Abstract
Mud volcano (MV) is one of the most important channels for deep seated volatile materials to migrate to Earth’s surface in the sedimentary basins at subduction zones. It plays a meaningful role of elemental budget in hydrosphere. Waters of MV fluid emitted from 46 mud pools in MV Lei-Gong-Huo (LGH) in eastern Taiwan were sampled from October 2015 to July 2016 monthly. Previous research indicates that the chemical characteristic of fluid expelled by MV LGH is dominated by two-end-member mixing of water–rock interaction with sedimentary and igneous rocks (Chao et al., Front Earth Sci 9:750436, 2022). Trace elements of 165 mud pool samples, including Al, Co, Ni, Cu, Zn, As, Br, Rb, Mo, Sb, I, Cs, Pb and U, were measured. The results show that waters of MV LGH reveal similar chemical characteristics to low-temperature ridge-flank hydrothermal spring and marine pore water in anoxic conditions coupled with the addition of clay dehydration. Principle component analysis (PCA) with previous published variables (Chao et al., Front Earth Sci 9:750436, 2022) indicates the important factors are the source of water–rock interaction, the surface ion exchange, the redox condition of mud pool, and the evaporation. In summary, the concentration of trace elements in the waters expelled by MV LGH is in the range of nM to pM. Their behaviors are dominated by secondary factors during and after migration such as surface ion exchange in response to changes in pressure and temperature, redox state of the mud pool, and evaporation. Trace elements in MV water are not suitable for fluid source or provenance studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The mud volcano is one of the most effective conduits for the fluids as well as volatile elements to migrate through thick sediments to the surface. It is a diapiric structure resulting from tectonic compaction where soft, thick, and fine-grained mud is rapidly deposited. The generation of hydrocarbon gas weakens the formations and increases the buoyancy of the fluid. Development of the mud diapir or the fault offers the channels for the fluid to migrate upward to the Earth’s surface (Milkov 2000; Dimitrov 2002; Kopf 2002; Mazzini and Etiope 2017). The MVs, including terrestrial and marine ones, are distributed on tectonically convergent region such as the Mediterranean Sea, the Alpine-Himalayan orogenic belt, the Caribbean Sea, and the Pacific Ring (Dia et al. 1999; Etiope et al. 2002, 2011a, 2011b; Planke et al. 2003; Shakirov et al. 2004; Yang et al. 2004; You et al. 2004; Lavrushin et al. 2005, 2015; Mazzini et al. 2007, 2009; Deville and Guerlais 2009; Farhadian Babadi et al. 2019). There are about 10 times more submarine MVs than terrestrial ones (Milkov 2000; Mazzini and Etiope 2017).
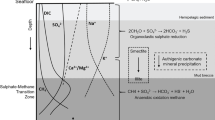
The materials expelled by MVs are composed of gas and mud. The composition of gas is mainly methane (> 90%) with nitrogen, argon, carbon dioxide, ethane, and higher hydrocarbon gases (Etiope et al. 2002, 2007, 2009; Shakirov et al. 2004; Yang et al. 2004; Chao et al. 2010; Sun et al. 2010). Globally, more than 76% of MVs emit thermogenic methane and only 4% emit microbial methane. The rest of the MVs emit mixed sourced gases (Etiope et al. 2009). The water separated from the mud originates from marine pore fluids. It may have experienced water–rock interaction and/or have been influenced by clay dehydration. Additionally, it may pass through halide formation and may mix with groundwater, surface runoff, and meteoric water near land surface (Bray and Karig 1985; Dia et al. 1999; Dahlmann and de Lange 2003; You et al. 2004; Mazzini et al. 2009). The chemical and isotopic composition may further vary through degradation, migration, and the conditions of mud pools/salsa during and after migration (Chao et al. 2011, 2013; this study). The mud breccias are mainly derived from the ambient sediments surrounding the fluid reservoirs with the addition materials from the fluid channel during migration. They are mostly clay minerals, such as smectite, illite, kaolinite and chlorite as well as other minerals like quartz and calcite (Dia et al. 1999; Kopf and Deyhle 2002; Farhadian Babadi et al. 2019). The three components are not necessarily from the same sources and can be decoupled from each other (Sun et al. 2010; Mazzini et al. 2018).
The evidence of major elements and 87Sr/86Sr indicates a two-end-member mixing scenario between igneous and sedimentary rocks dominants the chemical characteristic of waters expelled by MV LGH (Chao et al. 2022). Additionally, the predominantly gas, methane (> 90%), discharged by MV LGH is composed of a mixed thermogenic and microbial sources (Chao et al. 2010; Sun et al. 2010). Both gas and water show multiple origins, probably resulting from complex mélange formation at the source region.
There is limited data on dissolved trace element concentration in MV water (Chao et al. 2011, 2021; Lavrushin et al. 2015). The MV is an important geologic structure for the elements flowing back to the earth’s surface (e.g. You et al. 1993, 2004; Dimitrov 2002; Kopf 2002). They are one of the sources for seawater elemental budget. Marine mud volcanoes directly emit water with high concentration of trace elements such as Li, B and I into the ocean (You et al. 1993; Kopf 2002; Scholz et al. 2010). Although it is difficult to separate the trace element contribution in river water from weathering, water discharged by terristrial mud volcanoes does show notable influence on river water chemistry (e.g. Chao et al. 2015).
