Abstract
Extensive bone defects can not be repaired with traditional porous blocks composed of graft ceramics concerning the low strength of the materials. In this way, zirconia porous blocks become alternative bone graft material for repairing of extensive bone sites considering their physical properties. The main aim of this study was to evaluate the compressive strength and morphological aspects of porous zirconia blocks embedded with platelet rich fibrin for enhanced bone healing. Porous blocks composed of yttria-stabilized zirconia polycrystals (Y-TZP) were manufactured by the replica method using a polyurethane sponge. Specimens were submitted to a heat treatment at 1 °C/min up to 1500 °C for 120 min. Half of specimens were embedded with an injectable platelet rich fibrin (i-PRF). On i-PRF, harvested blood was immediately centrifuged by using a high-quality table centrifuge at 2700 rpm (408 g) and at room temperature for 3 min. Random specimens were prepared for morphological analyses by optical and scanning electron microscopy. Groups of specimens were mechanically assessed by compressive strength and nano-indentation tests. Porous structures composed of (Y-TZP) revealed high strength values even though interconnected pores had large dimensions. The size of pores was proper for cell migration, bone ingrowth, and angiogenesis. The incorporation of platelet rich fibrin promoted an increase in compressive strength of the porous YTZP structure. However, a decrease in strength of the porous structures was noted with the increase of number and size of pores. Fragile porous zirconia blocks can reveal an adequate strength for surgical handling and tissue healing at extensive bone repair. Also, the incorporation of further bioactive absorbable materials such as platelet rich fibrin increases the strength of the porous structures. The combination of zirconia porous blocks and platelet rich fibrin can enhance the bone healing leading to low risks of clinical issues.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zirconia has been developed for biomedical applications regarding desired properties such as: strength, fracture toughness, chemical stability, and high biocompatibility [1,2,3,4,5]. Zirconium dioxide is doped with metal oxides such as yttria (Y2O3), magnesium oxide and calcium oxide, to stabilize tetragonal phase. Yttria stabilized zirconia polycrystals (Y-TZP) has an elastic modulus at 240–270 GPa, flexural strength at 1200 MPa, fracture toughness at 8 MPa.m1/2, and a high biocompatibility [6,7,8]. In the field of dentistry and orthopaedics, zirconia implants are used for bone repairing covered or not with bioactive materials for enhanced cell stimulation and mechanical properties [1, 3, 4]. Nevertheless, the use of porous blocks composed of zirconia can become a challenge concerning the pores’ network and dimensions associated with adequate mechanical properties. Dimensions, number, and interconnectivity of pores significantly influence the cell behavior, angiogenesis, and bone ingrowth into the porous structure [9]. Effective flow and transportation of nutrients through the interconnected pores enables the bone tissue growth considering that an increased surface area leads to higher bonding to host tissues [6, 10]. However, a high porosity and pores’ size of the scaffold or porous implant affect their mechanical properties. A scaffold or porous implant should withstand loading during handling and surgical procedures for bone tissue healing preventing fractures that could compromise the bone ingrowth process [7]. A balance between the desired mechanical and biological functions can be established by controlling the porosity and selection of biomaterials [10].
On large bone defects, bone substitutes such as blocks or particulate materials composed of bone-derived grafts or bioactive ceramics can be combined with collagen, blood, or blood platelet-rich products such as platelet rich fibrin (PRF) [11,12,13,14,15]. For instance, Leukocyte- and Platelet-rich fibrin (L-PRF) clots are manufactured by centrifuging blood in silica-coated tubes and in the presence of a physiological amount of fibrinogen and thrombin leading to a natural cross-linking of fibrin [16,17,18,19,20]. That results in a fibrin matrix, embedding leukocytes, platelets, cytokines, growth factors, and stem cells [16,17,18,19,20]. Nowadays, different types of protocols and centrifuges have been assessed in previous studies resulting in a broad group of PRF as follow: Leukocyte- and Platelet-rich fibrin (L-PRF); advanced Platelet-rich fibrin (A-PRF); injectable Platelet-rich fibrin (i-PRF); titanium prepared Platelet-rich fibrin (T-PRF); platelet-rich lysate (PRF-L). An injectable PRF, namely i-PRF, can be produced by changing the spin centrifugation forces, and using non-glass centrifugation tubes resulting in a slowly cross-linking of fibrin [11, 15, 21, 22]. Thus, i-PRF is a low-viscosity or flowable PRF which acts as an autologous fibrin binder to agglomerate particulate bone graft materials or blocks [11, 12]. The flowable i-PRF can be clinically handled with syringes considering its low-viscosity [15, 21, 23]. The flowable i-PRF is also rich in leukocytes, fibrinogen, platelets, fibrin, and growth factors as reported by previous studies [11, 23]. The mixture of chopped PRF membranes, i-PRF, and particulate graft or blocks results in manufacturing a bioactive composite block for enhanced bone healing [12, 15, 23]. Adequate mechanical properties and biological response can be achieved by involving PRF and porous blocks composed of biocompatible ceramics such as hydroxyapatite and zirconia.
