported rates of resistance ranging from 16.1% (gentamicin) to 51.6% (meropenem) [37]. High susceptibility was reported to ceftazidime-avibactam and ceftolozane-tazobactam among all P. aeruginosa isolates (93.5% and 96.8%, respectively).
3.2 Studies on Molecular Resistance Mechanisms from the Middle East/Arabian Gulf Regions
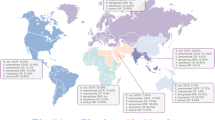
Figure 3 (data shown in Supplementary Table 4) shows the distribution of β-lactamase genes among P. aeruginosa from the Arabian Gulf countries, and other countries or regions. The variety of documented VIM and GES genes appears to be greater in the countries of the Arabian Gulf, compared with the other countries and regions. In contrast, fewer IMP-type genes were reported in the Arabian Gulf, compared with North America, Latin America, and Southeast Asia. Fewer types of NDM and KPC genes were documented among P. aeruginosa in the Arabian Gulf and the other countries and regions (Fig. 3, Supplementary Table 4). Molecular characterization studies conducted in the individual Arabian Gulf countries are described below.
Distribution of β-lactamase genes among P. aeruginosa from the Arabian Gulf countries and other countries/regions (2010–2021) (genotype data from each country/region are shown in Supplementary Table 4). Circles show the genotype number of each β-lactamase gene detected in a country or region and ‘type’ represents an identified β-lactamase gene that has not been sub-typed. EMR, Eastern Mediterranean Region; inc, including; excl, excluding; NDM, New Delhi metallo-β-lactamase; VIM, Verona integron-encoded metallo-β-lactamase; IMP, imipenemase; GES, Guiana extended-spectrum; KPC, Klebsiella pneumoniae carbapenemase; and UAE, United Arab Emirates
3.2.1 Bahrain, Kuwait and Qatar
The upregulated expression of genes mexB, mexD, mexF and mexY was identified in 8% of tested ciprofloxacin-resistant P. aeruginosa isolates [17]. The same isolates also showed a decrease in ciprofloxacin minimum inhibitory concentration (MIC) by the efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone. Among imipenem-resistant P. aeruginosa isolates collected from community, hospital, and ICU patients in Bahrain, 52.5% carried MBL genes; mostly VIM-type (90.4%) [16]. One isolate was NDM‑1-positive, and one isolate carried both VIM and NDM‑1 genes.
Data on the β-lactamase genes carried by P. aeruginosa isolates in Kuwait are scarce. A global antimicrobial surveillance study that included countries in the Middle East-Africa region showed that 11 isolates of VIM-2-positive and 3 VIM-4-positive P. aeruginosa were collected in Kuwait [8].
Among 75 MDR (defined according to [5]) P. aeruginosa isolates from two centers in Qatar, 96.0% possessed class C and/or class D β-lactamases, while MBLs were detected in 26.7% of the isolates (blaVIM-2, blaVIM-5 and blaIMP-2) [27]. One (1.3%) isolate co-carried both blaVIM-2 and blaIMP-1 and all four β-lactamase classes were present in three (4.0%) isolates. The ESBL gene blaVEB-9 (formerly known as blaVEB-1a) was identified as the most frequent ESBL gene (25.3%). High-risk clones ST235 (21.3%), ST357 (10.7%), ST389 and ST1284 (8.0% each) were mostly identified. Most ST235 isolates (93.8%) were resistant to all tested β-lactams. In an additional study from Qatar, sequencing of 78 MDR P. aeruginosa revealed 29 different sequence types with the predominance of ST235 (20.5%), followed by ST357 (10.3%), and ST389 and ST1284 (7.7% each) [38]. ST233 was associated with bloodstream infections and increased 30-day mortality, while all ST389 were isolated from cystic fibrosis patients.
3.2.2 Saudi Arabia
Two studies found that blaVEB genes were predominantly harbored by phenotypically ESBL-positive P. aeruginosa isolates (68.0% and 87.0%, respectively) [28, 29]. Tawfik et al. [28] also identified blaOXA-10 genes in 56.0% of ESBL-producing isolates, followed by blaGES in 20.0%. Both studies found that all MBL-producing P. aeruginosa isolates carried blaVIM genes but neither study found blaPER, blaTEM, blaSHV or blaCTX-M among the ESBL genes, nor blaIMP, blaGIM, blaSIM, blaSPM or blaNDM among the MBL genes [28, 29]. Tawfik et al. [28] suggested that the higher percentage of blaVEB than blaOXA-10 and blaGES ESBL genes could be due to residents from regions with a higher prevalence of blaVEB genes (such as blaVEB-1 in Southeast Asia) living in Saudi Arabia. In addition, the collection of clinical P. aeruginosa isolates was not dominated by any single clone and the identification of multiple bla genes did not solely reflect the spread of a single outbreak strain [28, 29].