The two-end-member mixing chemical characteristic of MV LGH provides a good chance to study the distribution of trace elements from two types of water-rock interaction. In this study, water samples from 46 mud pools at MV LGH were collected from October 2015 to July 2016 monthly. Trace elements including Al, Co, Ni, Cu, Zn, As, Br, Rb, Mo, Sb, I, Cs, Pb and U were measured carefully and confidently. The chosen trace elements are well studied in the laboratory and on the field observations (Garlick and Dymond 1970; Seyfried and Bischoff 1979; Lawrence and Gieskes 1981; Wheat et al. 2002; Wheat and Mottl 2000), and thus could be compared to those from this study. The results are going to be combined with previous reported data (Chao et al. 2022), and the systematic measurements will help to understand the behaviors of the trace elements in MV waters from two distinct lithologies (sedimentary and igneous).
2 Methodology
2.1 Geological background and site description
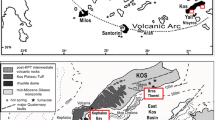
The Taiwan mountain belt formed as a result of arc-continent collision between the Philippine Sea Plate and the Eurasian Plate and one of the possible suture zones is the Longitudinal Valley, situated between the Central Range and the Coastal Range (Teng 1990). The highly deformed Lichi Mélange formation, which was the forearc basin and composed of chaotic mudstone mixed with fragments of oceanic crust (Chang et al. 2000), may provide the suitable environment and materials for the formation of MVs. All of the eastern Taiwan MVs are distributed along the hanging wall of Lichi Fault and the foothill of the Coastal Range on Lichi Mélange.
Mud Volcano Lei-Gong-Huo is the southmost MV in eastern Taiwan (Figs. 1 and 2). It has a shield appearance with a mud covered area approximate 150 m long and 50 m wide. Dozens of mud pools from about 10 cm to 2 m in diameter are distributed along the shield axis with the direction of approximately N15ºW (NNW–SSE). Seven groups in MV LGH are classified according to their locality (Fig. 3). Five clusters of mud pools, groups A to E, are distributed from north to south along the axis of the mud shield. Group F is the mud pools on the western flank. Group G is the mud pools in the northwestern corner. Previous study indicates groups D and E, located in the southeast, are dominated by the fluid experienced water–rock interaction with igneous rock (igneous fluid) while groups F and G, situated in the northwest, are dominated by the fluid experienced water–rock interaction with sedimentary rock (sedimentary fluid). The evidence of Sr isotopes also indicates possible residual ancient seawater emitted by the mud pools in group F (Chao et al. 2022).
Geologic map adopted from Central Geologic Survey, MOEA, Taiwan (www.moeacgs.gov.tw)
a Mud covered area of MV LGH (solid line) and the location of each sampled mud pool (black dots) and the groups from A to G. Arrow indicates the entrance of the MV field. b Photograph of group G facing west taken on 2016–06-21. c Photograph of group A facing east taken on 2015–06-21. d Photograph of mud pool F1 taken on 2016–02-23. e Photograph of mud pool F2 taken on 2016–03-22. f Photograph of group B (left) and C (right) facing east from the entrance taken on 2015–11-16. g Photograph of shield axis mud pools taken from group C facing north on 2016–05-24. h Photograph of group D taken on 2016–02-23. i Photograph of group E taken on 2016–02-23
2.2 Trace element composition in the MV water
A total of 46 mud pools in MV LGH were sampled from October 2015 to July 2016 monthly. Detailed sampling was performed on November 2015, May 2016 and July 2016. Only large, active, or long lasting mud pools were sampled on the rest of the visiting dates. Data of on site parameters such as temperature, pH, and oxidation–reduction potential (ORP) as well as major dissolved components, O, H, and triple Sr isotopes have been reported and discussed in the article by Chao et al. (2022).
Trace elements (Al, As, Br, Co, Cs, Cu, I, Mo, Ni, Pb, Rb, Sb, U, and Zn) were measured with Agilent 7500cx quadrupole inductively coupled plasma mass spectrometer (ICP-Q-MS). Due to high Cl, Na, Ca in the samples, matrix-matched calibration standards were prepared, the uncertainty is less than 3% and the quality assurance was obtained through 3 reference materials, SLRS-5, CASS-4, and NASS-5. The results Br, I, and Rb fall in the range of 10% certified values and the results of other elements fall in the range of 20% certified values except Co, Ni, Cu, Zn and As, which are deviated by isobaric interferences. Although Y and La show good results of reference materials, good correlation between Y and Sr as well as La and Ba indicate strong tailing effect on or hydride interference in both elements. The result of 2 elements is discarded. Numerical normalizations are applied to As, Co, Cu, Ni, and Zn due to low signal to noise ratio in collision mode. 75As has isobaric interference from 35Cl40Ar+. 59Co has interference from 43Ca16O+. 63Cu has strong interference from 23Na40Ar+; therefore, 65Cu is used in this study. 65Cu has interference from 130Ba2+ and 25Mg40Ar+. The separated test of matrix matched sample spiking with Ba and Mg indicates 130Ba2+ is the major source of interference and the contribution from MgAr is less than 1%. 60Ni has interference from 44Ca16O+ and 66Zn has interference from 26Mg40Ar+ and 132Ba2+. Similar to 65Cu, major contribution of interference is double charge of Ba, not MgAr. The 43/59, 43/60, 130/65, and 132/66 ratios of samples spiking with concentration matching Cl, Na, Ca and Ba were monitored before and after each 10 samples run. The intensity and ratios were applied to remove the potion from the interferences. The intensity of m/z 75 was deduced by the intensity of m/z 77 and multiplying by the 35Cl/37Cl ratio for the purpose of correcting the contribution from 35Cl40Ar+. Before the Cl correction, the intensity of m/z 77 contributed by Se was removed by monitoring m/z 82. After numerical normalization, the results of reference materials fall in 20% range of certified values for the 5 elements.
