Abstract
The role of inlet and outlet oxygenator pressure know as pressure drop (ΔP) in the elimination of the gaseous micro-emboli activity (GMEA) remains obscured during Extracorporeal Membrane Oxygenation (ECMO) and Minimally Invasive Extracorporeal Circulation (MiECC). There is conflicting literature on the role of pressure drop in oxygenators as well as on oxygenating performance, fluid dynamics and in the role of gaseous micro-embolic activity elimination. We analyzed 14 procedures, 10 MiECC with polypropylene fibers ALONE AF oxygenator and 4 ECMO with polymethylpentene Alone ECMO Oxygenator (Eurosets, SRL, Medolla,Italy), in the central and peripheral cannulation configuration, respectively, through the use of GAMPT BCC300 for air gas micro-emboli (GME) in relation to the ΔP of Oxygenator and incidence of cavitation. The groups of MiECC, Central Cannulation VS Peripheral Cannulation reported: mean values for cavitation n° of bubbles was 330 ± 12 vs 80 ± 13; 1.5 vs 0% reported a diameter > 500 μm; GME volume was 1.51 ± 0.2 vs 0.121 ± 0.2 (μL) in the arterial line, the mean ΔP of oxygenator reported was 89 ± 11 vs 130 ± 29 mmHg. The groups of ECMO, Central Cannulation VS Peripheral Cannulation reported: mean values for cavitation n° of bubbles was 470 ± 12 vs 140 ± 17; 2.1 vs 0% reported a diameter > 500 μm; GME volume was 2.5 ± 0.5 vs 0.355 ± 0.7 (μL) in the arterial line, the mean ΔP of oxygenator reported was 88 ± 15vs 150 ± 23 mmHg. In this report, patients with low pressure drop in the oxygenator reported a statistically significant increase in GMEA in the arterial line of the circuit for each cavitation phenomenon, compared to the group with high pressure drop in the oxygenator who reported a reduced GMEA. In this report, oxygenators with higher pressure drops demonstrated a significant reduction of GMEA, however, further studies are needed to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the realm of extracorporeal life support systems, the interplay between gaseous micro-embolic activity (GMEA) and pressure drop on oxygenators has become a subject of intense scrutiny. As medical advancements continue to push the boundaries of cardiovascular interventions, understanding the intricate dynamics between these phenomena is paramount for optimizing the performance and safety of oxygenation devices [1]. GMEA, characterized by the presence of tiny gas bubbles circulating within the bloodstream, has been implicated in various clinical scenarios [2].
Cavitation in extracorporeal technologies, particularly those employing centrifugal pumps, can occur due to low inlet pressures. This can lead to the formation of air bubbles, causing damage to blood cells and generation of GMEA [3]. Proper monitoring and material selection are essential to prevent cavitation and maintain optimal extracorporeal technologies performance as ECMO and MiECC. Notably, its correlation with pressure drop across oxygenators holds significant implications for the efficacy of oxygen exchange in extracorporeal membrane oxygenation (ECMO) and cardiopulmonary bypass (CPB) systems [4]. This article delves into the complex relationship between GMEA and pressure drop, aiming to unravel the physiological intricacies and clinical ramifications [5]. In this prospective study we analyzed the polypropylene ALONE AF oxygenator during MiECC and the polymethylpentene Alone ECMO Oxygenator (Eurosets, SRL, Medolla,Italy) on ECMO, we seek to contribute to the evolving understanding of these phenomena and pave the way for improved technologies in the field of cardiovascular support.
Materials and Methods
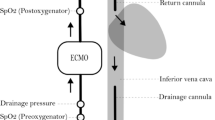
Preoperative data included patient demographic characteristics, ventricular ejection fraction, comorbidities, baseline Hb, logistic European System for Cardiac Operative Risk Evaluation II score and surgical procedures (Table 1). Perioperative data included: Cardiopulmonary Bypass (CPB) duration, nadir body temperature during CPB, nadir Hct and Hb values, RPM of the pump, Blood flow, negative pressure in the inlet of pump and central venous pressure (Table 2). We analyzed 14 procedures, 10 MiECC with polypropylene fibers ALONE AF oxygenator and 4 ECMO with polymethylpentene Alone ECMO Oxygenator (Eurosets, SRL, Medolla,Italy) (Fig. 1), with central and peripheral cannulation configuration.