To the best of our knowledge, there is a lack of studies on the effects of PRF on the mechanical behaviour of porous zirconia blocks or implants. Thus, the aim of this study was to evaluate the compressive strength and morphological aspects of porous zirconia blocks embedded with an injectable and flowable platelet rich fibrin for enhanced bone healing. In this way, the present study brings two significant novel approaches: the combination of an injectable platelet rich fibrin and zirconia blocks; the mechanical assessment of porous zirconia blocks in combination with platelet rich fibrin. It was hypothesized that an injectable platelet rich fibrin can increase the strength and surgical handling of porous zirconia blocks for bone healing.
Materials and Methods
Preparation of Zirconia Blocks
Yttria-stabilized zirconia polycrystals (Y-TZP) spray-dried powder (TZ-3YSB-E™, TOSOH Co., Tokyo, Japan) was assessed in this study. Y-TZP properties are shown in Table 1. According to the supplier, the powder has a maximum particle size of 120 µm and spherical agglomerates with an average size of 60 μm. The suspension was prepared by mixing 20 ml distilled water and 30 g zirconia powder. Then, the mixture was milled in a planetary ball mill (PM100, Retsch) at 400 rpm for 80 min to promote a powder deagglomeration and slurry homogenization. Afterwards, 1 wt% dispersant (Duramax 3005) was added to 60 wt% of Y-TZP powder achieving the desired pseudoplastic and thixotropic rheological behaviour.
On the development of an optimum combination of approaches, two groups of porous blocks were manufactured for embedment or not with an injectable and flowable PRF. Forty zirconia blocks were produced using polyurethane (PU) sponges of 60 ppi (10 × 10 mm) as temporary templates. Half of specimens were solely composed of zirconia (TZ-3YSB-E) which was the control group. Polyurethane sponges were manually soaked with the ceramic suspension without clogging the pores and kept in a stove at 100 °C for 20 min. A thermal treatment at 1 °C/min for 120 min was performed up to 1500 °C using a traditional laboratory high-temperature furnace to eliminate the PU sponge and pre-consolidate the scaffold [31]. At the end of the thermal treatment, fully sintered monolithic Y-TZP porous blocks were produced.
Preparation the Injectable and flowable Platelet-Rich Fibrin
Peripheral venous blood was harvested from four healthy, non-smoker, men volunteers (30–34 years old) (Fig. 1A). Volunteers with a history of using anti-coagulant or anti- inflammatory drugs within the previous 4 weeks were excluded from this study. Biochemical analyses were previously performed on the following markers: glucose, creatinine, total cholesterol, urea, triglycerides, uric acid, ALT/TGP, albumin, total proteins, and globulin. Volunteers who revealed any abnormality in the biochemical pattern results were also excluded. A total of 78 mL blood was harvested into 8 tubes within 18 mL into 6 plastic tubes without anticoagulants or silica coating (VACUETTE™, Greiner Bio-one GmbH, Kremsmünster, Austria) while 18 mL blood was harvested into 6 silica-coated tubes (BD Vacutainer Serum Becton Dickinson, Franklin Lakes, NJ, USA). The use of human blood was approved by the Human Research Ethics Committee at the Federal University of Santa Catarina (CEPSHUFSC), cod. 2229.085, that is in accordance with the Helsinki declaration of 1964. The volunteers signed the informed consent prior to the start of the study since the purpose of the study was described.