2.3 Statistical analysis
Principle component analysis (PCA) is a technique for analyzing large datasets and reducing the dimensionality of a dataset. It is used to examine the variability of the chemical results in waters of MV LGH. A varimax rotation with normalized data is applied to maximize a variance and to obtain a pattern of loading for each factor.
3 Results and discussion
The ICP–MS measureable elements sorted in order of the average molar concentration are Br, I, Al, Rb, Ni, Zn, Cu, Co, As, Mo, Cs, Sb, Pb and U (Additional file 1: Table S1). The results of elements Rb, Br, I and U are comparable to previous report which was collected in the year 2008 (Chao et al. 2011). Br, I, and Al are in the range of μM. Rb is in the range of sub-μM. The rest of the elements are in the range of nM to sub-nM. Br shows conservative behavior with limited variation, less than 5% of relative standard deviation. Cu, As, Rb, Mo, I, Cs, and U show variations smaller than 2 orders of magnitude. Al, Co, Ni, Zn, Sb, and Pb have great variations with more than 2 orders of magnitude.
3.1 Characteristic of trace elements
Waters expelled by MVs reveal similar chemical characteristics to deep marine pore fluids. They originate from ancient seawater and have been altered by early diagenesis, clay dehydration, and water–rock interaction (e. g. Dia et al. 1999; Dählmann and de Lange 2003; Hensen et al. 2004; You et al. 2004; Mazzini et al. 2007, 2009; Ray et al. 2013; Farhadian Babadi et al. 2019; Chen et al. 2020). Seawater is an important reference for chemical composition of MV waters. Chloride is one of the most important dissolved ions to decipher the source of the fluid other than seawater and the behavior of other elements because of its conservative characteristic. The average Al, Co, Cu, I and Pb over Cl ratios show at least ten folds higher than those of seawater (Additional file 2: Table S2). The average Ni, Zn, As, Sb and Cs over Cl ratios are slightly higher and the average Br/Cl is only 8% higher than those of seawater. The average Rb/Cl and Mo/Cl ratios are lower than those of seawater, and the average U/Cl ratio has less than 10% of the seawater value. Elements/Cl ratios higher than those of seawater indicate elements are released from the andesitic basement or sediments. Lower ratios indicate mechanisms that remove elements from the seawater such as uptake by alternation of the minerals, co-precipitation with secondary minerals or adsorption because of the variation of temperature, pressure, or redox conditions.
Due to high Ca/Cl and low Na/Cl and 87Sr/86Sr ratios, waters of MV LGH are speculated to derive from water–rock interaction of volcanogenic materials and have similar chemical characteristics to marine pore fluids near oceanic basement or low-temperature ridge-flank hydrothermal springs (Garlick and Dymond 1970; Seyfried and Bischoff 1979; Lawrence and Gieskes 1981; Wheat et al. 2002; Wheat and Mottl 2000). The laboratory simulation experiment and field investigation results show As/Cl, Rb/Cl, Cs/Cl, and Pb/Cl ratios higher than those of seawater in high temperature hydrothermal experiments (up to 350 ℃; You et al. 1996). Al/Cl ratio is higher than that of seawater in dissolution batch experiments at room temperature (e.g. Dixit et al. 2001). Mn/Cl, Mo/Cl, Co/Cl, Ni/Cl, and Zn/Cl ratios are higher than those of seawater, but Cu/Cl and U/Cl ratios are lower than those of seawater in low temperature ridge-flank hydrothermal spring waters (up to 64 ℃; Wheat et al. 2002). Br and I are released into pore fluids by the decomposition of organic matter with increasing degrees of diagenesis, raising Br/Cl and I/Cl ratios with depth (e.g. Gieskes and Mahn 2007).
Most of the element/Cl ratios match the pattern except Cu/Cl, Rb/Cl, and Mo/Cl ratios. Mo and U are redox sensitive elements and can be removed from the water under anoxic conditions easily. The results of on site ORP measurements indicate anoxic conditions in the MV waters (Chao et al. 2022) although Mo concentration shows no correlation with ORP and U has only low positive correlation (Additional file 3: Table S3. Mo removal requires H2S (e.g. Brüske et al. 2020), and H2S is not documented in the literature (Yang et al. 2004; Chao et al. 2010; Sun et al. 2010), which is a possible explanation of no correlation with the ORP. Low Cu/Cl in the spring water is inferred as being precipitated into sulfide phase (Wheat et al. 2002). The concentration of S in MV LGH is low possibly due to anaerobic oxidation of methane (AOM; Chang et al. 2012; Tu et al. 2017). With sufficient supply of methane, AOM is a common mechanism to remove sulfate in the MV water (e.g. Stadnitskaia et al. 2005; Wallmann et al. 2006; Lichtschlag et al. 2010). The absence of S may result in higher Cu content in the MV water. Rb and Cs are mobile elements in the high temperature hydrothermal fluid (Seyfried et al. 1998), and Cs is more mobile than Rb because the release of Cs from sediment is prior to Rb as temperature rises (You et al. 1996). Indeed, the Cs/Cl ratio is higher than Rb/Cl ratio relative to seawater, supporting the results of hydrothermal experiments (You et al. 1996; Seyfried et al. 1998). However, Rb/Cl ratio of MV LGH water is lower than that of seawater, indicating the uptake of Rb from water. Similar results have been reported in MVs from western Taiwan and Azerbaijan (Chao et al. 2011; Lavrushin et al. 2015) and low-temperature submarine springs (Wheat and Mottl 2000; Hulme et al. 2010). This uptake of Rb may couple with K uptake during smectite-illite transformation (Plank and Langmuir 1988; Hüpers and Kopf 2012) or water rock interaction with basalt (70 ℃; Seyfried and Bischoff 1979) at low temperature. Rb and Cs have strong positive correlation (r = 0.85), indicating the Rb uptake is stopped and Rb is released with Cs in the MV water. The evidence of high B and Li concentration as well as elevated δ18O in MV LGH waters (Chao et al. 2022) also supports the occurrence of smectite-illite transformation (e.g. Sokol et al. 2019; Baldermann et a., 2020; Chen et al. 2020). In summary, the general pattern of trace element distribution in waters of MV LGH is similar to low temperature submarine springs under anoxic conditions with the occurrence of clay dehydration.
