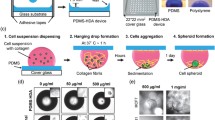
Abstract
Engineered three-dimensional (3D) tissue culture platforms are useful for reproducing and elucidating complex in vivo biological phenomena. Spheroids, 3D aggregates of living cells, are produced based on physicochemical or microfabrication technologies and are commonly used even in cancer pathology research. However, conventional methods have difficulties in constructing 3D structures depending on the cell types, and require specialized techniques/lab know-how to reproducibly control the spheroid size and shape. To overcome these issues, we have developed a fabrication method, which enables anyone to make and mature cancer spheroids using a superhydrophobic microwell made of the monolithic porous materials. Here, we characterize the biological behaviors of the breast cancer spheroids fabricated by our method under normoxic and hypoxic conditions. We found that the fabricated spheroid contracted to a certain size via activation of the actomyosin system. Cell proliferation induced a hypoxic state inside the spheroid (elevated expression of the hypoxia-inducible factor HIF-1α), followed by the formation of a necrotic core and cell escape from the spheroid. In addition, we observed a decrease in cancer spheroid contractility and cell escape from spheroids under hypoxic conditions compared to normoxic conditions, which were related to oxygen concentration-dependent cell motility. The fabricated spheroids perform as 3D tumor tissues in a highly reproducible manner and within a short culture period. Our findings indicate that this fabrication method has a wide range of applications in cancer research, such as elucidating the mechanisms of tumor invasion and metastasis and screening anticancer drugs, as with previous methods.





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within this article and its supplementary information files or from the corresponding author upon reasonable request.
References
Asghar W, Assal RE, Shafiee H, Pitteri S, Paulmurugan R, Demirci U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater Today. 2015;18:539–53. https://doi.org/10.1016/j.mattod.2015.05.002.
Kuo CT, Wang JY, Lin YF, Wo AM, Chen BPC, Lee H. Three-dimensional spheroid culture targeting versatile tissue bioassay using a PDMS-based hanging drop array. Sci Rep. 2017;7:4363. https://doi.org/10.1038/s41598-017-04718-1.
Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–84. https://doi.org/10.1002/biot.200700228.
Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev. 2014;69–70:29–41. https://doi.org/10.1016/j.addr.2014.03.001.
Nath S, Devi GR. Three-dimensional culture systems in cancer research: focus on tumor spheroid model. Pharmacol Ther. 2016;163:94–108. https://doi.org/10.1016/j.pharmthera.2016.03.013.
Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910–9. https://doi.org/10.5114/aoms.2016.63743.
Stock K, Estrada MF, Vidic S, Gjerde K, Rudisch A, Santo VE, Barbier M, Bolm S, Arundkar SC, Selvam I, Osswald A, Stein Y, Gruenewald S, Brito C, van Weerden W, Rotter V, Boghaert E, Oren M, Sommergruber W, Chong Y, de Hoogt R, Graeser R. Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep. 2016;6:28951. https://doi.org/10.1038/srep28951.
Thermo Fisher Scientific, Inc., 3D cell culture protocols. 2023. https://www.thermofisher.com/jp/ja/home/references/protocols/cell-culture/3-d-cell-culture-protocol.html. Accessed 13 Mar 2023.
Gao B, Jing C, Ng K, Pingguan-Murphy B, Yang Q. Fabrication of three-dimensional islet models by the geometry-controlled hanging-drop method. Acta Mech Sin. 2019;35:329–37. https://doi.org/10.1007/s10409-019-00856-z.
Marimuthu M, Rousset N, St-Georges-Robillard A, Lateef MA, Ferland M, Mes-Masson AM, Gervais T. Multi-size spheroid formation using microfluidic funnels. Lab Chip. 2018;18:304–14. https://doi.org/10.1039/C7LC00970D.
Moshksayan K, Kashaninejad N, Warkiani ME, Lock JG, Moghadas H, Firoozabadi B, Saidi MS, Nguyen NT. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens Actuators B Chem. 2018;263:151–76. https://doi.org/10.1016/j.snb.2018.01.223.
Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three-dimensional cell culturing by magnetic levitation. Nat Protoc. 2013;8:1940–9. https://doi.org/10.1038/nprot.2013.125.
Hayase G, Yoshino D. CNC-milled superhydrophobic macroporous monoliths for 3D cell culture. ACS Appl Bio Mater. 2020;3:4747–50. https://doi.org/10.1021/acsabm.0c00719.
McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87:20130676. https://doi.org/10.1259/bjr.20130676.
