Abstract
Objective
To investigate the feasibility and safety of early removal of gastric tube and thoracic drainage tube after minimally invasive esophagectomy.
Methods
A retrospective analysis was performed on 93 patients undergoing thoraco-laparoscopic assisted esophagectomy between January 2022 and September 2022. All patients were divided into two groups, the early group and the conventional group. The differences in drainage volume on the first day after operation, days of thoracic drainage tube indwelling, thoracic drainage tube replacement, incidence rate of pleural effusion, atelectasis, pneumothorax as well as pulmonary infection, respiratory insufficiency, and postoperative hospital stay were compared between the two groups. The criteria for thoracic drainage tube removal is the daily thoracic drainage volume of less than 250 ml in the early group. The gastric fluid volume on the first day after operation, the number of days of gastric tube indwelling, the rate of gastric tube replacement after removal, and the time of intestinal exhaust were compared between the two groups. In the meantime, the incidence of complications, such as anastomotic leakage and pulmonary infection, was observed in the two groups.
Results
The removal time of gastric tube and thoracic drainage tube was significantly earlier in the early group than in the control group (P < 0.05). There was no significant difference in the gastric fluid volume and pleural fluid between the early group and the conventional group. P > 0.05), but there were significant differences in postoperative anastomotic leakage between the two groups (P < 0.05). There was no significant difference in the incidence of pleural effusion, atelectasis, pneumothorax, and pulmonary infection (P > 0.05), nor in thoracentesis rate and gastric tube replacement (P > 0.05). The postoperative hospital stay was significantly shorter in the early group than in the conventional group (P < 0.05)
Conclusion
The early removal of the thoracic drainge tube and gastric tube is safe and effective, which does not increase the incidence of postoperative complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Enhanced recovery after surgery (ERAS) refers to the adoption of a series of optimization measures for perioperative management, to reduce the physical and psychological traumatic stress of surgical patients, thereby accelerating the recovery of patients. At present, as traditional open surgery for esophageal cancer surgery has been replaced by minimally invasive esophagectomy (MIE), ERAS has been successfully implemented in the surgical treatment of esophageal cancer. Based on the concept of ERAS, this case–control study has confirmed the feasibility and safety of early removal of gastric tube and thoracic drainage tube after video-assisted thoracoscopic esophagectomy, and the results are reported as follows.
1 Data and methods
1.1 Clinical data
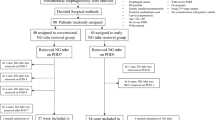
A total of 93 patients who underwent video-assisted (excluding robot-assisted) thoracoscopic resection of esophageal cancer in Shanghai Chest Hospital from January 2022 to September 2022 were selected.
Inclusion criteria were patients with esophageal cancer diagnosed by preoperative thoracic and abdominal CT, gastroscopy and pathology, absence of obvious distant metastasis, and absence of serious complications.
Exclusion criteria included persistent pulmonary leakage after operation, postoperative chylothorax, hemorrhagic thoracic fluid that was continuously drained after operation, the second operation in the postoperative perioperative period, postoperative gastric bleeding, open-chest operation converted from MIE, and perioperative death.
Patients were randomly assigned into two groups. One group was given early removal of thoracic drainage tube and gastric tube, which was set as early group (n = 31). The other group was given conventional extubation as conventional group (n = 62). There were no significant differences in gender, age, disease severity, surgical methods, intraoperative lymph node dissection, and postoperative pathological stage between the two groups (all P > 0.05), which were comparable (P > 0.05) (Tables 1 and 2).