3.2 Principle component analysis
Variables, including on site measurements, major elements, and Sr isotopes from Chao et al. (2022), are used with trace elements of this study for principle component analysis. Water isotopes are not used for PCA because not all of the samples have their water isotopes measured, and they are not correlated with any other parameters (Additional file 3: Table S3). There are 6 factors with eigenvalue higher than 1.5 (Table 1), and the accumulated variance of 6 factors is 68%.
The first factor (F1) from the PCA result has the highest variance by 29.71% and high positive loading for Li, Na, Mg, Sr, Ba, and 87Sr/86Sr and moderate positive loading for TA. B, S, Ca, and δ88Sr have high negative loading and Ni has moderate negative loading (Table 1; Fig. 4). 87Sr/86Sr is a robust tracer in hydrosphere indicating the rock that hosts the fluid reservoir. The high loading of 87Sr/86Sr in F1 clearly indicates that this factor can be assigned to the source of the fluid. Chao et al. (2022) illustrate that chemical characteristic of two-end-member mixing dominates chemical and Sr isotope distribution in the waters of MV LGH. The two end members are named sedimentary and igneous fluids, which are the fluid experienced water–rock interaction with sedimentary and igneous rocks respectively. The positive loading indicates the variables which are dominated by sedimentary fluid while negative loading indicates the variables which are dominated by igneous fluid. Groups F and G mud pools have high factor scores while D4 and group E mud pools have very negative scores (Additional file 2: Table S2; Figs. 5 and 6). The factor scores imply that sedimentary fluid dominates in mud pools of groups F and G in the northwest while igneous fluid dominates in mud pools of D4 and group E in the southeast (Fig. 6). The two-end-member characteristic are under stable condition through the whole study period while the mud pools located on the axis of mud shield (e.g. groups A, B and C) show relatively large temporal variation (Fig. 6). The temporal variation on the axis may imply the variation of end member contribution but the mechanism is not clear since the temporal resolution of the sampling is too low to detect the event.
The colored contour plot of F1 scores on 10 investigations. Black dots denote sampled mud pools. The unit of scale the bar is meter. Low score mud pools (red) dominate eastern and southeastern sides of the field, representing high contribution of igneous signals. High score mud pools (dark blue) dominate western and northwestern sides, representing contribution from sedimentary rocks. The southeastern side shows consistent condition during the sampling period while the northwestern side and the central part display variations. The end member sites are used as constrain points (i.e. D4, E1, F1, and F2)
Factor 2 (F2) has the variance by 11.88% with high positive loading for alkaline metal elements K, Rb and Cs and moderate positive loading for S, Ca, Cu, and Ba. TA and Na show moderate negative loading of F2 (Table 1). TA, B, Na, S, Ca, Cu, Rb, and Ba are both affected by F1 and F2 (Fig. 4). Mud pools with negative factor scores of F2 are distributed off the shield axis (i. e. D4 and F1; Figs. 5 and 7) and have relatively stable scores temporally (Fig. 7). Mud pools on the shield axis show positive scores and high score mud pools are distributed in the north with great variation spatially and temporally (Figs. 5 and 7). By comparing mud pools with great score variation (e.g. LGH-A7_2016-6 versus LGH-A7_2016-7, LGH-B2_2015-1 versus LGH-B2_2015-2, LGH-B2_2016-3 versus LGH-B3_2016-7, LGH-C1_2015-1 versus LGH-C1_2016-3), the high loading elements K, Rb and Cs show variations up to 26%, 66% and 80% respectively. However, major element Ca and Na, which show medium loadings, do not display co-variation with K because F1 has a greater influence on these elements. Additionally, 87Sr/86Sr ratios denote no loading on F2; indicating F2 does not represent another source. F2 is probably a secondary mechanism that occurs during fluid migration. Potassium can be affected by ion exchange reaction with the sediments due to the change in pressure and temperature (Wheat and Mottl 2000). This mechanism may occur to Rb and Cs because of similar ion radius. Factor score variation of the mud pools may indicate the variation of the mud flux. Mud pools on the flank show relatively low but stable fluxes and the axis pools display great variations.
The colored contour plot of F2 scores on 10 investigations. Black dots denote sampled mud pools. The unit of the scale bar is meter. High score mud pools (red) dominate the north side of the field and extend to the shield axis (central) on several sampling dates. Low score mud pools (dark blue) dominate flanks of the mud shield. Mud pools along the shield axis show great temporal and spatial variations while flank pools are in relatively stable condition. The end member sites are used as constrain points (i.e. D4, E1, F1, and F2)
Factor 3 (F3) has the variance by 10.26% with high positive loading for Si and Fe and moderate positive loading for total suspended solids (TSS) and As. ORP and U have moderate negative loading of F3 (Table 1). The negative correlation between ORP and TSS is interpreted as expelling activity (Chao et al. 2021). If the gas flux is higher than the threshold, the water and mud in the mud pool mix well. This scenario results in higher TSS in the sampling tubes and the ORP of the water will be low due to anaerobic oxidation of methane. The low ORP reduces the solubility of U but raises the solubility of Fe. More suspended particles and intensive blending in the mud pool release Si into aqueous phase. Therefore, F3 explains the activity of gas bubbling in the mud pool.