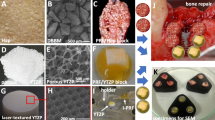
Preparation of PRF/zirconia blocks. A Blood harvesting from the volunteer and B centrifugation. C Tubes containing three different phases of the centrifuged blood. D, E L-PRF harvesting. F Preparation of L-PRF membranes and fragments. G Preparation and harvesting an injectable PRF (i-PRF). H Porous zirconia block. I Porous zirconia embedded with L-PRF and i-PRF. Schematics on the placement at J the surgical site for bone healing around dental implants
The harvested blood was immediately centrifuged by using a high-quality table centrifuge (IntraSpin™, IntraLock Systems, USA) at 2700 rpm (408 g) and at room temperature for 12 min, according to the L-PRF guidelines (Fig. 1B). After centrifugation in silica-coated tubes, three phases were identified: red blood corpuscles at the bottom of the tube, platelet-poor plasma (PPP) on the top, and an intermediate layer named clot (yellow layer) where most leucocytes and platelets are concentrated (Fig. 1C). Fibrin clots were harvested from the tubes using tweezers and the red blood cells were separated from the fibrin clot by using a periosteal elevator. Red blood corpuscles and PPP were discarded and then a yellow PRF clot was harvested from the middle of each tube (Fig. 1D). PRF clot was then compressed into membranes using a proper apparatus (Xpress kit™, IntraLock Systems, USA) to avoid any damage to the cells (Fig. 1F). The PRF membranes were cut using a surgical trephine and divided into smaller cylindric fragments with 1 × 1 mm.
Also, 3 plastic tubes were centrifuged at 2700 rpm for only 3 min, according to the i-PRF guidelines (Fig. 1B). After centrifugation, 3 plastic tubes were removed from the centrifuge and then the injectable PRF (yellow and fluid phase) was immediately harvested from the plastic tubes using plastic syringes (Fig. 1G) and injected into the porous zirconia specimens (Fig. 1H) towards the total immersion of the specimens. Then, ten L-PRF fragments were mixed with the injectable PRF surrounding the zirconia specimens (Fig. 1I). The zirconia specimens immersed in i-PRF were stirred gently for 10 s (Fig. 1I). Zirconia blocks embedded with i-PRF can also be seen in Fig. 2.
Mechanical Assessment
Cross-sectioned specimens embedded in epoxy resin were assessed by using a micro- and nano-scale indenter (NanoTest, MicroMaterial Limited, Wrexham, UK). The equipment has a diamond indenter on a Berkovich pyramidal tip (100 nm diameter). Load-depth curves were recorded at load rate of 5 N/s and at a maximum load up to 500 mN. Hardness and Young’s modulus were determined on the struts of the zirconia blocks.
Other groups of specimens were evaluated by compressive strength tests at a room temperature of 23 °C and at a crosshead speed of 1 mm/ min using a universal testing machine (Instron 8874, MA, USA), as seen in Fig. 2A. Forty specimens (n = 20) were then vertically positioned on the testing machine base and subjected to axial compressive loading up to fracture. The mean values of compressive strength were recorded using the Trapezium software (Shimadzu Corporation, Japan) and the compressive strength values were calculated from the equation F/pr2, where F is the maximum load at fracture and r the radius of the cylindrical specimen.
Morphological Analyses
Specimens were randomly divided into groups of ten specimens involving or not compressive testing. Specimens were placed in a 12-well culture plate for fixation in a solution containing 2.5 glutaraldehyde for 10 min and then washed in distilled water. The dehydration of specimens was achieved by immersing in a gradually series (25, 50, 75, 95, and 100%) of ethanol solutions for 5 min. Specimens were sputter-coated with a thin layer of AuPd film for scanning electron microscopy (SEM). The surface topography of specimens was analyzed by using a SEM unit (LEICA SEM-S360™, Cambridge, UK) coupled to energy dispersive spectroscopy (EDS). SEM analyses were performed at 15 kV on secondary electron (SE) and backscattering electron (BSE) mode.