1.2 Treatment methods
All cases underwent thoracoscopic esophagectomy, abdominal laparoscopy with free gastric incision for tubulized stomach or open abdominal surgery, and esophagogastric anastomosis in the neck. At the end of the operation, the chest anesthesiologist fully aspirated sputum, and the lung was expanded with positive airway pressure. A 32F silicone thoracic drainage tube was selected and placed in the 7th intercostal space of the right midaxillary line. The proximal medial thoracic hole was 2 cm away from the medial chest wall, and the distal end was connected with a water-sealed drainage bottle. In the meantime, a mediastinal ball was placed to connect to the negative pressure suction. After the cervical esophagogastric anastomosis was completed, the gastric tube and duodenal nutrition tube were placed at a depth of 15–20 cm below the anastomosis. On the next day after operation, both groups were given enteral nutrition via nasointestinal tube and encouraged to do off-bed activity. The next morning after surgery, bedside chest radiograph was performed to confirm sufficient lung distension. The amount, color and nature of drainage fluid were observed daily after operation. The criteria for thoracic drainage tube removal was the daily thoracic drainage volume of less than 250 ml and less than 100 ml in the early group and the conventional group respectively; X-ray examination was performed at 3–5 d after the removal of thoracic drainage tube in the two groups for pleural effusion, atelectasis and pulmonary infection. The differences in drainage volume on the first day after operation, the number of days of thoracic drainage tube indwelling, thoracic drainage tube re-placement, the incidence of pleural effusion, atelectasis, pneumothorax and pulmonary infection, respiratory insufficiency, and postoperative hospital stay were compared between the two groups. Criteria for gastric tube removal was removal that occurred within 16–72 h after operation in the early group regardless of intestinal exhaust, and the gastric fluid volume less than 30 ml per day in the control group after intestinal exhaust. The observation indexes were gastric fluid volume on the first day after operation, the number of days of gastric tube indwelling, the rate of gastric tube re-placement after removal, and the time of intestinal exhaust. In the meantime, the incidence of complications, such as anastomotic leakage and pneumonia, was observed in the two groups.
1.3 Statistical methods
SPSS 13.0 was used for statistical analysis. Measurement data are expressed as (x ± s) and t-test was used for data comparison. In addition, counting data were analyzed by Pearson χ2 test. P < 0.05 was considered statistically significant.
2 Results
There was no perioperative death in the two groups, and all patients recovered and discharged. The removal time of gastric tube and thoracic drainage tube in early group was significantly earlier than that in control group (P < 0.05). There was no significant difference in gastric fluid volume and thoracic fluid volume between the early group and the conventional group on the first day (P > 0.05). There was a significant difference in the incidence of anastomotic leakage between the two groups (P < 0.05). No significant differences were found in the incidence of pleural effusion, atelectasis, pneumothorax and pulmonary infection (P > 0.05), nor in thoracentesis rate and gastric tube replacement (P > 0.05). The postoperative hospital stay was significantly shorter in the early group than in the conventional group (P < 0.05) (Tables 3 and 4).
3 Discussion
The concept of fast track surgery (FTS) was proposed by Danish surgeon Kehlet in the 1990s [1], aiming to minimize the impact of surgery on the patient's body. This concept and model was first applied to colorectal surgery, and then widely used in other surgical fields [2, 3]. At present, with the change of surgical methods for esophageal cancer, MIE is mainly performed to replace the open thoracoabdominal incision for major trauma. MIE includes a variety of minimally invasive surgical methods aimed at reducing the trauma of esophageal surgery, with thoracolaparoscopic endoscopic esophagectomy as a typical representative. Regardless of the surgical method, the conventional placement of thoracic drainage tube and gastric tube becomes necessary and is adopted in all levels of hospitals and large medical centers. In the meantime, there is also “non-tube no fasting MIE” based on Li's Anastomosis, but due to the difficulty and safety of surgical technique, and long-term traditional concept of surgical treatment for esophageal cancer, it can not be effectively promoted in hospitals at all levels, even in some large medical centers, thus limiting its development.
The differences between this study and some previous studies are as follows: Before this prospective study, patients in the two groups had not been treated differently, such as preoperative bowel preparation for patients in one group, oral administration of glucose at 2–3 h before operation, which could appropriately reduce the incidence of postoperative insulin resistance without increasing the postoperative aspiration rate [4, 5]. In this study, gastric tube and thoracic drainage tube were removed early. This study can be applied in a large population, regardless of age, gender and preoperative induction therapy.