Factor 4 (F4) shows high positive loading for Mo and moderate positive loading for Sb. Mn has high negative loading of F4 (Table 1). Similar to F3, Mn, Mo and Sb are redox sensitive elements. Mn releases into aqueous phase under reducing condition but Mo is removed from aqueous phase. Although both U and Mo are removed from aqueous phase under anoxic condition, they show distinct differences in their removal pathways (Brüske et al. 2020). The release and uptake of Mn is also different from Fe. Therefore, F4 represents different redox pathways other than F3.
Factor 5 (F5) shows high positive loading for Co and Pb. F5 may represent the mechanism causing Co and Pb to vary. Factor 6 (F6) shows high positive loading for Cl and Br (Table 1). Cl and Br are conservative elements in MV LGH water. F6 implies evaporation and meteoric water dilution. Mud pool G5_16-3 has the lowest Cl and Br concentrations as well as the most negative factor scores (Additional file 2: Table S2).
3.3 Comparison with MV LS and MVs in Azerbaijan
Trace element distribution of MV waters obtained by ICP-MS is limited in the literatures. MV LS, which is located in the north, shows lower chloride concentration and slightly higher δ18O (Δ18OLS-LGH = 1.00 ‰; Chao et al. 2022) than that of MV LGH, possibly resulting from more dehydrated water addition. However, MV LS water shows lower to similar trace element over chloride ratios to MV LGH water (Chao et al. 2021; Additional file 2: Table S2). The proposed differences made by different degree of clay dehydration or sedimentary contribution in LGH water are not clear from the point of view of trace elements. Additionally, possible great variation of trace elements in two-end-member mixing dominated MV LGH waters is not detected since MV LGH does not display significant greater standard deviation than that of MV LS. As discussed in Sect. 3.2, trace element concentrations lower than μM scale are easily altered by secondary mechanisms during fluid migration or the condition of the mud pool. They are not suitable for fluid source tracing or provenance studies.
The average values of trace element over chloride ratios in Azerbaijan MV waters show higher Al/Cl, Zn/Cl/ Br/Cl, Mo/Cl, Sb/Cl, Pb/Cl, and U/Cl ratios, similar Cu/Cl, and lower Rb/Cl and Cs/Cl ratios relative to LGH waters (Lavrushin et al. 2015; Baldermann et al. 2020; Additional file 2: Table S2). Azerbaijan MVs are distributed on the area similar to Taiwan Island. The major source lithology of MV fluid is sedimentary rock. The addition of brines or dissolution of evaporates occurs in some MVs. It is difficult to compare MV LGH water to Azerbaijan MVs directly due to different spatial scale and lithology. However, the similar pattern of Al, Cu, Zn, As, Sb, Cs, and Pb enrichment and depletion of Rb related to seawater may indicate the general pattern of MV fluid worldwide. The discrepancy between Eastern Taiwan and Azerbaijan MVs may be induced by different lithology, degree of clay dehydration, proportion of water–rock interaction, degradation during migration, and condition of mud pool/salsa. Global database of the chemical composition of MV waters displays great diversity due to the variation of primary and secondary factors involved in the MVs (Nikitenko and Ershov 2021).
4 Conclusion
A total of 165 water samples from 46 mud pools of MV LGH in eastern Taiwan were collected from October 2015 to July 2016 monthly. Their dissolved trace elements, including Al, Co, Ni, Cu, Zn, As, Br, Rb, Mo, Sb, I, Cs, Pb and U, were analyzed. The results of Br, Rb, I and U concentrations agree with limited previous findings (Chao et al. 2011). Our major results are summarized as follows:
-
1.
The measurable concentration of trace elements in waters expelled by MV LGH ranges in the scale from sub μM to pM. The average trace elements over chloride ratios indicate similar pattern to low-temperature ridge-flank hydrothermal spring and marine pore water in anoxic condition. The low Rb/Cl ratio implies uptake of Rb from MV water, probably because of consumption with K during smectite to illite transformation.
-
2.
Combined with previous reported parameters (Chao et al. 2022), PCA results indicate the major factors of chemical variability in the waters of MV LGH is the source of water–rock interaction (sedimentary versus igneous rocks), ion exchange with sediments during fluid migration, the redox condition during fluid migration and in the mud pool, and evaporation. Besides the fluid source controlled factor F1, the rest of the factors are the representative of secondary mechanisms and contribute more variance than F1. It indicates that secondary mechanisms actually play a more important role than the source to vary trace elements in MV waters.
-
3.
There is large discrepancy between trace element concentrations in MV LGH and limited previous reports, including MV LS, 35 km north, and MVs in Azerbaijan (Lavrushin et al. 2015; Baldermann et al. 2020; Chao et al. 2021). Although trace elements in MV waters are release through water–rock interaction, they are easily masked by secondary mechanisms during migration and occurrence in the mud pools. Therefore, fluid source identity or provenance studies through trace elements are not recommended.