Cavitation and GME Measurements
In our clinical practice, we have identified the phenomenon of cavitation during the procedures by identifying [3]:
-
0 or < 1 Liter (L/min) Blood flow (BF)/Pump RPM > 2500.
-
Negative pressure less > 200 (-mmHg).
-
Visive Chattering of venous tube and cannula.
-
Mean Arterial pressure less < 50 (mmHg).
-
Generation and delivery of GMEA before and after pump.
The GAMPT BCC300 for GMEA evaluation was used, the ultrasounds probes were positioned in venous line, in the pump outlet and in arterial line, for measurement and recording of the number of gaseous micro-emboli (n°), the diameter of GME (μm), and the volume (μL) (Fig. 2), in relation to the pressure Drop (ΔP) of Oxygenator (mmHg) and incidence of cavitation.
MiECC Components
The MiECC type I circuit (MiECTIS classification) was used with Bio-console 560 (Medtronic Bio-Medicus, Inc., Eden Prairie, MN) with the AP40 Affinity CP centrifugal pump (Medtronic Bio-Medicus, Inc., Eden Prairie, MN, USA) and polypropylene (PPT) Oxygenator alone AF (Eurosets, Medolla, Italy) (Fig. 3).
ECMO Components
The ECMOLIFE console (Eurosets, Medolla, Italy) with the ECMOLIFE centrifugal pump (Eurosets, Medolla, Italy) and polymethylpentene (PMP) Oxygenator ECMO alone AF (Eurosets, Medolla, Italy) were used (Fig. 3).
Central Cannulation
The central venous Cannulation was made with 32/40 Fr atrio-caval (Medtronic, Inc., Eden Prairie, MN, USA). The arterial cannulation was made with arterial cannula EOPA 24 Fr (Medtronic, Inc., Eden Prairie, MN, USA).
Peripheral Cannulation
The peripheral cannulation was made with RAP femoral venous cannula 22–23/25 Fr (Livanova, London,UK). The arterial cannulation was made with arterial cannula biomedicus 19 Fr (Medtronic,Biomedicus, Inc., Eden Prairie, MN, USA).
Statistical Analysis
Continuous data are expressed as a mean ± standard deviation or a median with the interquartile range, and categorical data are expressed as percentages. All reported p values are two-sided, and p values of < 0.05 were considered to indicate statistical significance. All statistical analyses were performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
All patients during the procedures had a means values from a minimum of one to a maximum of twelve cavitation phenomena during extracorporeal procedures, in particular the low pressure drop of oxygenator contributed to increase the gaseous micro-emboli activity in terms of volume and number of micro-emboli in the arterial line in relation to cavitation greater than peripheral cannulation. The groups of MiECC, 5 Central Cannulation VS 5Peripheral Cannulation reported: mean values for cavitation n° of bubbles was 336 ± 5 vs 296 ± 16, 6.4% vs 6.01 reported a diameter > 500 μm, GME volume was 5.9 ± 2 vs 5.2 ± 2 (μL) in the venous inlet line of the pump; n° of bubbles was 900 ± 33 vs 790 ± 21, 3.3 vs 3.2% reported a diameter > 500 μm; GME volume was 5.11 ± 0.3 vs 4.93 ± 0.3 (μL) in the outlet line of centrifugal pump; n° of bubbles was 330 ± 12 vs 80 ± 13; 1.5 vs 0% reported a diameter > 500 μm; GME volume was 1.51 ± 0.2 vs 0.121 ± 0.2 (μL) in the arterial line, the mean ΔP of oxygenator reported was 89 ± 11 vs 130 ± 29 mmHg. The groups of ECMO, 2 Central Cannulation VS 2 Peripheral Cannulation reported: mean values for cavitation n° of bubbles was 329 ± 5 vs 280 ± 39, 7.2% vs 6.5 reported a diameter > 500 μm, GME volume was 6.1 ± 3 vs 5.9 ± 3 (μL) in the venous inlet line of the pump; n° of bubbles was 1100 ± 50 vs 920 ± 29, 3.8 vs 3.6% reported a diameter > 500 μm; GME volume was 6.21 ± 0.3 vs 5.95 ± 0.7 (μL) in the outlet line of centrifugal pump; n° of bubbles was 470 ± 12 vs 140 ± 17; 2.1 vs 0% reported a diameter > 500 μm; GME volume was 2.5 ± 0.5 vs 0.355 ± 0.7 (μL) in the arterial line, the mean ΔP of oxygenator reported was 88 ± 15vs 150 ± 23 mmHg (Table 2).