3.1 Early removal of thoracic drainage tube after esophageal cancer surgery
The purpose of placing thoracic drainage tube after MIE for esophageal cancer is to observe intrathoracic hemorrhage, pulmonary air leakage, and chylothorax, and to prevent the occurrence of atelectasis. However, the placement of thoracic drainage tube itself will also limit ventilation due to pain and affect early ambulation of patients [6, 7]. In the meantime, the thoracic drainage tube, as a foreign body in the chest, will stimulate the pleura to leak more fluid, increasing the chance of infection. Therefore, the time point of thoracic drainage tube removal is crucial to ensure the safety of patients after operation. The traditional indication for thoracic drainage tube removal is a 24-h drainage volume of less than 50 ml. With the rapid development of modern medical technology and the upgrading of medical equipment, the indication for extubation has been gradually expanded to be < 100 ml/d. Younes et al. [8] reported that it was safe to remove the thoracic drainage tube when the thoracic drainage fluid was < 200 ml/d after thoracotomy. McKenna et al. [9] reported that extubation was safe when the drainage was < 300 ml/d after video-assisted thoracoscopic surgery. Recently, several scholars believe that extubation is also safe when drainage fluid is < 450 ml/d after thoracotomy [10]. But not all of these studies were focused on esophagectomy. This study showed that removal of the thoracic drainage tube did not increase the risk of pleural effusion when the drainage volume of thoracic fluid was < 250 ml on a daily basis after MIE. In this study, there were 2 cases of pleural effusion in the early group after extubation, which was very small and cured by pleural absorption without treatment. Compared with the conventional group, there was no increase in complications such as pneumonia, atelectasis, and pulmonary infection due to early removal of thoracic drainage tube. Therefore, early removal of thoracic drainage tube after MIE is safe and feasible, which can significantly relieve the postoperative pain and discomfort, and shorten the postoperative hospital stay, thus benefiting patients.
3.2 Early removal of gastric tube after esophageal cancer surgery
Gastric tube and enteral nutrition tube should be placed through nose for gastrointestinal decompression and early enteral nutrition in patients with esophageal cancer after operation. The purpose of gastrointestinal decompression is to promote the recovery of gastrointestinal function, prevent postoperative abdominal distension, enable the gastrointestinal anastomosis to heal under hypotonic condition, reduce the occurrence of anastomotic leakage, and observe presence of bleeding at the site of anastomosis and tubular stomach at the early stage. However, because the two tubes are in the same nasal cavity, the patient's discomfort is aggravated. Some patients may be accompanied by nausea and discomfort, which also affects patients' cough and expectoration, and increases the possibility of epistaxis and pulmonary infection [11,12,13]. Currently, most data show that early removal of the gastric tube is beneficial to the recovery of patients undergoing surgery for lower gastrointestinal diseases. Studies on the application of ERAS in gastric surgery reported that no indwelling gastric tube after operation did not increase the incidence of anastomotic leakage and other complications [14, 15]. At present, several studies on the application of ERAS in esophageal cancer surgery revealed that the time of gastric tube removal was 3 days in most patients and 2 days in a few patients, which did not increase the incidence of anastomotic leakage [16, 17]. In the meantime, studies have also shown that early enteral nutrition after surgery can lead to reduced postoperative abdominal distension, faster recovery of intestinal function, shorter postoperative intestinal exhaust time, and reduced gastrointestinal pressure and postoperative anastomotic leakage. Based on these findings, in the ERAS group, the gastric tube was removed at 16–72 h after operation, that is, extubation before intestinal exhaust, which was different from the traditional extubation after intestinal exhaust. The results of this study showed that, the incidence of postoperative anastomotic leakage in the early group was significantly lower than that of the control group, which might be related to the following factors: first, early extubation promoted early ambulation and gastrointestinal peristalsis, which may reduce the influence of gastric dilation and acid retention on anastomotic fistula; second, early ambulation promotes sputum expulsion, reduces the incidence of pulmonary complications which was associated with anastomotic leakage; third, insufficient sample size; fourth, prospective randomized controlled trial are needed to confirm whether early extubation can help reduce leakage. However, the early extubation did not increase the incidence of postoperative anastomotic leakage in the current study. This suggests that early removal of the gastric tube before postoperative intestinal exhaust is safe and feasible.