References
Baldermann A, Abbasov OR, Bayramova A, Abdullayev E, Dietzel M (2020) New insights into fluid-rock interaction mechanisms at mud volcanoes: implications for fluid origin and mud provenance at Bahar and Zenbil (Azerbaijan). Chem Geol 537:119479. https://doi.org/10.1016/j.chemgeo.2020.119479
Bray CJ, Karig DE (1985) Porosity of sediments on accretionary prisms and some implications for dewatering processes. J Geophys Res 90:768–787. https://doi.org/10.1029/JB090iB01p00768
Brüske A, Weyer S, Zhao MY, Planavsky NJ, Wegwerth A, Neubert N, Dellwig O, Lau KV, Lyons TW (2020) Correlated molybdenum and uranium isotope signatures in modern anoxic sediments: implications for their use as paleo-redox proxy. Geochim Cosmochim Acta 270:449–474. https://doi.org/10.1016/j.gca.2019.11.031
Chang CP, Angelier J, Huang CY (2000) Origin and evolution of a mélange: the active plate boundary and suture zone of the longtitudunal Valley, Taiwan. Tectonophysics 325:43–62. https://doi.org/10.1016/S0040-1951(00)00130-X
Chang YH, Cheng TW, Lai WJ, Tsai WY, Sun CH, Lin LH, Wang PL (2012) Microbial methane cycling in a terrestrial mud volcano in eastern Taiwan. Environ Microbio 14(4):895–908. https://doi.org/10.1111/j.1462-2920.2011.02658.x
Chao HC, You CF, Sun CH (2010) Gases in Taiwan mud volcanoes: chemical composition, methane carbon isotopes, and gas fluxes. Appl Geochem 25:428–436. https://doi.org/10.1016/j.apgeochem.2009.12.009
Chao HC, You CF, Wang BS, Chung CH, Huang KF (2011) Boron isotopic composition of mud volcano fluids: Implications for fluid migration in shallow subduction zones. Earth Planet Sci Lett 305:32–44. https://doi.org/10.1016/j.epsl.2011.02.033
Chao HC, You CF, Liu HC, Chung CH (2013) The origin and migration of mud volcano fluids in Taiwan: evidence from hydrogen, oxygen, and strontium isotopic compositions. Geochim Cosmochim Acta 114:29–51. https://doi.org/10.1016/j.gca.2013.03.035
Chao HC, You CF, Liu HC, Chung CH (2015) Evidence for stable Sr isotope fractionation by silicate weathering in a small sedimentary watershed in southwestern Taiwan. Geochim Cosmochim Acta 165:324–341. https://doi.org/10.1016/j.gca.2015.06.006
Chao HC, You CF, Lin IT, Lu HY, Liu HC, Chung CH (2021) Water of Eastern Taiwan mud volcanoes. Part I. H, triple O, triple Sr isotopes and trace elements of Lo-Shan Mud Volcano. Terr Atmos Ocean Sci. 32(6):1227–1253. https://doi.org/10.3319/TAO.2021.12.26.01
Chao HC, You CF, Lin IT, Liu HC, Chung LH, Huang CC, Chung CH (2022) Two-end-member mixing in the fluid emitted from Mud Volcano Lei-Gong-Huo, Eastern Taiwan: evidence from Sr isotopes. Front Earth Sci 9:750436. https://doi.org/10.3389/feart.2021.750436
Chen NC, Yang TF, Hong WL, Yu TL, Lin IT, Wang PL, Lin SW, Su CC, Shen CC, Wang YS, Lin LH (2020) Discharge of deeply rooted fuids from submarine mud volcanism in the Taiwan accretionary prism. Sci Rep 10:381. https://doi.org/10.1038/s41598-019-57250-9
Dahlmann A, de Lange GJ (2003) Fluid-sediment interactions at Eastern Mediterranean mud volcanoes: a stable isotope study from ODP Leg 160. Earth Planet Sci Lett 212:377–391. https://doi.org/10.1016/S0012-821X(03)00227-9
Deville E, Guerlais SH (2009) Cyclic activity of mud volcanoes: evidences from Trinidad (SE Caribbean). Mar Petrol Geol 26:1681–1691. https://doi.org/10.1016/j.marpetgeo.2009.03.002
Dia AN, Rouelle MC, Boulegue J, Comeau P (1999) Trinidad mud volcanoes: where do the expelled fluids come from? Geochim Cosmochim Acta 63:1023–1038. https://doi.org/10.1016/S0016-7037(98)00309-3
Dimitrov L (2002) Mud volcanoes-the most important pathway for degassing deeply buried sediments. Earth Sci Rev 59:49–76. https://doi.org/10.1016/S0012-8252(02)00069-7
Dixit S, Van Vappellen P, van Bennekom AJ (2001) Processes controlling solubility of biogenic silica and pore water build-up of silicic acid in marine sediments. Mar Chem 73:333–352. https://doi.org/10.1016/S0304-4203(00)00118-3
Etiope G, Caracausi A, Favara R, Italiano F, Baciu C (2002) Methane emission from the mud volcanoes of Sicily (Italy). Geophys Res Lett 29:14340–14343. https://doi.org/10.1029/2001GL014340
Etiope G, Martinelli G, Caracausi A, Italiano F (2007) Methane seeps and mud volcanoes in Italy: gas origin, fractionation and emission to the atmosphere. Geophys Res Lett. https://doi.org/10.1029/2007GL030341
Etiope G, Feyzullayev A, Baciu CL (2009) Terrestrial methane seeps and mud volcanoes: a global perspective of gas origin. Mar Petrol Geol 26:333–344. https://doi.org/10.1016/j.marpetgeo.2008.03.001
Etiope G, Baciu CL, Schoell M (2011a) Extreme methane deuterium, nitrogen and helium enrichment in natural gas from the Homorod seep (Romania). Chem Geol 280:89–96. https://doi.org/10.1016/j.chemgeo.2010.10.019
