Abstract
Background
Abdominal desmoid tumors (DTs) are rare soft-tissue neoplasms known for their relatively high local recurrence rate. This study aims to elucidate the clinicopathological features and investigate prognostic factors related to abdominal DTs across various tumor locations.
Methods
From January 2000 to January 2021, patients diagnosed with abdominal DTs who underwent complete resection at the Cancer Hospital, Chinese Academy of Medical Sciences, were included in this study. Thorough collection and review of clinicopathologic characteristics and follow-up data were performed. Prognostic factors, including age at presentation, sex, tumor location, size, and proximity to nerves or vasculature, were meticulously analyzed to assess their impact on recurrence-free survival.
Results
A cohort of 226 patients diagnosed with abdominal DTs was categorized into two groups based on tumor site: the abdominal wall group (n = 132) and the intra-abdominal cavity group (n = 94). Distinct clinicopathological features and prognoses were observed between abdominal wall DTs and intra-abdominal DTs. During a median follow-up of 60 months, 24 patients (10.2%) experienced local recurrence. Univariate and multivariate analyses identified intra-abdominal tumors, tumor size >10cm, and positive margins as independent risk factors associated with poor prognosis.
Conclusion
Abdominal wall DTs demonstrate different clinicopathological characteristics and better prognoses compared to intra-abdominal DTs. By achieving negative margins, patients with abdominal wall DTs can achieve favorable therapeutic outcomes and prognosis through curative resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
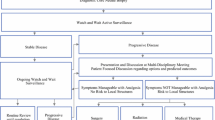
Desmoid tumors (DTs), known as aggressive fibromatosis, are rare neoplasms characterized by clonal proliferation, speculated to arise from mesenchymal stem cells [1, 2]. Despite lacking metastatic potential or malignancy, DTs demonstrate considerable local aggressiveness and tend to recur, posing a substantial risk of local relapse and significant functional impairments [3,4,5]. Hence, in clinical practice, surgeons frequently choose to pursue more assertive therapeutic modalities, including extensive surgical excision [6,7,8], radiation therapy [9, 10], systemic treatments [11,12,13], and the utilization of neoadjuvant radiation either alone or combined with chemotherapy [14, 15]. Nonetheless, due to the infrequency of Desmoid tumors and their unpredictable clinical trajectory, determining the most effective therapeutic approaches remains a daunting task.
Desmoid tumors (DTs) exhibit diverse clinical manifestations throughout the body. They are classified into extra-abdominal, abdominal wall, and intra-abdominal types based on their anatomical locations. Symptoms of intra-abdominal DTs may include intestinal obstruction, fistula formation, or dysuria, whereas extra-abdominal DTs often present with neuropathic pain [16]. Various research investigations have pinpointed age, surgical margin status, tumor size, location, and the administration of adjuvant radiotherapy as clinicopathological factors predictive of recurrence [17,18,19,20]. However, abdominal wall and intra-abdominal DTs are generally characterized by minimal invasiveness and a relatively lower recurrence risk [16, 21, 22]. Moreover, distinct biological compositions and genetic profiles suggest that abdominal DTs may represent a distinct disease entity compared to DTs occurring in other body regions [8, 16, 23]. Despite limited data on managing abdominal DTs and the lack of specific prognostic factors, this multicenter study enrolled 226 patients to investigate the clinicopathologic features and identify predictive factors for recurrence-free survival (RFS) in abdominal DTs.
1.1 Patients
This study utilized clinical data extracted from electronic medical records, covering a range of variables including age at diagnosis, gender, body mass index (BMI), concurrent medical conditions, history of prior abdominal surgeries, admission status, tumor characteristics (such as location, size, and stage), surgical margins, and proximity of the tumor to critical blood vessels or nerves. Moreover, surgical outcomes, encompassing operative time, intraoperative blood loss, postoperative complications, and duration of hospitalization, were meticulously documented and subjected to analysis. Tumor localization was classified into intra-abdominal and abdominal wall sites, with retroperitoneal lesions categorized separately as extra-abdominal DTs and consequently excluded from the research cohort. Tumor diameter and surgical margin status were assessed by two dedicated pathologists specializing in abdominal tumors, with a positive margin diagnosis assigned if cancerous tissue was detected within 0.5 cm from the inked margin on pathological examination. Following surgical intervention, adjuvant radiation therapy was prescribed, typically delivering a median total dose of 55 Gy, as per the evaluation by radiologists and consideration of the patient's general health status. Subsequent to treatment, patients were scheduled for routine follow-up evaluations at intervals of 1-2 years, conducted during outpatient appointments until either recurrence, mortality, or the end date of December 31, 2022. Detection of local recurrence, determined by magnetic resonance imaging (MRI) or computed tomography (CT) scans [24, 25], served as the principal endpoint for this investigation.