Discussion
The observed correlation between high pressure drop in the oxygenator during MiECC and ECMO and the reduction of gaseous micro-emboli raises important implications for clinical practice. This finding suggests a potential avenue for optimizing perfusion strategies to enhance patient outcomes.
The reduction in gaseous micro-embolic activity may contribute to improved cerebral and systemic perfusion, which could be particularly beneficial in scenarios where micro-emboli-related complications are a concern [6, 7]. Further research is warranted to elucidate the specific mechanisms behind this correlation and to validate its reproducibility in diverse patient populations. It's essential to acknowledge the limitations of our study, such as the retrospective nature and the need for prospective trials to establish causation definitively. Additionally, exploring the long-term effects of reduced GMEA on clinical outcomes would be valuable. In conclusion, our findings open avenues for refining perfusion strategies during MiECC and ECMO, with the potential to enhance patient safety and outcomes [8]. Future research endeavors should focus on elucidating the underlying mechanisms and conducting prospective studies to validate these observations in different clinical settings. Cavitation in the context of ECMO refers to the formation and collapse of small vapor-filled bubbles in the blood as it passes through the pump [3]. When the pressure drops too low, bubbles can form, and when pressure rises again, these bubbles collapse, potentially causing damage to blood cells and the pump itself. To prevent this, maintaining adequate pressure across the oxygenator is crucial, as it helps mitigate the risk of air microemboli, which can be harmful to the patient. Proper management of pressure differentials is essential for the safe and effective operation of the ECMO system [7]. Several hypotheses can explain how a high pressure drop in the oxygenator reduces gaseous micro-emboli: (1) Degassing Hypothesis: The increased pressure drop promotes efficient degassing of blood, causing dissolved gases, including microbubbles, to escape. This reduces the likelihood of micro-emboli formation [9]. (2) Bubble Coalescence Hypothesis: High pressure drop may encourage smaller bubbles to merge into larger, less harmful bubbles. This coalescence can make the bubbles less likely to cause micro-emboli-related issues [10]. (3) Enhanced Gas Exchange Hypothesis: A more significant pressure drop enhances the efficiency of gas exchange in the oxygenator, minimizing the presence of gas bubbles in the bloodstream and decreasing the potential for micro-emboli formation [11, 12]. (4) Improved Bubble Removal Hypothesis: The pressure drop may facilitate better removal of gas bubbles within the oxygenator, preventing their passage into the patient's circulatory system [13, 14]. These hypotheses collectively suggest that optimizing pressure conditions in the oxygenator plays a crucial role in reducing the risk of gaseous micro-emboli and their associated complications [15]. Acknowledging the study limitations is crucial. The relatively low number of cases and the reliance on a single oxygenator model highlight the need for cautious interpretation of the results. The generalizability of our findings may be limited by the specific characteristics of the patient cohort and the chosen oxygenator. Expanding the study to include a larger and more diverse sample, encompassing various oxygenator models, would strengthen the external validity of our observations. This would provide a more comprehensive understanding of the relationship between pressure drop and gaseous micro-embolic reduction across different clinical scenarios. Despite these limitations, our study serves as a valuable initial exploration of the potential correlation. It lays the groundwork for future investigations with a broader scope, emphasizing the importance of collaborative efforts within the research community to corroborate and build upon these preliminary findings.