Etiope G, Nakada R, Tanaka K, Yoshida N (2011b) Gas seepage from Tokamachi mud volcanoes, onshore Niigata Basin (Japan): origin, post-genetic alterations and CH4–CO2 fluxes. Appl Geochem 26:348–359. https://doi.org/10.1016/j.apgeochem.2010.12.008
Farhadian Babadi M, Mehrabi B, Tassi F, Cabassi J, Vaselli O, Shakeri A, Pecchioni E, Venturi S, Zelenski M, Chaplygin I (2019) Origin of fluids discharged from mud volcanoes in SE Iran. Mar Petrol Geol 106:190–205. https://doi.org/10.1016/j.marpetgeo.2019.05.005
Garlick GD, Dymond JR (1970) Oxygen isotope exchange between volcanic materials and ocean water. Geol Soc Am Bull 81(7):2137–2142. https://doi.org/10.1130/0016-7606(1970)81[2137:OIEBVM]2.0.CO;2
Gieskes JM, Mahn C (2007) Halide systematics in interstitial waters of ocean drilling sediment cores. Appl Geochem 22:515–533. https://doi.org/10.1016/j.apgeochem.2006.12.003
Hensen C, Wallmann K, Schmidt M, Ranero CR, Suess E (2004) Fluid expulsion related to mud extrusion off Costa Rica—a window to the subducting slab. Geology 32:201–204. https://doi.org/10.1130/G20119.1
Hulme SM, Wheat CG, Fryer P, Mottl MJ (2010) Pore water chemistry of the Mariana serpentinite mud volcanoes: a window to the seismogenic zone. Geochem Geophys Geosyst 11:Q01X09. https://doi.org/10.1029/2009GC002674
Hüpers A, Kopf AJ (2012) Effect of smectite dehydration on pore water geochemistry in the shallow subduction zone: an experimental approach. Geochem Geophys Geosyst 13:Q0AD26. https://doi.org/10.1029/2012GC004212
Kopf AJ (2002) Significance of mud volcanism. Rev Geophys 40:1–51. https://doi.org/10.1029/2000RG000093
Kopf A, Deyhle A (2002) Back to the roots: boron geochemistry of mud volcanoes and its implications for mobilization depth and global B cycling. Chem Geol 192:195–210. https://doi.org/10.1016/S0009-2541(02)00221-8
Lavrushin VY, Dubinina EO, Avdeenko AS (2005) Isotopic composition of oxygen and hydrogen in mud-volcanic waters from Taman (Russia) and Kakhetia (Eastern Georgia). Lithol Miner Resour 40:123–137. https://doi.org/10.1007/s10987-005-0014-z
Lavrushin VY, Guliev IS, Kikvadze OE, Aliev AA, Pokrovsky BG, Polyak BG (2015) Waters from mud volcanoes of Azerbaijan: Isotopic-geochemical properties and generation environments. Lithol Miner Resour 50:1–25. https://doi.org/10.1134/S0024490215010034
Lawrence JR, Gieskes JM (1981) Constraints on water transport and alteration in the oceanic crust from the isotopic composition of pore water. J Geophys Res 86:7924–7934. https://doi.org/10.1029/JB086iB09p07924
Lichtschlag A, Felden J, Wenzhöfer F, Schubotz F, Ertefai TF, Boetius A, de Beer D (2010) Methane and sulfide fluxes in permanent anoxia: in situ studies at the Dvurechenskii mud volcano (Sorokin Trough, Black Sea). Geochim Cosmochim Acta 74:5002–5018. https://doi.org/10.1016/j.gca.2010.05.031
Mazzini A, Etiope G (2017) Mud volcanism: an updated review. Earth Sci Rev 168:81–112. https://doi.org/10.1016/j.earscirev.2017.03.001
Mazzini A, Svensen H, Akhmanov GG, Aloisi G, Planke S, Malthe-Sorenssen A, Istadi B (2007) Triggering and dynamic evolution of the LUSI mud volcano, Indonesia. Earth Planet Sci Lett 261:375–388. https://doi.org/10.1016/j.epsl.2007.07.001
Mazzini A, Svensen H, Planke S, Guliyev I, Akhmanov GG, Fallik T, Banks D (2009) When mud volcanoes sleep: Insight from seep geochemistry at the Dashgil mud volcano, Azerbaijan. Mar Petrol Geol 26:1704–1715. https://doi.org/10.1016/j.marpetgeo.2008.11.003
Mazzini A, Scholz F, Svensen HH, Hensen C, Hadi S (2018) The geochemistry and origin of the hydrothermal water erupted at Lusi, Indonesia. Mar Petrol Geol 90:52–66. https://doi.org/10.1016/j.marpetgeo.2017.06.018
Milkov AV (2000) Worldwide distribution of submarine mud volcanoes and associated gas hydrates. Mar Geol 167:29–42. https://doi.org/10.1016/S0025-3227(00)00022-0
Nikitenko OA, Ershov VV (2021) Geochemical patterns of mud volcanic waters: reviewed worldwide data. Geochem Int 59:922–937. https://doi.org/10.1134/S0016702921090044
Plank T, Langmuir CH (1998) The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem Geol 145:325–394. https://doi.org/10.1016/S0009-2541(97)00150-2
Planke S, Svensen H, Hovland M, Banks DA, Jamtveit B (2003) Mud and fluid migration in active mud volcanoes in Azerbaijan. Geo Mar Lett 23:258–268. https://doi.org/10.1007/s00367-003-0152-z
Ray JS, Kumar A, Sudheer AK, Deshpande RD, Rao DK, Patil DJ, Awasthi N, Bhutani R, Bhushan R, Dayal AM (2013) Origin of gases and water in mud volcanoes of Andaman accretionary prism: implications for fluid migration in forearcs. Chem Geol 347:102–113. https://doi.org/10.1016/j.chemgeo.2013.03.015
Scholz F, Hensen C, De Lange GJ, Haeckel M, Liebetrau V, Meixner A, Reitz A, Romer RL (2010) Lithium isotope geochemistry of marine pore waters—insights from cold seep fluids. Geochim Cosmochim Acta 74:3459–3475. https://doi.org/10.1016/j.gca.2010.03.026