A subsequent study by Al-Agamy et al. [39] also found that the predominant ESBL was VEB-1 (47.1%), followed by OXA-10 (41.2%) and GES (8.8% [GES-1, GES-4 and GES-6]) among CR P. aeruginosa isolates. As previously found, the most common MBLs were VIM-type, but in this later study, IMP-7 was also found [28, 29, 39]. Further analysis showed the downregulation of OprD porin gene expression in nine (26.5%) isolates and an upregulation of MexAB efflux pump gene expression in five (14.7%) isolates [39]. Six different serotypes and 14 different pulsotypes were detected; of which all 9 serotype O:15 strains were found to have the same pulsotype (F) and the same mechanism of resistance (OXA-10 and VEB-1a).
A multicenter study investigating the carriage of MBL genes in 39 CR P. aeruginosa isolates found that 28.2% were phenotypic carbapenemase-producers, with VIM being the dominant MBL detected (72.7% [VIM-2, VIM-6 and VIM-28]). Three isolates carried the blaGES-5 gene. No IMP-producing genes were detected among any P. aeruginosa isolates [31].
In a study of digestive tract colonization by P. aeruginosa in ICU patients, 5 of 13 CR P. aeruginosa isolates from rectal swabs were shown to carry MBL carbapenemases (4 with NDM and 1 with VIM) [40]. AmpC overexpression, which has been linked with carbapenem resistance when coupled with porin mutations, was detected in eight isolates of CR P. aeruginosa. The overexpression of blaAmpC in these isolates may have arisen due to the overuse of β-lactams, such as ceftazidime, which can cause derepression of AmpC. The authors reported that this was the first study in the Arabian Gulf region to document AmpC overexpression in CR P. aeruginosa isolates [40].
3.2.3 United Arab Emirates
In a cross-sectional survey on carbapenem-non-susceptible P. aeruginosa, the VIM-type MBL carbapenemases were most common among a subset of 37 that were analyzed (32.4% [VIM-2, VIM-30, VIM-31 and VIM-42]) [36]. A single VIM-2-carrying isolate was also GES-9-positive, while one VIM-42-positive isolate co-carried GES-5. No other investigated MBL genes were detected, nor were KPC carbapenemases. Outer membrane impermeability was observed in 73% of isolates and 75.6% displayed overproduced MexAB-OprM efflux pump. Seven distinct clones were identified, one of which comprised 81.1% of isolates across all hospitals (including 11 out of the 12 isolates that were VIM- and GES-positive), suggesting clonal dissemination [36].
3.3 Changes in Antimicrobial Resistance Since 2011 in the Arabian Gulf Region
3.3.1 Kuwait
Between 2012 and 2021, there were net increases of antimicrobial resistance to amikacin (3.2%), ceftazidime (15.1%), cefepime (5.6%), and piperacillin-tazobactam (7.1%) [53] (Table 2). Resistance to levofloxacin remained stable (−0.4% net change), and to meropenem (−7.4% net change). Following 2019, the rates to all agents appeared to decrease annually.
3.3.2 Qatar
Between 2011 and 2018, the rates of non-susceptibility to amikacin and ceftazidime remained stable (net changes, 0.0% and −1.0%, respectively), whereas non-susceptibility to cefepime, meropenem and piperacillin-tazobactam increased (by 4.0%, 4.0%, and 13.0%, respectively) [56] (Table 2). ATLAS 2021 data showed higher rates of non-susceptibility than in 2011 for all tested agents except amikacin [53] (Table 2).
