Abstract
Monkeypox is a DNA virus that belongs to the orthopox virus family with two distinct clades known as West African and Congo Basin. This virus was initially discovered in crab-eating Macaques in 1958 and in 1970s it spread among people in the Democratic Republic of the Congo. Initially, there were several outbreaks of the disease reported in Africa and other regions of the world. The simultaneous spread in 19 countries in 2022 prompted severe worries. The monkeypox virus is closely related to smallpox, which was responsible for the highest fatality rate in human history, and a preconceived thought combined with fear is enough to make us shiver. Furthermore, the virus is often mistaken for a sexually transmitted infection or the Varicella zoster virus. The emergence of outbreaks outside of Africa is indicative of the disease’s global footprint. Increased detection and monitoring of monkey pox cases as well as understanding the disease’s ever-changing epidemiology is critical. Furthermore, intensive research is yet to identify the exact small mammal reservoir for monkeypox virus. Identifying the exact reservoir may aid in the identification of previously unknown high-risk activities for getting orthopoxvirus infections. Finally, a better understanding of the potential/suspected monkeypox viral transmission pathways is required so that public health officials can develop and implement interventions to lower the risk of human infection. This review focuses on the genetic, clinical, molecular, diagnostic, and therapeutic perspectives of monkeypox.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monkeypox virus (MPXV) is an etiological agent of monkeypox that is thought to have originated in Central/West Africa [1]. MPXV is an orthopoxvirus, which is related to the variola virus [2]. It is a pathogen of medical importance due to its increasing occurrence [1]. The MPXV’s genome, which is around 197 kbp long, is nearly identical to the smallpox variola virus. MPXV is a cytoplasmic virus that is brick-shaped and enveloped. MPXV enters host cells through binding to glycosaminoglycans [3]. As an enveloped virus, it is thought to use the typical apoptotic mimicking method for entry into host cells. However, despite being closely related, neither the MPXV nor the variola virus appear to be the ancestor of the other [4]. The detection of MPXV infection, however, is difficult due to similarities between smallpox and varicella-zoster viruses [5]. This is due to the fact that the pathophysiologic process and clinical symptoms are nearly comparable to those of smallpox. Generally, orthopoxviruses are large in size, double-stranded DNA viruses that include various species such as vaccinia virus, MPXV, variola virus, cowpox virus, and other discovered species (e.g., Akhmeta virus and Alaskapox virus) [6,7,8,9].
MPXV was first discovered in 1958 during an outbreak in an animal facility in Copenhagen, Denmark [10,11,12,13]. Despite its name, monkeypox is actually a misnomer since it most commonly infects small African mammals and rodents. The virus got its name after being discovered in 1958 when 2 outbreaks of a pox-like disease were discovered in colonies of crab-eating macaques that were being used for research purposes [3, 14]. However, the first human case actually came from the Democratic Republic of Congo (DRC) in 1970 [5, 15, 16]. Following that, it became endemic in Central Africa (Congo) and West Africa (Nigeria), with periodic cluster outbreaks [17]. The 2017–2019 outbreak in Nigeria was the most severe [15, 18]. Then, in 2022, cluster epidemics resurfaced in Europe, US, and in the Middle East [3, 18].
The MPXV is classified into two genetically distinct clades: the Congo Basin (CA) clade and the West African (WA) clade [19]. The Congo Basin clade is more virulent than the West African clade, with a case fatality rate of 10.6% vs 3.6% for the West African clade [20, 21]. Comparative genomic investigations found a 0.55–0.56% nucleotide difference between the two strains [22], with the CA strain containing 173 functionally distinct genes compared to 171 in the West African strain. The two strains were determined to be 99.4% similar, with 170 protein orthologs shared and no substantial changes in transcriptional regulatory sequences [22, 23]. In both strains, 53 of the 56 virulence genes were discovered, with 61 conserved, 93 non-conserved, and 121 silent amino acid alterations [22].
The occurrence of zoonotic infectious disease outbreaks poses a serious public health concern. Again, a number of viral infections with pandemic potential pose a threat to global health security. Zoonotic viruses, in particular, have been the source of multiple outbreaks in recent years, resulting in significant morbidity and mortality. The COVID-19 pandemic demonstrated that any virus epidemic capable of transmitting human-to-human or cross-species transmission might pose a major risk and contribute to a worldwide pandemic [24].
Although it is still a relatively rare virus, the growing number of MPXV cases in non-endemic countries and across Europe is causing concern around the world. Epidemiological studies, transmission dynamics, and illness ecology are not fully understood and require more attention. To deal with emerging or re-emerging viral threats in a sustainable manner, awareness campaigns, promoting precautionary measures, educating health care workers, and advancing public health preparedness through proactive continuous extensive surveillance, rapid risk assessments, response activities, early detection, and contact tracing should be prioritized [24]. This review focuses on the genetic, clinical, molecular, diagnostic, and therapeutic perspectives of monkeypox.
Overview of outbreak trends
The United Kingdom (UK) reported an imported case of MPXV in a person traveling from Nigeria on May 7, 2022 [3, 25]. The subject reported a rash-like disease on April 29, 2022, and traveled from Lagos to London on May 3–4, 2022. On May 6, 2022, the UK Health Security Agency Rare and Imported Pathogens Laboratory verified the diagnosis using MPXV polymerase chain reaction (PCR) on a vesicular swab [3, 26]. Since then, more than 1500 cases in 45 countries have been suspected and/or confirmed (as of June 11, 2022), prompting the World Health Organization (WHO) to organize an emergency meeting to combat the virus’ uncontrolled spread [3].