Seyfried JWE, Bischoff JL (1979) Low temperature basalt alteration by sea water: an experimental study at 70°C and 150°C. Geochim Cosmochim Acta 43:1937–1947. https://doi.org/10.1016/0016-7037(79)90006-1
Seyfried JWE, Chen X, Chan LH (1998) Trace element mobility and lithium isotope exchange during hydrothermal alteration of seafloor weathered basalt: an experimental study at 350 C, 500 bars. Geochim Cosmochim Acta 62:949–960. https://doi.org/10.1016/S0016-7037(98)00045-3
Shakirov R, Obzhirov A, Suess E, Salyuk A, Biebow N (2004) Mud volcanoes and gas vents in the Okhotsk sea area. Geo Mar Lett 24:140–149. https://doi.org/10.1007/s00367-004-0177-y
Sokol EV, Kokh SN, Kozmenko OA, Lavrushin VY, Belogub EV, Khvorov PV, Kikvadze OE (2019) Boron in an onshore mud volcanic environment: case study from the Kerch Peninsula, the Caucasus continental collision zone. Chem Geol 525:58–81. https://doi.org/10.1016/j.chemgeo.2019.07.018
Stadnitskaia A, Muyzer G, Abbas B, Coolen MJL, Hopmans EC, Baas M, van Weering TCE, Ivanov MK, Poludetkina E, Sinninghe Damsté JS (2005) Biomarker and 16S rDNA evidence for anaerobic oxidation of methane and related carbonate precipitation in deep-sea mud volcanoes of the Sorokin Trough, Black Sea. Mar Geol 217:67–96. https://doi.org/10.1016/j.margeo.2005.02.023
Sun CH, Chang SC, Kuo CL, Wu JC, Shao PH, Oung JN (2010) Origins of Taiwan’s mud volcanoes: evidence from geochemistry. J Asian Earth Sci 37:105–116. https://doi.org/10.1016/j.jseaes.2009.02.007
Teng LS (1990) Geotectonic evolution of late Cenozoic arc-continent collision in Taiwan. Tectonophysics 183:57–76. https://doi.org/10.1016/0040-1951(90)90188-E
Tu TH, Wu LW, Lin YS, Imachi H, Lin LH, Wang PL (2017) Microbial community composition and functional capacity in a Terrestrial Ferruginous, Sulfate-Depleted Mud Volcano. Front Microbiol 8:2137. https://doi.org/10.3389/fmicb.2017.02137
Wallmann K, Drews M, Aloisi G, Bohrmann G (2006) Methane discharge into the Black Sea and the global ocean via fluid flow through submarine mud volcanoes. Earth Planet Sci Lett 248:545–560. https://doi.org/10.1016/j.epsl.2006.06.026
Wheat CG, Mottl MJ (2000) Composition of pore and spring waters from Baby Bare: global implications of geochemical fluxes from a ridge flank hydrothermal system. Geochim Cosmochim Acta 64:629–642. https://doi.org/10.1016/S0016-7037(99)00347-6
Wheat CG, Mottl MJ, Rudnicki M (2002) Trace element and REE composition of a low-temperature ridge-flank hydrothermal spring. Geochim Cosmochim Acta 66:3693–3705. https://doi.org/10.1016/S0016-7037(02)00894-3
Yang TF, Yeh GH, Fu CC, Wang CC, Lan TF, Lee HF, Chen CH, Walia V, Sung QC (2004) Composition and exhalation flux of gases from mud volcanoes in Taiwan. Environ Geol 46:1003–1011. https://doi.org/10.1007/s00254-004-1086-0
You CF, Spivack AJ, Smith JH, Gieskes JM (1993) Mobilization of boron in convergent margins: implications for the boron geochemical cycle. Geology 21:207–210. https://doi.org/10.1130/0091-7613(1993)021%3c0207:MOBICM%3e2.3.CO;2
You CF, Castillo PR, Gieskes JM, Chan LH, Spivack AJ (1996) Trace element behavior in hydrothermal experiments: implications for fluid processes at shallow depths in subduction zones. Earth Planet Sci Lett 140:41–52. https://doi.org/10.1016/0012-821X(96)00049-0
You CF, Gieskes JM, Lee T, Yui TF, Chen HW (2004) Geochemistry of mud volcano fluids in the Taiwan accretionary prism. Appl Geochem 19:695–707. https://doi.org/10.1016/j.apgeochem.2003.10.004
Acknowledgements
This study was supported by MOST (Ministry of Science and Technology, Taiwan) grants MOST109-2116-M-194-009 and MOST110-2116-M-194-012 to HC Chao. The author wishes to thank Prof. HY Lu for the machine time of ICP-MS. Constructive comments by two reviewers are greatly appreciated, as well as the editors for their effective handling of this manuscript.
Author information
Authors and Affiliations
Contributions
HCC contributed to the conception and design of the study, did the field work, performed the chemical measurements and wrote the manuscript. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Trace element concentrations of MV LGH waters.
Additional file 2: Table S2.
Trace elements over chloride ratios and factor scores of PCA results of MV LGH waters, chloride concentration adopted from Chao et al. (2022).
Additional file 3: Table S3.
Matrix of Pearson’s correlation coefficient of all MV LGH samples (n = 165), r higher than 0.7 or lower than -0.7 in red color.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chao, HC. Water of Eastern Taiwan mud volcanoes: part II—trace element distribution of Lei-Gong-Huo mud volcano. Terr Atmos Ocean Sci 34, 1 (2023). https://doi.org/10.1007/s44195-022-00033-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44195-022-00033-z