Palacio-Castañeda V, Velthuijs N, Le Gac S, Verdurmen WPR. Oxygen control: The often overlooked but essential piece to create better in vitro systems. Lab Chip. 2022;22:1068–92. https://doi.org/10.1039/D1LC00603G.
Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–7. https://doi.org/10.1126/science.1081412.
Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. https://doi.org/10.1038/40187.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. https://doi.org/10.1038/nmeth.2089.
Murphy KC, Hung BP, Browne-Bourne S, Zhou D, Yeung J, Genetos DC, Leach JK. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface. 2017;14:20160851. https://doi.org/10.1098/rsif.2016.0851.
Grimes DR, Kelly C, Bloch K, Partridge M. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface. 2014;11:20131124. https://doi.org/10.1098/rsif.2013.1124.
Xing W, Yin M, Lv Q, Hu Y, Liu C, Zhang J. Oxygen solubility, diffusion coefficient, and solution viscosity. In: Xing W, Yin G, Zhang J, editors. Rotating electrode methods and oxygen reduction electrocatalysts. Elsevier; 2014. pp. 1–31. https://doi.org/10.1016/B978-0-444-63278-4.00001-X.
McMurtrey RJ. Analytic models of oxygen and nutrient diffusion, metabolism dynamics, and architecture optimization in three-dimensional tissue constructs with applications and insights in cerebral organoids. Tissue Eng Part C Methods. 2016;22:221–49. https://doi.org/10.1089/ten.tec.2015.0375.
Subczynski WK, Hopwood LE, Hyde JS. Is the mammalian cell plasma membrane a barrier to oxygen transport? J Gen Physiol. 1992;100:69–87. https://doi.org/10.1085/jgp.100.1.69.
Dotson RJ, McClenahan E, Pias SC. Updated evaluation of cholesterol’s influence on membrane oxygen permeability. In: Nemoto EM, Harrison EM, Pias SC, Bragin DE, Harrison DK, LaManna JC, editors. Oxygen Transport to Tissue XLII. Advances in Experimental Medicine and Biology, vol 1269. Cham: Springer; 2021. 23–30. https://doi.org/10.1007/978-3-030-48238-1_4.
Riffle S, Pandey RN, Albert M, Hegde RS. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer. 2017;17:338. https://doi.org/10.1186/s12885-017-3319-0.
Costa EC, Moreira AF, de Melo-Diogo D, Gaspar VM, Carvalho MP, Correia IJ. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol Adv. 2016;34:1427–41. https://doi.org/10.1016/j.biotechadv.2016.11.002.
Zhao L, Xiu J, Liu Y, Zhang T, Pan W, Zheng X, Zhang X. A 3D printed hanging drop dripper for tumor spheroids analysis without recovery. Sci Rep. 2019;9:19717. https://doi.org/10.1038/s41598-019-56241-0.
Ham SL, Joshi R, Luker GD, Tavana H. Engineered breast cancer cell spheroids reproduce biologic properties of solid tumors. Adv Healthc Mater. 2016;5:2788–98. https://doi.org/10.1002/adhm.201600644.
Koens R, Tabata Y, Serrano JC, Aratake S, Yoshino D, Kamm RD, Funamoto K. Microfluidic platform for three-dimensional cell culture under spatiotemporal heterogeneity of oxygen tension. APL Bioeng. 2020;4:016106. https://doi.org/10.1063/1.5127069.
Cox TR, Rumney RMH, Schoof EM, Perryman L, Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, Huggins ID, Lang G, Linding R, Gartland A, Erler JT. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;552:106–10. https://doi.org/10.1038/nature14492.
Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SFT, Csiszar K, Hendrix MJC, Krischmann DA. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 2005;65:11429–36. https://doi.org/10.1158/0008-5472.CAN-05-1274.
Xu H, Yuan Y, Wu W, Zhou M, Jiang Q, Niu L, Ji J, Liu N, Zhang L, Wang X. Hypoxia stimulates invasion and migration of human cervical cancer cell lines HeLa/SiHa through the Rab11 trafficking of integrin αvβ3/FAK/PI3K pathway-mediated Rac1 activation. J Biosci. 2017;42:491–9. https://doi.org/10.1007/s12038-017-9699-0.
Silva P, Mendoza P, Rivas S, Díaz J, Moraga C, Quest AFG, Torres VA. Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget. 2016; 7: 29548–29562. https://doi.org/10.18632/oncotarget.8794.
Chin VT, Nagrial AM, Chou A, Biankin AV, Gill AJ, Timpson P, Pajic M. Rho-associated kinase signalling and the cancer microenvironment: Novel biological implications and therapeutic opportunities. Expert Rev Mol Med. 2015;17:E17. https://doi.org/10.1017/erm.2015.17.