On the other hand, ten dehydrated zirconia blocks mixed or not with PRF were embedded into autopolymerizing polyether modified resin (Technovit 400™; Kulzer GmbH, Germany) and then cross-sectioned at 90 degrees relative to the plane of zirconia surface. Surfaces were wet ground down to 2400 Mesh using SiC abrasive papers and then polished with 1 µm Al2O3 particles. Then, surfaces were ultrasonically cleaned in isopropyl alcohol for 10 min and then in distilled water for 10 min. At first, cross-sectioned specimens were inspected by optical microscopy at magnification ranging from × 10 up to × 500. Microstructural analyses were performed using an optical microscope (Leica DM 2500 MTM; Leica Microsystems, Germany) connected to a computer for image processing, using Leica Application Suite™ software (Leica Microsystems, Germany). Three micrographs were acquired at × 500 magnification on different regions for each specimen (n = 30). The software Adobe Photoshop™ (Adobe Systems Software, Ireland) was used to analyze black and white images, with the black regions representing the pores and the white regions representing the material struts. Image J™ software (National Institutes of Health, USA) was used to quantify the porosity percentage of the cross-sections. Then, surfaces were sputter coated with a AgPd thin layer for SEM analyses (JSM-6010 LV™, JEOL, Japan) coupled to energy dispersive spectroscopy (EDS). The morphological aspects and pores of the specimens were evaluated at high magnification ranging from × 1000 up to × 20,000 on (SE) secondary and (BSE) backscattered electrons.
Statistical Analysis
Results were statistically analyzed by normality test Shapiro–Wilk and two-way ANOVA to determine statistical differences in the compressive strength values between groups. The t student test was used to compare the compressive strength results of zirconia embedded or not with PRF. A probability value < 0.05 was considered significant. The power analysis performed by t student test or ANOVA, to determine the number of specimens for each group (n), revealed a test power of 100% in the present study. Statistical analyses were carried out using Origin Lab statistical software (Origin Lab, Northampton, MA, USA).
Results
As seen in Fig. 3, images acquired by optical microscopy revealed the size of the pores and the presence of PRF. The diameter of the pores ranged from 70 up to 300 µm as seen in Fig. 3B and D, while the mean thickness of the zirconia struts ranged from 20 up to 90 µm. The mean value of the pores’ diameter was measured at 171 µm. The porosity of the zirconia free of PRF was recorded at 89 ± 6.4%. However, PRF filled most of the zirconia pores as seen in the reddish regions in Figs. 3C and D). The porosity of the zirconia embedded with PRF was recorded at 33 ± 4.6%. % that indicated the filling of the porous structure. Details of the morphological aspects of the zirconia blocks embedded with PRF are shown in Fig. 4.
SEM images of a porous zirconia block embedded or not with a flowable and injectable PRF (i-PRF). Porous zirconia block at A × 50 and B × 2,000 magnification). Porous zirconia block embedded with a flowable and injecatble PRF at C × 50 and D × 2,000 magnification). E, F Fibrin filaments and leukocytes covering the surface of the zirconia (× 2000 magnification)
On the microscopic analyses of the zirconia blocks, it should be highlighted that the sponge format has been reproduced without significant defects on the zirconia struts. As seen in Fig. 4A, SEM images reveals the network of the struts leading to an interconnected pores’ structure. The surface of the zirconia struts also revealed pores at micro-scale (Fig. 4B). In Fig. 4C–F, PRF components were detected covering the zirconia surface and embedding the porous structure. A dense fibrin network was the major component of the PRF as noted in Fig. 4D and F. Leukocytes and platelets were entrapped in the fibrin mesh (Fig. 4E and F).
On nano-indentation tests, the zirconia struts revealed a Young’s modulus at around 88 ± 8.6 GPa while the hardness was recorded at 13.8 ± 3.6 GPa. Mean values of the compressive strength of the porous zirconia blocks embedded or not with PRF are shown in Fig. 5. The t student test confirmed there were differences in the strength mean values between groups regarding the incorporation of PRF (p < 0.05). The mean values of compressive strength of zirconia blocks free of PRF was recorded at around 0.28 ± 0.06 MPa while the zirconia embedded with PRF showed higher compressive strength of 0.42 ± 0.05 MPa as seen in Fig. 5. A lower standard deviation was noticed for the group embedded with PRF when compared to the group free of PRF.