1.2 Statistical analysis
For data analysis, the Statistical Package for the Social Sciences (SPSS version 24.0, IBM Corp., Armonk, NY, United States) was employed. Continuous variables were represented as mean ± standard deviation and subjected to paired Student’s t-tests for analysis. Categorical variables and ordinal data were depicted as numerical values and percentages, undergoing comparison via appropriate statistical tests such as χ2 tests or Mann–Whitney U-tests. The period of recurrence-free survival (RFS) follow-up was defined as the duration between the date of pathological diagnosis and the identification of recurrence. Survival analysis was executed utilizing the Kaplan-Meier method, with comparisons facilitated through the log-rank test. Multivariate analyses were conducted utilizing the Cox proportional hazards regression model to discern independent prognostic factors. Statistical significance was defined at P < 0.05.
2 Results
2.1 Clinical and pathological characteristics
Successful surgery was performed on all 226 patients, achieving complete macroscopic resection (Fig. 1A, B). Table 1 presents detailed clinical and pathological characteristics of the study population. The median age at diagnosis was 37 years (range 14-73), with a predominance of female individuals (73.5%, n = 166). The average BMI stood at 22.3 ± 3.3 kg/m2. The majority of patients (86.7%, n = 196) presented with primary tumors, with a median tumor diameter of 5.5 cm (range 1.4-30). Common initial symptoms included abdominal pain (14.2%, n = 32), alterations in bowel habits (5.3%, n = 12), obstruction (2.7%, n = 6), anemia (2.7%, n = 6), and hydronephrosis (1.8%, n = 4). Positive margins were identified in 18 cases (8.0%), and postoperative radiotherapy was administered to 18 patients (8.0%). Table 2 outlines surgical outcomes and postoperative complications. The average duration of surgery was 138.4 ± 83.5 minutes (range 40-360), with intraoperative blood loss averaging 70.5 ± 114.3 milliliters (range 10-400). Postoperative complications were noted in 42 patients (18.6%), primarily chronic pain (7.1%, n = 16), followed by wound infection (6.2%, n = 14), anastomotic leakage (3.5%, n = 8), ileus (2.7%, n = 6), incisional hernia (1.8%, n = 4), and neurological issues (0.9%, n = 2).
The 226 patients were divided into two groups based on tumor locations: the abdominal wall (AW) group (n = 132) and the intra-abdominal cavity (IAC) group (n = 94). A notably lower proportion of male patients was evident in the AW group in comparison to the IAC group (6.1% vs. 55.3%, P < 0.001). Patients aged ≥30 years were markedly less prevalent in the AW group than in the IAC group (63.6% vs. 91.5%, P < 0.001). Moreover, a higher percentage of patients in the AW group had a history of cesarean section compared to those in the IAC group (34.8% vs. 6.4%, P < 0.001). Additionally, patients in the AW group were less likely to exhibit clinical symptoms than those in the IAC group (13.6% vs. 40.4%, P < 0.001). The proportion of patients with lesions exceeding 10 cm was significantly higher in the IAC group than in the AW group (27.7% vs. 7.6%, P < 0.001), and lesions in the IAC group were more prone to adhering to vital nerves or vasculature (42.6% vs. 13.6%, P < 0.001). Regarding surgical outcomes, patients in the IAC group experienced significantly longer operation durations (193.5 ± 89.3 vs. 98.6 ± 50.0 min, P < 0.001) and greater intraoperative blood loss compared to those in the AW group (136.0 ± 149.3 vs. 24.0 ± 41.0 ml, P < 0.001).