Close DA, Johnston PA. Detection and impact of hypoxic regions in multicellular tumor spheroid cultures formed by head and neck squamous cell carcinoma cells lines. SLAS Discov. 2022;22:39–54. https://doi.org/10.1016/j.slasd.2021.10.008.
Vleugel MM, Greijer AE, Shvarts A, van der Groep P, van Berkel M, Aarbodem Y, van Tinteren H, Harris AL, van Diest PJ, van der Wall E. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J Clin Pathol. 2005;58:172–7. https://doi.org/10.1136/jcp.2004.019885.
Ermis M. Photo-crosslinked gelatin methacrylate hydrogels with mesenchymal stem cell and endothelial cell spheroids as soft tissue substitutes. J Mater Res. 2021;36:176–90. https://doi.org/10.1557/s43578-020-00091-4.
Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, Poh YC, Tang K, Wang N, Huang B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater. 2012;11:734–41. https://doi.org/10.1038/nmat3361.
Choi JY, Jang YS, Min SY, Song JY. Overexpression of MMP-9 and HIF-1α in breast cancer cells under hypoxic conditions. J Breast Cancer. 2011; 14: 88–95. https://doi.org/10.4048/2Fjbc.2011.14.2.88.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. https://doi.org/10.1016/j.cell.2010.03.015.
Indelicato M, Pucci B, Schito L, Reali V, Aventaggiato M, Mazzarino MC, Stivala F, Fini M, Russo MA, Tafani M. Role of hypoxia and autophagy in MDA-MB-231 invasiveness. J Cell Physiol. 2010;223:359–68. https://doi.org/10.1002/jcp.22041.
Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Investig. 2022;132:e159839. https://doi.org/10.1172/JCI159839.
Däster S, Amatruda N, Calabrese D, Ivanek R, Turrini E, Droeser RA, Zajac P, Fimognari C, Spagnoli GC, Iezzi G, Mele V, Muraro MG. Induction of hypoxia and necrosis in multicellular tumor spheroids is associated with resistance to chemotherapy treatment. Oncotarget. 2017;8:1725–1736. https://doi.org/10.18632/oncotarget.13857.
Hagemann J, Jacobi C, Hahn M, Schmid V, Welz C, Schwenk-Zieger S, Stauber R, Baumeister P, Becker S. Spheroid-based 3D cell cultures enable personalized therapy testing and drug discovery in head and neck cancer. Anticancer Res. 2017;37:2201–2210. https://doi.org/10.21873/anticanres.11555.
Shi W, Kwon J, Huang Y, Tan J, Uhl CG, He R, Zhou C, Liu Y. Facile tumor spheroids formation in large quantity with controllable size and high uniformity. Sci Rep. 2018;8:6837. https://doi.org/10.1038/s41598-018-25203-3.
Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2021;276:37258–37265. https://doi.org/10.1074/jbc.m106089200
Acknowledgements
The human pancreatic cancer cell line, MIA Paca2 (RCB2094) and human breast adenocarcinoma cell line, MCF7 (RCB1902), were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT/AMED, Japan. We thank Dr. Yoshitsugu Aoki, Director of the Department of Molecular Therapy, National Center of Neurology and Psychiatry, for providing the fluorescence microscope system. This study was partly supported by grants from the Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering and the JSPS KAKENHI (No. 21K19893) to D.Y.
Author information
Authors and Affiliations
Contributions
D.Y. conceived and designed the research. Y.I. conducted most of the experiments. N.U. was in charge of most of the immunoblotting. M.M. and D.S. conducted mathematical modeling and calculation for oxygen concentration in spheroids. Y.I. and S.H. performed the analysis of the distribution of protein fluorescence intensity. N.S. and M.S. provided technical support in Immunohistochemistry. K.F. supported technical methods for hypoxic exposure experiments on cancer spheroids. G.H. developed, prepared, and provided the superhydrophobic substrates for spheroid fabrication. All authors discussed the data. Y.I. and D.Y. wrote the manuscript. D.Y. directed and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest directly relevant to the content of this article.
Additional information
Highlights
- Cancer spheroids of the desired size can be produced in a highly reproducible manner.
- The fabricated MDA-MB-231 spheroid contracts via actomyosin system activation.
- Cell proliferation induces a highly hypoxic state inside the spheroid.
- Necrotic core formation in the inner layer and cell escape from the spheroid can be reproduced.
- Spheroid behavior is influenced by oxygen concentration-dependent changes in cell motility.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iijima, Y., Uenaka, N., Morimoto, M. et al. Biological characterization of breast cancer spheroid formed by fast fabrication method. In vitro models 3, 19–32 (2024). https://doi.org/10.1007/s44164-024-00066-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44164-024-00066-3