3.3.3 Saudi Arabia
In a 6-year, multicenter national antimicrobial surveillance study, the trend was increased resistance to aztreonam, imipenem and meropenem, with overall percentage increases during the study period of 17.3%, 12.3% and 11.6%, respectively [41]. Overall percentage decreases in resistance during the study period were shown to netilmicin, amikacin and tobramycin (by 10.0%, 5.9% and 5.0%, respectively). Resistance to the other antimicrobials on the test panel (including ciprofloxacin, piperacillin-tazobactam, ceftazidime, levofloxacin, cefepime, colistin and gentamicin), remained stable with <5% overall change. In another 6-year antimicrobial surveillance study, the susceptibility of. P. aeruginosa to ceftriaxone, ceftazidime and meropenem decreased over the study period, but remained stable to cefepime, amikacin, ciprofloxacin, and increased to gentamicin and trimethoprim-sulfamethoxazole [41].
ATLAS antimicrobial resistance data were not collected in 2014 or 2017, but resistance rates to all tested agents were higher in 2011–2013 than 2018–2021. Between 2018 and 2021, there appeared to be a decrease in resistance to all agents tested [53] (Table 2).
3.3.4 United Arab Emirates
In the UAE, P. aeruginosa showed a horizontal trend for resistance to fluoroquinolones, and to third- and fourth-generation cephalosporins, and showed a decreasing trend for resistance to aminoglycosides. Amikacin and piperacillin-tazobactam resistance decreased by 1.8% and 3.8%, respectively, and resistance to ceftazidime, cefepime and meropenem remained stable (net changes, 0.8%, −1.0%, and 0.1%, respectively) (Table 2). Nevertheless, from 2016 to 2020, both carbapenems, imipenem and meropenem, showed a decreasing trend of resistance. The percentage of MDR, XDR, and possible PDR isolates generally declined from 2010 to 2020 [57].
4 Discussion
The studies above describe important published findings on antimicrobial resistance among isolates of P. aeruginosa in the Arabian Gulf countries since 2010. More information is now known about the genes and mechanisms driving resistance among P. aeruginosa in these countries, and about the antimicrobial agents that could be used against P. aeruginosa infections in a hospital or ICU setting. Recent data (2016–2021) on antimicrobial resistance among clinical isolates of P. aeruginosa show that resistance to meropenem was highest (10.3–45.7%) among the agents presented [53]. In Kuwait, there was an overall increase in resistance rates from 2012 to 2021 to most antimicrobial agents presented, although levofloxacin and meropenem resistance appeared to fluctuate [53]. Data collected over the last 12 years from Saudi Arabia showed an increase in resistance to aztreonam, imipenem and meropenem, and a decrease in susceptibility of. P. aeruginosa to ceftriaxone and ceftazidime [41, 42]. The reported increase in resistance rates is alarming, and continued surveillance is needed to monitor these upward trends. The significantly higher rate of resistance to meropenem than to other tested agents among P. aeruginosa in one study from Saudi Arabia was suggested to be due to over-prescribing of broad-spectrum antimicrobial agents, such as carbapenems [32]. Prior antibiotic treatment was found to be associated with MDR P. aeruginosa infections, according to studies in Saudi Arabia and Qatar [24, 34]. Further studies are warranted to correlate antimicrobial use in P. aeruginosa infections with the current resistance profiles in the Arabian Gulf countries.
In treatment guidance from the IDSA, DTR among P. aeruginosa is defined as non-susceptibility to piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin and levofloxacin [8]. The most common MDR profile among P. aeruginosa in the study by Karlowsky et al. [14] was non-susceptibility to aztreonam, ceftazidime, cefepime, ciprofloxacin, imipenem and piperacillin-tazobactam. These resistance profiles leave few antimicrobial agents with known activity against P. aeruginosa, one of which is colistin. Colistin demonstrated the highest antimicrobial activity among the studies reported in this review, including those with subsets of antimicrobial-resistant phenotypes; however, owing to the increased use of colistin as a last-resort agent for infections caused by MDR and XDR strains, colistin resistance among MDR and XDR P. aeruginosa is emerging worldwide. Thus, resistance mechanisms in these antimicrobial-resistant phenotypes are being investigated [58,59,60]. Therefore, colistin resistance among P. aeruginosa requires continued surveillance and suitable monitoring systems to report the dissemination rate of these resistance genes.