Zirconia specimens were morphologically evaluated by SEM after compressive strength testing (Fig. 6). A catastrophic fracture occurred on the porous zirconia specimens considering the macro- and micro-scale pores are regions of stresses concentration. However, the presence of PRF into the pores increased the compressive strength of the zirconia/PRF blocks (Fig. 5). The fracture zone of the porous zirconia embedded with PRF is shown in Fig. 7. A dense coating of PRF was noticeable although there was a strength threshold of the hybrid material. Cracks were detected at the fibrin matrix of the PRF along the porous zirconia, as seen in Fig. 7C and D.
Discussion
The morphological aspects and strength of porous zirconia was assessed in this study regarding the incorporation of a flowable injectable platelet rich fibrin (PRF). The results of the present study support the hypothesis of the present work since differences in compressive strength between groups were detected. In fact, the incorporation of a flowable injectable PRF allowed the filling of the porous network of zirconia leading to an increased strength of the zirconia/PRF block. The morphological aspects of the zirconia filled with PRF revealed a dense fibrin network entrapping red blood cells, lymphocytes, and platelets.
In this study, monolithic zirconia blocks (free of PRF) showed a high porosity and the diameter of pores were proper for the angiogenesis, cell response, and bone healing. On the zirconia block manufacturing, the polyurethane sponge pattern was reproduced without significant defects within the zirconia struts. The proportion of interconnected pores and pores’ dimensions of the zirconia depend on some factors such as polyurethane pattern, zirconia powder, and thermal processing. As the number of sintering cycles increases, the size and number of pores decreases. Specifically, porosity can decrease from around 90% down to 70% when the sintering cycles increases from 1 up to 7. Specimens with a higher porosity have shown well inter-connected pores, while specimens with low porosity revealed pores with limited interconnectivity. Clogged pores can appear in the porous structures treated with 5 sintering cycles. Previous studies reported that a porosity above 70% is beneficial to osteogenic differentiation and therefore a macro-scale pore size ranging from 100 up to 400 μm promotes cell ingrowth and angiogenesis into the porous structure [1,2,3,4, 7, 8, 10, 24,25,26,27,28]. Also, the size of the pores enhances the transferring of nutrients and oxygen among the osteogenic cells. Pores at macro-scale dimension (1–50 μm) provide an increase in the wettability of the porous structure as well as the adhesion of proteins and cells [1, 26] [29]. Blood fluid flow, cell migration, and angiogenesis also depend on the interconnectivity of the pores at different macro- and micro-scale dimensions thereby establishing a 3D-vascular network [10, 12, 25, 30]. In vivo studies reported a faster bone formation into porous structures with a higher porosity. New bone ingrowth started by lining the surfaces and gradually filling the entire pore volume from the periphery of the porous structures towards the core [7, 24, 27]. Radiographic examination showed clear boundaries of surrounding bone to zirconia interfaces. After healing time, the bone to zirconia porous region reveal a transition zone due to the gradual deposition and ingrowth of bone tissue [1, 24].
The control of the elastic modulus and wettability of zirconia can be achieved with the manufacturing of zirconia porous structures. Thus, a zirconia with a high porosity (~ 85%) still maintain a moderate compressive strength even though the standard deviation can be higher than that recorded for a zirconia with low porosity [4, 7]. Biocompatible ceramic scaffolds support the in vitro and in vivo cell growth although the mechanical properties for extensive bone repair is still a challenge. In this study, the compressive strength the porous zirconia and elastic modulus of struts achieved mean values as reported by previous studies [31, 32]. The 3-D open cell (interconnected pores) structures show the most interesting design for bone tissue healing due to their similar structure to the trabecular bone. For instance, the spongy shape of the trabecular bone can be acquired via the replica method to manufacture zirconia foams with different porous design [33]. Furthermore, recent developments in computer-aid design (CAD) and rapid prototyping methods have become a feasible solution since the 3D-design can be carefully planned at macro- and micro-scale dimensions prior to the manufacturing of porous structures or mesh implants [9, 27, 33]. Free-form fabrication, which uses a 3D-printing principle, is an effective method to control pores’ architecture (size, shape, and interconnectivity) of porous structures for specific clinical applications [9]. Previous experimental studies on the bone response to different ceramic materials have shown results revealing not only the effects of the material’s chemical composition but also the effects of the 3-D design on the biological and mechanical response of the scaffolds or mesh implants [27]. It should be emphasized the mechanical performance of porous structures is crucial for bone repair in the case of extensive bone loss such as at jaw body or ramus [2, 7]. Thus, the balance of porosity and strength must be accomplished for enhanced bone healing [2, 7, 10, 12, 25].