2.2 Survival analysis
The median follow-up duration was 60 months (range 8-255). At the last follow-up, only four patients had deceased, with only one death attributed to recurrence caused by abdominal DTs. Throughout the follow-up period, 24 patients experienced recurrence post-surgery with or without adjuvant radiotherapy. Among these, 20 cases (83.3%) of recurrence occurred in the intra-abdominal cavity (IAC) group, while 4 cases (16.7%) occurred in the abdominal wall (AW) group. The 5- and 10-year recurrence-free survival (RFS) rates for the entire cohort were estimated at 92.8% and 89.8%, respectively, as illustrated in Fig. 2. Remarkably, recurrence manifested in two patients even after a decade post-surgery. Furthermore, among the 24 patients admitted with recurrent disease, none experienced further recurrence during the follow-up period subsequent to surgical resection with or without radiotherapy.
Upon univariate analysis, it was observed that tumor size, location, and margin status significantly influenced RFS (P < 0.05). Patients with intra-abdominal tumors, tumor size exceeding 10 cm, and positive margins (R1) demonstrated notably poorer RFS (Fig. 3A-C). Subsequent multivariate analysis pinpointed tumor location (HR: 8.407; 95% CI, 1.649-42.865; P = 0.010), tumor size (HR: 17.437; 95% CI, 3.648-83.346; P < 0.001), and margin status (HR: 8.045; 95% CI, 2.388-27.099; P = 0.001) as independent risk factors for RFS. The results of univariate and multivariate analyses are shown in Table 3.
3 Discussion
Desmoid tumors (DTs) are rare soft-tissue neoplasms originating from clonal myofibroblasts, typically emerging from musculoaponeurotic structures, fascial planes, and ligaments throughout the body [1, 2]. Despite lacking metastatic potential or malignancy, DTs demonstrate local aggressiveness towards adjacent structures and a propensity for recurrence. The local recurrence rate can range from 17.6% to 30.7% [16, 21, 23, 26]. Desmoid tumors (DTs) are categorized according to their localization into extra-abdominal, abdominal wall, and intra-abdominal types. The biological behaviors of DTs vary across different anatomical sites, with previous research indicating a significantly better prognosis for abdominal DTs compared to extra-abdominal DTs [21, 23, 25, 26]. To delve deeper into the clinicopathologic characteristics and prognostic factors of this uncommon tumor, a retrospective multicenter study was undertaken, with the objective of offering valuable insights for clinical practice.
We categorized abdominal DT patients into two groups: the abdominal wall (AW) and intra-abdominal cavity (IAC) groups, with the aim of comparing their clinicopathological characteristics and survival outcomes. Our investigation revealed that the AW group predominantly comprised young women with a history of cesarean section, consistent with prior findings [8, 23, 27]. Additionally, we noted a significantly larger tumor diameter in the IAC group compared to the AW group. Most individuals in the IAC group exhibited noticeable clinical symptoms upon initial diagnosis, likely due to the subtle nature of intra-abdominal DTs and the absence of specific symptoms in early disease stages. As the tumor enlarges and exerts pressure on surrounding tissues, symptoms such as ileus, abdominal pain, and hydronephrosis may ensue [28]. Moreover, our results suggested a higher propensity for lesions in the IAC group to adhere to vital nerves or vasculature compared to those in the AW group, possibly indicating the insidious nature of intra-abdominal DTs and the heightened local aggressiveness of the tumor upon diagnosis.