It has been suggested that the Middle East region could act as a secondary reservoir for NDM carbapenemases, on account of population flow to the Middle East from countries of the Asian subcontinent, such as India or Pakistan [61, 62]. While NDM- and VIM-type β-lactamase genes are predominant in the Asian subcontinent, only four isolates included in this review were found to harbor NDM-type genes (one from Bahrain and three from Saudi Arabia) [16, 40]. The lack of molecular data and proper representation could be a contributing factor to this observation. Further research and enhanced surveillance efforts are needed to fully understand the distribution and prevalence of NDM-type genes. Although the VIM β-lactamase appears to be dominant in the Arabian Gulf, multiple resistance mechanisms were found to cause carbapenem resistance in most P. aeruginosa isolates from Saudi Arabia and the UAE [28, 29, 31, 36, 39]. Multiple clones of MDR P. aeruginosa were also identified in Bahrain, Qatar, Saudi Arabia, and the UAE, including ST235, ST233 and ST357 which are deemed as high-risk clones [27, 38]. Their associated MDR phenotypes are a cause for concern. Clonal dissemination was also discussed by Ayoub Moubareck et al. [36] regarding their study findings of seven distinct clones among carbapenem-non-susceptible P. aeruginosa isolates from the UAE. This is also concerning as clonal dissemination contributes to the spread of P. aeruginosa pathogens that carry β-lactamase genes and are antimicrobial-resistant.
When comparing antimicrobial susceptibility or resistance data from different studies of antimicrobial surveillance or prevalence (whether national, regional or global), it is vital to be aware of the limitations of comparisons made. We express similar limitations to the observations noted by the European Centre for Disease Prevention and Control on inter-country comparisons and national trends of surveillance data on antimicrobial resistance, such as population coverage, sampling, laboratory routines [63]. Some of the most important potential sources of bias are the various protocols for antimicrobial susceptibility testing, the use of guidelines for clinical breakpoints, various isolate sources and numbers of isolates, the sizes of the hospitals (and whether single- or multi-center), and single versus multiple study years. Details on isolate collection and susceptibility testing, where available, for each study are noted in Supplementary Table 2. Data from individual non-national studies may not reflect the trends of the whole country and might generate inaccurate reporting bias for prevalence rates, microbiological characteristics, or mechanisms of genetic resistance. Furthermore, it is important to acknowledge that excluding papers published in Arabic language may limit the scope of the study and the perspectives represented.
Despite these limitations, the sharing of data on the local, national, and international levels of antimicrobial-resistant P. aeruginosa may serve to improve public health, inform health policies, provide evidence for developing treatment guidelines, and monitor the trends and spread of resistance. Local epidemiology data can inform the implementation of infection prevention and control, and antimicrobial stewardship programs in the respective healthcare institutions. Together, these factors need to be addressed as a matter of urgency to establish a more comprehensive and representative antimicrobial resistance surveillance system to monitor the threat of the opportunistic pathogen P. aeruginosa.
Data Availability
The datasets generated and/or analyzed during the current study are included in this published article [and its supplementary information files], or available from the corresponding author on reasonable request.
Abbreviations
- AmpC:
-
Ampicillin class C
- bla:
-
β-Lactamase gene
- CDC:
-
Centers for Disease Control and Prevention
- CLSI:
-
Clinical and Laboratory Standards Institute
- CR:
-
Carbapenem-resistant
- DTR:
-
Difficult-to-treat resistance
- ESBL:
-
Extended-spectrum β-lactamase
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- GES:
-
Guiana extended-spectrum
- IDSA:
-
Infectious Diseases Society of America
- I:
-
Intermediate
- ICMR:
-
Indian Council of Medical Research
- ICU:
-
Intensive care unit
- IMP:
-
Imipenemase
- KPC:
-
Klebsiella pneumoniae carbapenemase
- MBL:
-
Metallo-β-lactamase
- MDR:
-
Multidrug-resistant
- mex:
-
Multidrug efflux pump
- MIC:
-
Minimum inhibitory concentration
- NDM:
-
New Delhi metallo-β-lactamase
- NS:
-
Non-susceptible
- Opr:
-
Outer membrane porin
- OXA:
-
Oxacillinase
- PDR:
-
Pandrug-resistant
- PER:
-
Plasmid-mediated extended spectrum
- R:
-
Resistant
- S:
-
Susceptible
- SPM:
-
São Paulo metallo-β-lactamase
- ST:
-
Sequence type
- TZP:
-
Piperacillin-tazobactam
- UAE:
-
United Arab Emirates
- VAP:
-
Ventilator-associated pneumonia
- VEB:
-
Vietnamese extended-spectrum β-lactamase
- VIM:
-
Verona integron-encoded metallo-β-lactamase
- XDR:
-
Extensively drug-resistant
References
Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, et al. Global prevalence of nosocomial infection: a systematic review and meta-analysis. PLoS ONE. 2023;18: e0274248. https://doi.org/10.1371/journal.pone.0274248.
GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10369):2221–48. https://doi.org/10.1016/S0140-6736(22)02185-7.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55. https://doi.org/10.1016/S0140-6736(21)02724-0.
Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4):e00031-19. https://doi.org/10.1128/CMR.00031-19.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. AT Threats; 2019.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27. https://doi.org/10.1016/S1473-3099(17)30753-3.
Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;60:1067–78. https://doi.org/10.1128/AAC.02379-15.
Kiratisin P, Kazmierczak K, Stone GG. In vitro activity of ceftazidime-avibactam and comparators against carbapenemase-producing Enterobacterales and Pseudomonas aeruginosa isolates collected globally between 2016 and 2018. J Glob Antimicrob Resist. 2021;S2213–7165(21):00202–12. https://doi.org/10.1016/j.jgar.2021.08.010.
Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–14. https://doi.org/10.1093/cid/ciy378.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72:e169–83. https://doi.org/10.1093/cid/ciaa1478.
Karlowsky JA, Bouchillon SK, El Mahdy KR, Mohamed N, Stone GG, Sahm DF. In vitro activity of ceftazidime/avibactam against clinical isolates of Enterobacterales and Pseudomonas aeruginosa from Middle Eastern and African countries: ATLAS global surveillance programme 2015–2018. JAC Antimicrob Resist. 2021;3:dlab067. https://doi.org/10.1093/jacamr/dlab067.
Nichols WW, de Jonge BL, Kazmierczak KM, Karlowsky JA, Sahm DF. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012–2014). Antimicrob Agents Chemother. 2016;60:4743–9. https://doi.org/10.1128/AAC.00220-16.
Karlowsky JA, Lob SH, Young K, Motyl MR, Sahm DF. Activity of imipenem/relebactam against Pseudomonas aeruginosa with antimicrobial-resistant phenotypes from seven global regions: SMART 2015–2016. J Glob Antimicrob Resist. 2018;15:140–7. https://doi.org/10.1016/j.jgar.2018.07.012.
Moise PA, Gonzalez M, Alekseeva I, Lopez D, Akrich B, DeRyke CA, et al. Collective assessment of antimicrobial susceptibility among the most common Gram-negative respiratory pathogens driving therapy in the ICU. JAC Antimicrob Resist. 2021;3:dlaa129. https://doi.org/10.1093/jacamr/dlaa129.
Joji RM, Al-Rashed N, Saeed NK, Bindayna KM. Detection of VIM and NDM-1 metallo-beta-lactamase genes in carbapenem-resistant Pseudomonas aeruginosa clinical strains in Bahrain. J Lab Phys. 2019;11:138–43. https://doi.org/10.4103/JLP.JLP_118_18.
Al Rashed N, Joji RM, Saeed NK, Bindayna KM. Detection of overexpression of efflux pump expression in fluoroquinolone-resistant Pseudomonas aeruginosa isolates. Int J Appl Basic Med Res. 2020;10:37–42. https://doi.org/10.4103/ijabmr.IJABMR_90_19.
Alfouzan W, Dhar R, Nicolau DP. In vitro activity of newer and conventional antimicrobial agents, including fosfomycin and colistin, against selected Gram-negative bacilli in Kuwait. Pathogens. 2018;7:75. https://doi.org/10.3390/pathogens7030075.
Alhubail A, Sewify M, Messenger G, Masoetsa R, Hussain I, Nair S, et al. Microbiological profile of diabetic foot ulcers in Kuwait. PLoS ONE. 2020;15: e0244306. https://doi.org/10.1371/journal.pone.0244306.
Al Rahmany D, Albeloushi A, Alreesi I, Alzaabi A, Alreesi M, Pontiggia L, et al. Exploring bacterial resistance in Northern Oman, a foundation for implementing evidence-based antimicrobial stewardship program. Int J Infect Dis. 2019;83:77–82. https://doi.org/10.1016/j.ijid.2019.04.004.
Balkhair A, Al-Farsi YM, Al-Muharrmi Z, Al-Rashdi R, Al-Jabri M, Neilson F, et al. Epidemiology of multi-drug resistant organisms in a teaching hospital in Oman: a 1-year hospital-based study. ScientificWorldJournal. 2014;2014: 157102. https://doi.org/10.1155/2014/157102.