In conclusion, early removal of thoracic drainage tube and gastric tube after MIE esophageal cancer surgery is safe and effective, which does not increase the incidence of postoperative anastomotic leakage and pulmonary infection. Due to the few restrictions in this study, it can be applied in a large population, which is worthy of popularization.
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Kehlet H, Wilmore DW. Fast-track surgery. Br J Surg. 2005;92(1):3–4.
Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–41.
Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322(7284):473–6.
Xie WK. Clinical application of fast track surgery in laparoscopic radical resection of colon cancer. Pract J Cancer. 2013;28(4):367–9.
Li Y. Effect of health education on esophageal fast track surgery. Chin J Pract Med. 2010;5(22):242–3.
Nomori H, Horio H, Suemasu K. Early removal of chest drainage tubes and oxygen support after a lobectomy for lung cancer facilitates earlier recovery of the 6-minute walking distance. Surg Today. 2001;31(5):395–9.
Refai M, Brunelli A, Salati M, et al. The impact of chest tube removal on pain and pulmonary function after pulmonary resection. Eur J Cardiothorac Surg. 2012;41(4):820–3. https://doi.org/10.1093/ejcts/ezrl26.
Younes RN, Gross JL, Aguiar S, et al. When to remove a chest tube? A randomized study with subsequent prospective consecutive validation. J Am Coll Surg. 2002;195(5):658–62.
McKenna RJ Jr, Mahtabifard A, Pickens A, et al. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. Ann Thorac Surg. 2007;84(5):1663–7.
Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg. 2008;135(2):269–73.
Mistry RC, Vijayabhaskar R, Karimundaekal G, et al. Effect of short-term vs prolonged nasogastrie decompression on major postesophagectomy complications: a parallel-group, randomized trial. Arch Surg. 2012;147(8):747–51. https://doi.org/10.1001/arehsurg.2012.1008.
Purl V, Hu Y, Guthrie T, et al. Retrograde jejunogastric decompression after esophagectomy is superior to nasogastric drainage. Ann Thorac Surg. 2011;92(2):499–503. https://doi.org/10.1016/j.athoracsur.2011.03.082.
Sato T, Takayama T, So K, et al. Is retention of a nasogastric tube after esophagectomy a risk factor for postoperative respiratory tract infection? J Infect chemother. 2007;13(2):109–13.
Feng F, Ji G, Li JP, et al. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol. 2013;19(23):3642–8. https://doi.org/10.3748/wig.v19.i23.3642.
Choi JW, Xuan Y, Hur H, et al. Outcomes of critical pathway in laparoscopic and open surgical treatments for gastric cancer patients: patients selection for fast-track program through retrospective analysis. J Gastric Cancer. 2013;13(2):98. https://doi.org/10.5230/jgc.2013.13.2.98.
Wang Q, Wu M, Shen G, et al. Application of fast track surgery in minimally invasive resection of esophageal cancer. Chin J Thorac and Cardiovasc Surg. 2013;29(1):34–353. https://doi.org/10.3760/ema.j.issn.1001-4497.2013.06.009.
You ZB, Xu DF, Ji J, et al. Application of concept of fast track surgery in the treatment of esophageal cancer. Chin J Gastrointest Surg. 2012;15(6): 561–563. (in Chinese). https://doi.org/10.3760/cma.j.issn.1671-0274.2012.06.012.
Acknowledgements
Thanks to all medical staff of departments of thoracic surgery , both in Shanghai Chest Hospital and The first hospital of Hebei Medical University, for data collection and analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Qiang Wang, HuiNing Liu and Xufeng Guo. The first draft of the manuscript was written by Qiang Wang and Xufeng Guo, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Shanghai Chest Hospital, School of medicine, Shanghai Jiao Tong University. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Liu, H. & Guo, X. A case–control study on early removal of thoracic drainage tube and gastric tube after MIE. Holist Integ Oncol 2, 28 (2023). https://doi.org/10.1007/s44178-023-00052-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-023-00052-x