Conclusion
In this report, patients with low pressure drop in the oxygenator reported a statistically significant increase in GMEA in the arterial line for each cavitation phenomenon, compared to the group with high pressure drop in the oxygenator who reported a reduced GMEA. In this report, oxygenators with higher pressure drops demonstrated a significant reduction of GMEA, however, further studies are needed to confirm these results.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Y.M. Ganushchak, E.P. Körver, J.G. Maessen, Is there a “safe” suction pressure in the venous line of extracorporeal circulation system? Perfusion 35(6), 521–528 (2020)
J.B. Atkinson, P. Emerson, R. Wheaton, C.M. Bowman, A simplified method for autoregulation of blood flow in the extracorporeal membrane oxygenation circuit. J. Pediatr. Surg. 24(3), 251–252 (1989)
I. Condello, M. Basile, G. Speziale, Theoretical considerations on cavitation during microaxial left ventricular assist device. Ann. Thorac. Surg. S0003–4975(22), 00542–00552 (2022)
A.R. Fisher, M. Baker, M. Buffin, P. Campbell, S. Hansbro, S. Kennington, A. Lilley, M. Whitehorne, Normal and abnormal trans-oxygenator pressure gradients during cardiopulmonary bypass. Perfusion 18, 25–30 (2003)
G.J. Myers, P.R. Weighell, B.J. McCloskey, A.M. Holt, S. McTeer, S.L. Maxwell, A multicenter investigation into the occurrence of high-pressure excursions. J Extra Corpor Technol 35, 127–132 (2003)
A. Ündar, W.R. Owens, M.C. McGarry, D.L. Surprise, V.D. Kilpack, M.W. Mueller, E.D. McKenzie, J.C.D. Fraser, Comparison of hollow-fiber membrane oxygenators in terms of pressure drop of the membranes during normothermic and hypothermic cardiopulmonary bypass in neonates. Perfusion 20, 135–138 (2005)
R. Izumi, To reduce complications in pediatric extracorporeal circulation. Jpn. J. Extra Corpor. Technol. 46, 274 (2019). (In Japanese)
S. Iwaki, S. Tani, Y. Yoshida, H. Taka, Why do the excessive pressure drops in pediatric membrane oxygenator not decrease? Jpn. J. Extra Corpor. Technol. 46, 283 (2019). (In Japanese)
K. Sakai, M. Yanagisawa, N. Hosoya, T. Ohmura, M. Sakagami, K. Kuwano, H. Nakanishi, Comparison of oxygenation and flow characteristics of inside and outside blood flow membrane oxygenators. Artif. Organs Today 3, 57–80 (1993)
N. Matsuda, K. Sakai, Blood flow and oxygen transfer rate of an outside blood flow membrane oxygenator. J. Membr. Sci. 170, 153–158 (2000)
N. Matsuda, K. Sakai, K.-I. Yamamoto, H. Iwasaki, Effects of hollow fiber packing fraction on blood flow pattern and gas transfer rate of an intravascular oxygenator (IVOX). J. Membr. Sci. 179, 231–241 (2000)
A. Funakubo, I. Taga, J.W. McGillicuddy, Y. Fukui, R.B. Hirschl, R.H. Bartlett, Flow vectorial analysis in an artificial implantable lung. ASAIO J. 49, 383–387 (2003)
G. Catapano, Turbulent flow technique for the estimation of oxygen diffusive permeability of membranes for the oxygenation of blood and other cell suspensions. J. Membr. Sci. 230, 131–139 (2004)
J. Zhang, T.D. Nolan, T. Zhang, B.P. Griffith, Z.J. Wu, Characterization of membrane blood oxygenation devices using computational fluid dynamics. J. Membr. Sci. 288, 268–279 (2007)
H. Tabesh, G. Amoabediny, A. Poorkhalil, A. Khachab, A. Kashefi, K. Mottaghy, A theoretical model for evaluation of the design of a hollow-fiber membrane oxygenator. J. Artif. Organs 15, 347–356 (2012)
Funding
None.
Author information
Authors and Affiliations
Contributions
IC analyzed the data and wrote the original draft. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Ignazio Condello is Consultant for Eurosets SRL, Medolla, Italy.
Review Involving Human and Animal Participants
This study was conducted according to the guidelines of the Declaration of Helsinki. The GVM Care and Research review board approved the study (internal protocol; decision 24 August 2018) and need for patient consent was waived due to the retrospective nature of the study.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Condello, I. Exploring the Nexus: Gaseous Micro-embolic Activity and Pressure Drop on ALONE ECMO Oxygenator. Biomedical Materials & Devices (2024). https://doi.org/10.1007/s44174-024-00173-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44174-024-00173-5