As seen in Figs. 3 and 4, the porous zirconia structure was filled with a flowable platelet rich fibrin. Most of pores were filled although some pores remained free of PRF. Even though the porous zirconia was not entirely filled with PRF, the compressive strength increased by around 34 % revealing the PRF-zirconia block is useful for clinical handling. Also, adequate mechanical properties are important to avoid fracture and tissue damage over the period of bone healing. In Fig. 4, leukocytes, red blood cells, platelets are noticeable. Cells are entrapped into a dense fibrin network and therefore the morphological aspects of the PRF corroborates the findings from previous studies [11, 15, 23]. Fibrin contains binding sites for integrins, growth factors, and other extracellular matrix components including fibronectin, that provides molecular signals to direct cell function [23, 34]. Previous studies reported that an injectable PRF, namely i-PRF, revealed a similar cell composition, growth factors, and 3D-fibrin network when compared to a L-PRF membrane [11, 23]. Also, the capability of i-PRF to release higher content of various growth factors (e.g. PDGF, TGF-β) has been reported in literature [11, 21]. Growth factors from i-PRF stimulates the migration of fibroblasts and osteogenic cells when compared to platelet-rich plasma (PRP).
However, different viscosity, cell content, and physiologic aspects of platelet-rich fibrin can occur by using specific protocols and centrifuges. Several factors related to the centrifuge has been reported in previous studies such as the radius inclination and vibration amplitude of the tube holders [23, 34]. An increase in temperature occurs on vibration of the tube holders that negatively affect the morphological and biological integrity of the platelet-rich fibrin. Also, various centrifugation forces can be implemented on producing PRF [23, 34]. Consequently, findings on a certain centrifugation protocol may not be compared or inferred to other protocols [23, 34].
Conclusions
The present study revealed the manufacturing of porous zirconia structures by using a replica method and powder sintering. The control of the size and percentage of pores can be achieved by selecting a polyurethane pattern with a desired macro-scale size of interconnected pores. Considering the mechanical properties of zirconia, a high porosity at approximately 89% and macro-scale pores ranging from 70 up to 300 μm can be accomplished without compromising the mechanical integrity of zirconia porous structures in extensive surgical bone sites. Macro-scale pores size allows cell ingrowth and angiogenesis into the porous structures, while pores at micro-scale provide an increase in the wettability, protein adsorption, and cell adhesion. The pores of zirconia structure were partially filled with a flowable and injectable platelet rich fibrin. Therefore, the strength of the porous zirconia increased by 34% due to the embedment of the porous structure by platelet rich fibrin components. Further studies should be carried out concerning the optimum balance between the porosity and the mechanical properties of the porous zirconia structures. Also, the hybrid bioactive graft involving zirconia and platelet rich fibrin could be explored since the platelet rich fibrin significantly improve the adsorption of proteins and osteogenic cells as well as the cell differentiation and angiogenesis leading to an enhanced bone healing.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
R.-X. Shao, R.-F. Quan, T. Wang, W.-B. Du, G.-Y. Jia, D. Wang et al., Effects of a bone graft substitute consisting of porous gradient HA/ZrO2 and gelatin/chitosan slow-release hydrogel containing BMP-2 and BMSCs on lumbar vertebral defect repair in rhesus monkey. J. Tissue Eng. Regen Med. 12, e1813–e1825 (2018)
Y. Zhu, R. Zhu, J. Ma, Z. Weng, Y. Wang, X. Shi et al., In vitro cell proliferation evaluation of porous nano-zirconia scaffolds with different porosity for bone tissue engineering. Biomed. Mater. 10, 55009 (2015)