Balkhair A, Al-Muharrmi Z, Al’Adawi B, Al Busaidi I, Taher HB, Al-Siyabi T, et al. Prevalence and 30-day all-cause mortality of carbapenem-and colistin-resistant bacteraemia caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: description of a decade-long trend. Int J Infect Dis. 2019;85:10–5. https://doi.org/10.1016/j.ijid.2019.05.004.
AbdulWahab A, Zahraldin K, Sid Ahmed MA, Jarir SA, Muneer M, Mohamed SF, et al. The emergence of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis patients on inhaled antibiotics. Lung India. 2017;34:527–31. https://doi.org/10.4103/lungindia.lungindia_39_17.
Sid Ahmed MA, Hassan AAI, Abu Jarir S, Abdel Hadi H, Bansal D, Abdul Wahab A, et al. Emergence of multidrug- and pandrug-resistant Pseudomonas aeruginosa from five hospitals in Qatar. Infect Prev Pract. 2019;1: 100027. https://doi.org/10.1016/j.infpip.2019.100027.
Sid Ahmed MA, Abdel Hadi H, Abu Jarir S, Al Khal AL, Al-Maslamani MA, Jass J, et al. Impact of an antimicrobial stewardship programme on antimicrobial utilization and the prevalence of MDR Pseudomonas aeruginosa in an acute care hospital in Qatar. JAC Antimicrob Resist. 2020;2:dlaa050. https://doi.org/10.1093/jacamr/dlaa050.
Sid Ahmed MA, Abdel Hadi H, Hassan AAI, Abu Jarir S, Al-Maslamani MA, Eltai NO, et al. Evaluation of in vitro activity of ceftazidime/avibactam and ceftolozane/tazobactam against MDR Pseudomonas aeruginosa isolates from Qatar. J Antimicrob Chemother. 2019;74:3497–504. https://doi.org/10.1093/jac/dkz379.
Sid Ahmed MA, Khan FA, Sultan AA, Söderquist B, Ibrahim EB, Jass J, et al. Beta-lactamase-mediated resistance in MDR-Pseudomonas aeruginosa from Qatar. Antimicrob Resist Infect Control. 2020;9:170. https://doi.org/10.1186/s13756-020-00838-y.
Tawfik AF, Shibl AM, Aljohi MA, Altammami MA, Al-Agamy MH. Distribution of Ambler class A, B and D β-lactamases among Pseudomonas aeruginosa isolates. Burns. 2012;38:855–60. https://doi.org/10.1016/j.burns.2012.01.005.
Al-Agamy MH, Shibl AM, Tawfik AF, Elkhizzi NA, Livermore DM. Extended-spectrum and metallo-beta-lactamases among ceftazidime-resistant Pseudomonas aeruginosa in Riyadh. Saudi Arabia J Chemother. 2012;24:97–100. https://doi.org/10.1179/1120009X12Z.00000000015.
Somily AM, Absar MM, Arshad MZ, Al Aska AI, Shakoor ZA, Fatani AJ, et al. Antimicrobial susceptibility patterns of multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii against carbapenems, colistin, and tigecycline. Saudi Med J. 2012;33:750–5.
Memish ZA, Assiri A, Almasri M, Roshdy H, Hathout H, Kaase M, et al. Molecular characterization of carbapenemase production among gram-negative bacteria in Saudi Arabia. Microb Drug Resist. 2015;21:307–14. https://doi.org/10.1089/mdr.2014.0121.
Khan MA, Faiz A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann Saudi Med. 2016;36:23–8. https://doi.org/10.5144/0256-4947.2016.23.
Ibrahim ME. High antimicrobial resistant rates among Gram-negative pathogens in intensive care units. A retrospective study at a tertiary care hospital in Southwest Saudi Arabia. Saudi Med J. 2018;39:1035–43. https://doi.org/10.15537/smj.2018.10.22944.
Alhussain FA, Yenugadhati N, Al Eidan FA, Al Johani S, Badri M. Risk factors, antimicrobial susceptibility pattern and patient outcomes of Pseudomonas aeruginosa infection: a matched case-control study. J Infect Public Health. 2021;14:152–7. https://doi.org/10.1016/j.jiph.2020.11.010.
Bukhari SZ, Hussain WM, Banjar AA, Fatani MI, Karima TM, Ashshi AM. Application of ventilator care bundle and its impact on ventilator associated pneumonia incidence rate in the adult intensive care unit. Saudi Med J. 2012;33:278–83.