Previously reported prognostic factors affecting the survival of DT patients include younger age, tumor location (particularly extra-abdominal sites), larger tumor size, and close or positive surgical margins, as outlined by He et al [26]. Similarly, Mullen et al. underscored the importance of margin status as a prognostic indicator for postoperative recurrence in DT patients undergoing surgical excision [21]. Focusing on abdominal DTs, our study findings reveal that intra-abdominal localization, larger tumor size, and achieving R0 resection were all independent predictors for recurrence-free survival (RFS) in abdominal DT patients. Prior research has established that patients with abdominal wall DTs generally have better long-term survival outcomes compared to those with intra-abdominal DTs [27,28,29]. Wilkinson et al. conducted a study involving 50 patients with abdominal wall DTs who underwent complete surgical resection. Over a median follow-up period of 5 years, only 8% (4/50) experienced local recurrence. Among the 46 patients who remained disease-free, 13 underwent uncomplicated pregnancies following abdominal mesh repair or without tumor recurrence [27]. In line with these findings, our study demonstrated a significantly higher RFS rate among patients with abdominal wall DTs compared to those with intra-abdominal DTs, with only 3% (4/132) of patients in the AW group experiencing local recurrence during follow-up. Conversely, the local recurrence rate among patients in the IAC group was notably higher at 23.1% (20/94), with all 20 patients experiencing tumor recurrence having tumors larger than 5 cm, and eight patients had tumors exceeding 10 cm in size.
Moreover, among the 20 patients with tumor recurrence in the IAC group, pathology confirmed positive margins in 10 patients. We propose that unlike abdominal wall DTs situated superficially, intra-abdominal lesions, due to their lower incidence and absence of specific initial clinical symptoms, tend to manifest prominently at diagnosis and are closely associated with surrounding vital organs and structures. Achieving radical resection to maximize protection of surrounding organ function is challenging, leading to a significantly heightened risk of local recurrence. Moreover, the muscular and fascial defects arising from extensive resection in abdominal wall DTs can be efficiently addressed through the utilization of synthetic or biological mesh, providing a dependable technical approach to attain R0 resection [30, 31].
Several limitations were observed in the current study. Firstly, being retrospective in nature, our study was susceptible to biases stemming from patient selection and information collection. Secondly, over the nearly 20-year duration of our study, treatment strategies for DTs have evolved, potentially influencing outcomes. Furthermore, initial pathological assessments might lack the thorough evaluation observed in current reports, leading to the absence of crucial information, such as SMA, β-catenin, Desmin, and Ki-67 data, in certain patients. This limitation hindered our ability to assess their prognostic value accurately.
4 Conclusion
Distinct clinicopathological features characterize abdominal wall DTs, typically yielding more favorable prognoses in contrast to intra-abdominal DTs. Through the assurance of achieving negative margins, radical resection can result in satisfactory therapeutic outcomes and prognosis for patients with abdominal wall DTs.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- DTs:
-
Abdominal desmoid tumors
- RFS:
-
Recurrence-free survival
- BMI:
-
Body mass index
- AW:
-
Abdominal wall
- CT:
-
Computed tomography
- IAC:
-
Intra-abdominal cavity
References
Wu C, Amini-Nik S, Nadesan P, et al. Aggressive fibromatosis (desmoid tumor) is derived from mesenchymal progenitor cells. Cancer Res. 2010;70(19):7690–8.
Kasper B, Baumgarten C, Garcia J, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma Patients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399–408.
Mankin HJ, Hornicek FJ, Springfield DS. Extraabdominal desmoid tumors: a report of 234 cases. J Surg Oncol. 2010;102(5):380–4.
Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, et al. A nation-wide study comparing sporadic and familial adenomatous polyposis–related desmoid-type fibromatoses. Int J Cancer. 2011;129(1):256–61.
Wood TJ, Quinn KM, Farrokhyar F, et al. Local control of extra-abdominal desmoid tumors: systematic review and meta-analysis. Rare Tumors. 2013;5(1):e2.
Seinen JM, Niebling MG, Bastiaannet E, et al. Four different treatment strategies in aggressive fibromatosis: a systematic review. Clin Transl Radiat Oncol. 2018;12:1–7.
Bonvalot S, Ternes N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol. 2013;20(13):4096–102.
Martínez Trufero J, Pajares Bernad I, Torres Ramón I, et al. Desmoid-type fibromatosis: who, when, and how to treat. Curr Treat Options Oncol. 2017;18(5):29.
Kim JS, Kim HJ, Lee MY, et al. Survival outcomes after adjuvant radiotherapy for aggressive fibromatosis depend on time frame and nuclear β-catenin. Radiat Oncol J. 2019;37(1):37–42.
Fiore M, Colombo C, Radaelli S, et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer. 2015;51(18):2800–7.
Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25(13):1785–91.
Bertagnolli MM, Morgan JA, Fletcher CD, et al. Multimodality treatment of mesenteric desmoid tumours. Eur J Cancer. 2008;44(16):2404–10.
Chugh R, Wathen JK, Patel SR, et al. Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res. 2010;16(19):4884–91.
O’Dea FJ, Wunder J, Bell RS, et al. Preoperative radiotherapy is effective in the treatment of fibromatosis. Clin Orthop Relat Res. 2003;415:19–24.
Goy BW, Lee SP, Eilber F, et al. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int J Radiat Oncol Biol Phys. 1997;39(3):659–65.
Crago AM, Denton B, Salas S, et al. A Prognostic Nomogram for Prediction of Recurrence in Desmoid Fibromatosis. Ann Surg. 2013;258(2):347–53.
Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol. 2011;29(26):3553–8.
Garbay D, Le Cesne A, Penel N, et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group(FSG). Ann Oncol. 2012;23(1):182–6.
Guadagnolo BA, Zagars GK, Ballo MT. Long-term outcomes for desmoid tumors treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;71(2):441–7.
Zlotecki RA, Scarborough MT, Morris CG, et al. External beam radiotherapy for primary and adjuvant management of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 2002;54(1):177–81.
Mullen JT, Delaney TF, Kobayashi WK, et al. Desmoid tumor: analysis of prognostic factors and outcomes in a surgical series. Ann Surg Oncol. 2012;19(13):4028–35.
Huang K, Fu H, Shi YQ, et al. Prognostic factors for extra-abdominal and abdominal wall desmoids: a 20-year experience at a single institution. J Surg Oncol. 2009;100(7):563–9.
Grignol VP, Pollock R, Howard JH, et al. Management of desmoids. Surg Clin North Am. 2016;96(5):1015–30.
Hartley JE, Church JM, Gupta S, et al. Significance of incidental desmoids identified during surgery for familial adenomatous polyposis. Dis Colon Rectum. 2004;47(3):334–40.
Overhaus M, Decker P, Fischer HP, et al. Desmoid tumours of the abdominal wall. World J Surg Oncol. 2003;1(1):11–5.
He XD, Zhang YB, Wang L, et al. Prognostic factors for the recurrence of sporadic desmoid-type fibromatosis after macroscopically complete resection: analysis of 114 patients at a single institution. Eur J Surg Oncol. 2015;41(8):1013–9.
Wilkinson MJ, Chan KE, Hayes AJ, et al. Surgical outcomes following resection for sporadic abdominal wall fibromatosis. Ann Surg Oncol. 2014;21(7):2144–9.
Isik A, Soyturk M, Süleyman S, et al. Correlation of bowel wall thickening seen using computerized tomography with colonoscopies: a preliminary study. Surg Laparosc Endosc Percutan Tech. 2017;27(3):154–7.
Couto Netto SD, Teixeira F, Menegozzo CAM, et al. Sporadic Abdominal Wall Desmoid type Fibromatosis: treatment paradigm after thirty two years. BMC Surg. 2018;18(1):37.
Abbonante F, Ribuffo D, Vitagliano T, et al. Abdominal desmoid tumors. A new reconstructive approach. Ann Hal Chir. 2015;86(1):78–84.
Fei Y, Li JY. Excision of giant desmoid in the abdominal wall, method of abdominal wall reconstruction, and follow-up of long-termed effect. Zhonghua Wai Ke Za Zhi. 2018;56(1):52–5.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (82072734).
Author information
Authors and Affiliations
Contributions
YJJ conceived and designed the project. HTH, WKL, and YML analyzed the data. YL and YTT revised the manuscript for important intellectual content. YJJ and XXS participated in the collection of patient information. All authors have reviewed and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for the study was obtained from the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences (NCC2023C-657). Informed consent was considered unnecessary for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, Y., Shao, X., Li, W. et al. Investigation of clinicopathological characteristics and prognosis of abdominal Desmoid tumors. Holist Integ Oncol 3, 20 (2024). https://doi.org/10.1007/s44178-024-00093-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00093-w