R.-X. Shao, R.-F. Quan, X.-L. Huang, T. Wang, S.-J. Xie, H.-H. Gao et al., Evaluation of porous gradient hydroxyapatite/zirconia composites for repair of lumbar vertebra defect in dogs. J. Biomater. Appl. 30, 1312–1321 (2016)
S.-H. An, T. Matsumoto, H. Miyajima, A. Nakahira, K.-H. Kim, S. Imazato, Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 28, 1221–1231 (2012)
A. Alizadeh, F. Moztarzadeh, S.N. Ostad, M. Azami, B. Geramizadeh, G. Hatam et al., Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering. Artif cells, nanomed. Biotechnol. 44, 66–73 (2016)
C. Hadjicharalambous, E. Mygdali, O. Prymak, A. Buyakov, S. Kulkov, M. Chatzinikolaidou, Proliferation and osteogenic response of MC3T3-E1 pre-osteoblastic cells on porous zirconia ceramics stabilized with magnesia or yttria. J. Biomed. Mater. Res. A 103, 3612–3624 (2015)
H.-W. Kim, S.-Y. Shin, H.-E. Kim, Y.-M. Lee, C.-P. Chung, H.-H. Lee et al., Bone formation on the apatite-coated zirconia porous scaffolds within a rabbit calvarial defect. J. Biomater. Appl. 22, 485–504 (2008)
Y.-G. Song, I.-H. Cho, Characteristics and osteogenic effect of zirconia porous scaffold coated with beta-TCP/HA. J. Adv. Prosthodont. (South) 6, 285–294 (2014)
K. Grandfield, A. Palmquist, F. Ericson, J. Malmstrom, L. Emanuelsson, C. Slotte et al., Bone response to free-form fabricated hydroxyapatite and zirconia scaffolds: a transmission electron microscopy study in the human maxilla. Clin. Implant Dent. Relat. Res. 14, 461–469 (2012)
C. Hadjicharalambous, A. Buyakov, S. Buyakova, S. Kulkov, M. Chatzinikolaidou, Porous alumina, zirconia and alumina/zirconia for bone repair: fabrication, mechanical and in vitro biological response. Biomed. Mater. 10, 25012 (2015)
H.A. Varela, J.C.M. Souza, R.M. Nascimento, R.F.J. Araújo, R.C. Vasconcelos, R.S. Cavalcante et al., Injectable platelet rich fibrin: cell content, morphological, and protein characterization. Clin. Oral Investig. 23, 1309–1318 (2019)
M. Noronha Oliveira, H.A. Varela, J. Caramês, F. Silva, B. Henriques, W. Teughels et al., Synergistic benefits on combining injectable platelet-rich fibrin and bone graft porous particulate materials. Biomed. Mater. Dev. (2022). https://doi.org/10.1007/s44174-022-00004-5
X. Wang, Y. Zhang, J. Choukroun, S. Ghanaati, R.J. Miron, Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP. Int. J. Mol. Sci. 18(2), 331 (2017)
V. Lekovic, I. Milinkovic, Z. Aleksic, S. Jankovic, P. Stankovic, E.B. Kenney et al., Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res. 47, 409–417 (2012)
S. Cortellini, A.B. Castro, A. Temmerman, J. Van Dessel, N. Pinto, R. Jacobs et al., Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: a proof-of-concept study. J Clin Periodontol. 45, 624–634 (2018)
A. Temmerman, J. Vandessel, A. Castro, R. Jacobs, W. Teughels, N. Pinto et al., The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 43, 990–999 (2016)
A.B. Castro, N. Meschi, A. Temmerman, N. Pinto, P. Lambrechts, W. Teughels et al., Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J. Clin. Periodontol. 44, 67–82 (2017)
A.B. Castro, N. Meschi, A. Temmerman, N. Pinto, P. Lambrechts, W. Teughels et al., Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clin. Periodontol. 44, 225–234 (2017)
C.-L. Wu, S.-S. Lee, C.-H. Tsai, K.-H. Lu, J.-H. Zhao, Y.-C. Chang, Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 57, 207–212 (2012)
J. Choukroun, A. Diss, A. Simonpieri, M.O. Girard, C. Schoeffler, S.L. Dohan et al., Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg., Oral Med. Oral Pathol Oral Radiol. Endodontol. 101(3), e56-60 (2006)
R.J. Miron, M. Fujioka-Kobayashi, M. Hernandez, U. Kandalam, Y. Zhang, S. Ghanaati et al., Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin. Oral Investig. 21, 2619–2627 (2017)
A. Kubesch, M. Barbeck, S. Al-Maawi, A. Orlowska, P.F. Booms, R.A. Sader et al., A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets 30(3), 329–340 (2019)