Ayoub Moubareck C, Hammoudi Halat D, Akkawi C, Nabi A, AlSharhan MA, AlDeesi ZO, et al. Role of outer membrane permeability, efflux mechanism, and carbapenemases in carbapenem-nonsusceptible Pseudomonas aeruginosa from Dubai hospitals. Int J Infect Dis. 2019;84:143–50. https://doi.org/10.1016/j.ijid.2019.04.027.
Alatoom A, Elsayed H, Lawlor K, AbdelWareth L, El-Lababidi R, Cardona L, et al. Comparison of antimicrobial activity between ceftolozane-tazobactam and ceftazidime-avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis. 2017;62:39–43. https://doi.org/10.1016/j.ijid.2017.06.007.
Sid Ahmed MA, Hadi HA, Jarir SA, Khan FA, Arbab MA, Hamid JM, et al. Prevalence and microbiological and genetic characteristics of multidrug-resistant Pseudomonas aeruginosa over 3 years in Qatar. Antimicrob Stewardship Healthc Epidemiol. 2022;2:E96. https://doi.org/10.1017/ash.2022.226.
Al-Agamy MH, Jeannot K, El-Mahdy TS, Samaha HA, Shibl AM, Plésiat P, et al. Diversity of molecular mechanisms conferring carbapenem resistance to Pseudomonas aeruginosa isolates from Saudi Arabia. Can J Infect Dis Med Microbiol. 2016;2016:4379686. https://doi.org/10.1155/2016/4379686.
Abdalhamid B, Elhadi N, Alabdulqader N, Alsamman K, Aljindan R. Rates of gastrointestinal tract colonization of carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa in hospitals in Saudi Arabia. New Microbes New Infect. 2016;10:77–83. https://doi.org/10.1016/j.nmni.2016.01.014.
Somily A, Balkhy HH, Mas E, Althawadi SI, Alawi M, Al Johani SM, et al. Antimicrobial resistance trends of non-fermenter Gram negative bacteria in Saudi Arabia: a 6-year national study. J Infect Public Health. 2021;14:1144–50. https://doi.org/10.1016/j.jiph.2021.07.007.
Al-Tawfiq JA, Rabaan AA, Saunar JV, Bazzi AM. Antimicrobial resistance of gram-negative bacteria: a 6-year longitudinal study in a hospital in Saudi Arabia. J Infect Public Health. 2020;13:737–45. https://doi.org/10.1016/j.jiph.2020.01.004.
Garg A, Garg J, Kumar S, Bhattacharya A, Agarwal S, Upadhyay GC. Molecular epidemiology & therapeutic options of carbapenem-resistant Gram-negative bacteria. Indian J Med Res. 2019;149:285–9. https://doi.org/10.4103/ijmr.IJMR_36_18.
Indian Council of Medical Research (ICMR). AMR surveillance network Indian Council of Medical Research 2018. Annual report: Antimicrobial Resistance Surveillance Network January 2018-December 2018. https://main.icmr.nic.in/sites/default/files/reports/AMRSN_Annual_Report_2018_0.pdf. Accessed 01 October 2021.
Pragasam AK, Veeraraghavan B, Anandan S, Narasiman V, Sistla S, Kapil A, et al. Dominance of international high-risk clones in carbapenemase-producing Pseudomonas aeruginosa: Multicentric molecular epidemiology report from India. Indian J Med Microbiol. 2018;36:344–51. https://doi.org/10.4103/ijmm.IJMM_18_294.
Lee YL, Ko WC, Hsueh PR. Geographic patterns of carbapenem-resistant Pseudomonas aeruginosa in the Asia-Pacific region: results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) program, 2015–2019. Antimicrob Agents Chemother. 2022;66(2): e0200021. https://doi.org/10.1128/AAC.02000-21.
Ahmed N, Ali Z, Riaz M, Zeshan B, Wattoo JI, Aslam MN. Evaluation of antibiotic resistance and virulence genes among clinical isolates of Pseudomonas aeruginosa from cancer patients. Asian Pac J Cancer Prev. 2020;21:1333–8. https://doi.org/10.31557/APJCP.2020.21.5.1333.
Saleem S, Bokhari H. Resistance profile of genetically distinct clinical Pseudomonas aeruginosa isolates from public hospitals in central Pakistan. J Infect Public Health. 2020;13:598–605. https://doi.org/10.1016/j.jiph.2019.08.019.