A. Castro, S. Cortellini, A. Temmerman, X. Li, N. Pinto, W. Teughels et al., Characterization of the leukocyte- and platelet-rich fibrin block: release of growth factors, cellular content, and structure. Int. J. Oral Maxillofac. Implants 34(4), 855–864 (2019)
M.N. Aboushelib, R. Shawky, Osteogenesis ability of CAD/CAM porous zirconia scaffolds enriched with nano-hydroxyapatite particles. Int. J. Implant Dent. 3, 21 (2017)
A. Teimouri, R. Ebrahimi, R. Emadi, B.H. Beni, A.N. Chermahini, Nano-composite of silk fibroin-chitosan/Nano ZrO2 for tissue engineering applications: fabrication and morphology. Int. J. Biol. Macromol. 76, 292–302 (2015)
J. Malmstrom, C. Slotte, E. Adolfsson, O. Norderyd, P. Thomsen, Bone response to free form-fabricated hydroxyapatite and zirconia scaffolds: a histological study in the human maxilla. Clin. Oral Implants Res. 20, 379–385 (2009)
J. Malmstrom, E. Adolfsson, L. Emanuelsson, P. Thomsen, Bone ingrowth in zirconia and hydroxyapatite scaffolds with identical macroporosity. J. Mater. Sci. Mater. Med. 19, 2983–2992 (2008)
H.-W. Kim, H.-E. Kim, V. Salih, J.C. Knowles, Dissolution control and cellular responses of calcium phosphate coatings on zirconia porous scaffold. J. Biomed. Mater. Res. A. 68, 522–530 (2004)
B. Gaihre, A.C. Jayasuriya, Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 91, 330–339 (2018)
D. Mondal, S. So-Ra, S.K. Sarkar, Y.K. Min, H.M. Yang, B.T. Lee, Fabrication of multilayer ZrO(2)-biphasic calcium phosphate-poly-caprolactone unidirectional channeled scaffold for bone tissue formation. J. Biomater. Appl. 28, 462–472 (2013)
P.F. Gouveia, J. Mesquita-Guimarães, M.E. Galárraga-Vinueza, J.C.M. Souza, F.S. Silva, M.C. Fredel et al., In-vitro mechanical and biological evaluation of novel zirconia reinforced bioglass scaffolds for bone repair. J. Mech. Behav. Biomed. Mater. 114, 104164 (2021)
D. Fabris, J. Mesquita-Guimarães, P. Pinto, J.C.M. Souza, M.C. Fredel, F.S. Silva et al., Mechanical properties of zirconia periodic open cellular structures. Ceram. Int. 45, 15799–15806 (2019)
E. Askari, I.F. Cengiz, J.L. Alves, B. Henriques, P. Flores, M.C. Fredel et al., Micro-CT based finite element modelling and experimental characterization of the compressive mechanical properties of 3-D zirconia scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 102, 103516 (2020)
A. Scarano, F. Inchingolo, G. Murmura, T. Traini, A. Piattelli, F. Lorusso, Three-dimensional architecture and mechanical properties of bovine bone mixed with autologous platelet liquid, blood, or physiological water: an in vitro study. Int. J. Mol. Sci. 19(4), 1230 (2018)
Acknowledgements
The authors acknowledge the financial support provided by the Portuguese Foundation for Science and Technology (FCT) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was funded by FCT in the subject of the following projects: UIDB/04436/2020, UIDP/04436/2020, SFRH/BPD/123769/2016, and LaserMULTICER (POCI-01-0145-FEDER-031035). This work was also partially funded by CAPES (Brazil) in the subject of the project CAPES-PRINT/88881.310728/2018-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interests.
Ethical Approval
All the procedures performed involving human participants followed the ethical standards of the research committee of the Federal University of Santa Catarina (CEPSH, UFSC, Brazil) and therefore with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (cod. 2229.085).
Informed Consent
Informed consent was not necessary since all data were processed anonymously.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sampaio, N., Noronha Oliveira, M., Carvalho, O. et al. Porous Zirconia Blocks Embedded with Platelet Rich Fibrin for Enhanced Bone Healing: Mechanical and Morphological Assessment. Biomedical Materials & Devices 1, 979–989 (2023). https://doi.org/10.1007/s44174-023-00076-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-023-00076-x