Awan AB, Yan A, Sarwar Y, Schierack P, Ali A. Detection of synergistic antimicrobial resistance mechanisms in clinical isolates of Pseudomonas aeruginosa from post-operative wound infections. Appl Microbiol Biotechnol. 2021. https://doi.org/10.1007/s00253-021-11680-6.
Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BL, Bouchillon SK, et al. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother. 2016;60:4490–500. https://doi.org/10.1128/AAC.00107-16.
Kazmierczak KM, de Jonge BLM, Stone GG, Sahm DF. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013–17. J Antimicrob Chemother. 2020;75:1165–73. https://doi.org/10.1093/jac/dkz571.
McCracken MG, Adam HJ, Blondeau JM, Walkty AJ, Karlowsky JA, Hoban DJ, et al. Characterization of carbapenem-resistant and XDR Pseudomonas aeruginosa in Canada: results of the CANWARD 2007–16 study. J Antimicrob Chemother. 2019;74(Suppl 4):iv32-iv38. https://doi.org/10.1093/jac/dkz285.
ATLAS (Antimicrobial Testing Leadership and Surveillance) database. Pfizer. Available from https://atlas-surveillance.com/. Accessed 13 Jan 2023.
European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters–Version 10.0. January 2020. http://www.eucast.org/clinical_breakpoints/.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: 30th informational supplement, M100. 30th ed. Wayne, PA: CLSI; 2020.
Hamad Medical Corporation. Annual cumulative report of the antimicrobial susceptibility rates of common microbial pathogens to antimicrobials available in Hamad General Hospital Formulary. (2020).
United Arab Emirates Ministry of Health and Prevention (UAE MOHAP). National Sub-Committee for AMR Surveillance. United Arab Emirates Surveillance of Antimicrobial Resistance Annual Report 2022. Document ref. number: AMR/NSR 2022. https://mohap.gov.ae/assets/download/ade73514/National%20AMR%20Surveillance%20Report%202022%20MOHAP.pdf.aspx. Accessed 4 Oct 2022.
Del Barrio-Tofiño E, López-Causapé C, Cabot G, Rivera A, Benito N, Segura C, et al. Genomics and susceptibility profiles of extensively drug-resistant Pseudomonas aeruginosa isolates from Spain. Antimicrob Agents Chemother. 2017;61:e01589-17. https://doi.org/10.1128/AAC.01589-17.
Abd El-Baky RM, Masoud SM, Mohamed DS, Waly NG, Shafik EA, Mohareb DA, et al. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. Infect Drug Resist. 2020;13:323–32. https://doi.org/10.2147/IDR.S238811.
Torrens G, van der Schalk TE, Cortes-Lara S, Timbermont L, Del Barrio-Tofiño E, Xavier BB, et al. Susceptibility profiles and resistance genomics of Pseudomonas aeruginosa isolates from European ICUs participating in the ASPIRE-ICU trial. J Antimicrob Chemother. 2022;77:1862–72. https://doi.org/10.1093/jac/dkac122.
Nordmann P, Poirel L, Toleman MA, Walsh TR. Does broad-spectrum β-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J Antimicrob Chemother. 2011;66:689–92. https://doi.org/10.1093/jac/dkq520.
Patel G, Bonomo RA. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol. 2013;4:48. https://doi.org/10.3389/fmicb.2013.00048.
European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/about-us/networks/disease-networks-and-laboratory-networks/ears-net-data. Accessed 31 May 2023.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: 31st informational supplement, M100. 32nd ed. Wayne, PA: CLSI; 2022.
Funding
This work is funded by Pfizer. Medical writing support was provided by Neera Hobson, PhD of Micron Research Ltd. (Ely, UK), and was funded by Pfizer Gulf FZ LLC.
Author information
Authors and Affiliations
Contributions
All authors were involved in the article design and data interpretation, as well as drafting and reviewing the manuscript. All authors read and approved the final submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
AA, BA, MA, CAM, WJ, AS, AMS, and HZ declare no competing interests. AH and NM are current Pfizer employees. AK is a former employee of Pfizer.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alatoom, A., Alattas, M., Alraddadi, B. et al. Antimicrobial Resistance Profiles of Pseudomonas aeruginosa in the Arabian Gulf Region Over a 12-Year Period (2010–2021). J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00191-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00191-y