On May 13, 2022, the UK recorded two new cases of MPXV that were not related to the single imported case from Nigeria that was reported on May 7, 2022. PCR testing on vesicle swabs verified the cases [16]. A third family member had previously suffered a rash but had totally healed. None of the people in this cluster had traveled or had contact with somebody who had traveled [26]. On May 15, 2022, the UK recorded four new cases of monkeypox (MPX), which were verified by PCR. There were no known epidemiological linkages between any of these patients and the imported case from Nigeria (announced on May 7, 2022) or the family cluster (announced on May 13, 2022). The four cases included males who had intercourse with men and developed a vesicular rash-like condition [27]. They were discovered while attending genitourinary medical clinics. The cases were treated at high-risk infectious disease units in the UK [26].
In general, episodes of MPXV continue to be documented in West and Central African countries [8]. Cameroon reported an MPXV epidemic in December 2021, and 3 confirmed and 25 suspected cases, including 2 deaths, were reported as of February 17, 2022. Again, multiple cases have been recorded from countries in the continent’s central, northwestern, and southwestern areas. Moreover, cases of MPXV have been reported in Cameroon on an irregular basis, with more than 50% of Africa’s geographical areas reporting at least one incident during 2020 and 2022 [26].
In 2003, the US experienced an epidemic of 47 verified and likely cases associated with a shipment of animals from Ghana [28]. Everyone afflicted with MPXV became unwell after coming into contact with contaminated pet prairie dogs housed near the imported small mammals [3, 25, 29]. Seven previous instances of MPX had been documented in the UK (in 2018, 2019, and 2021), primarily among people who had traveled to endemic countries [30]. The first MPXV epidemic outside of Africa was discovered in the United States (US) in 2003, following the importation of infected animals from Ghana [24, 25, 31, 32]. Surprisingly, due to the spread of COVID-19 and Lassa fever, no instances of MPX were reported in 2020. The COVID-19 pandemic caused a lockdown and social isolation, which may have contributed to the MPVX spreading slowly or not at all [25].
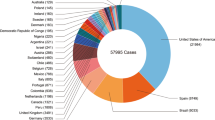
Figure 1 depicts the progression of MPXV infection [20].
The progression of MPXV infection. The number of confirmed, probable, and/or potential MPX cases is shown by decade [20]
Genetic history
MPXV was isolated and identified in 1958 in cynomolgus monkeys in a laboratory environment in Copenhagen, Denmark [2, 4, 33]. This was the world’s first encounter with a human orthopox virus other than smallpox, prompting public health professionals to wonder where and when MPXV would occur next. The first human case was reported in 1970 in the DRC’s Basankusu area in a 9-month-old boy who presented with symptoms similar to smallpox but was later confirmed as MPXV [25, 34,35,36]. This incidence served as an early warning sign to public health professionals that the virus had the potential to infect more people [1].
As the number of MPXV cases increased, it became obvious that humans were a particularly susceptible host to MPXV infection. During this period, scientists discovered two MPXV strains: a WA and a CA strain [3, 37]. This was the first sign that MPXV had mutated and was developing to a more infectious disease, alerting public health professionals to the gravity of this emerging zoonotic virus and the need for intensified surveillance. Previous research findings suggested that MPXV is naturally maintained in rodent populations and, to a lesser extent, non-human primates, but the true reservoir host for the virus was unknown [1, 2, 5, 38]. Rodents are most likely the MPXV reservoir [4, 24]. African rodents and nonhuman primates may harbor the virus and infect people [15]. According to the findings of a study conducted by Mary Walker, the Giant Gambian Rat, the African Rope Squirrel, and various other squirrels (as a group) were the most likely to be identified as prospective reservoir hosts for MPXV. These species were cited in 26.80%, 19.59%, and 15.46% of the papers that proposed a potential reservoir host, respectively [1].
In September 2017, Nigeria saw the greatest WA MPX outbreak in history [25]. For the first 11.5 months of the outbreak, no exported cases were documented; however, from September 2 to September 23, 2018, three unrelated visitors afflicted with MPXV left Nigeria and landed in two countries. In Singapore, a Nigerian national became ill from MPX 7 months later [25]. These exportations were the first reported instances of a human host transferring MPXV from the African continent; nevertheless, since its discovery in 1958, there had been a few cases of MPX outbreaks in animals in laboratories and zoos with no clearly established source of infection [8]. The source of the 2003 MPX outbreak in the US was identified as a shipment of rodents from West Africa [8].
Molecular pathogenesis
The molecular process involves viral entrance, fusion, replication, and release, during which the virus creates two infectious forms: extracellular enveloped virions (EV) and intracellular mature virions (MV). While MV are single-membrane bound virions that are only released upon host cell lysis, EV are specialized intracellular mature virions that are bound by an antigenically unique triple membrane [39, 40]. In fact, it has been established that vaccines and antibodies that do not create or target extracellular wrapped virions antigens provide less protection than those that do [41, 42].
The early stages of infection might differ based on the infectious form of the virus, virus strain, and host target cell type, among other considerations [43]. Virus proteins target host cell glycosaminoglyans or laminins during the initial attachment process [44,45,46]. After EV binding, the outer membrane allows the entry of fusion complex on the MV membrane to engage with host membrane proteins, resulting in viral fusion [47, 48]. Following this, viral cores are released into the cytoplasm, where virus replication occurs. The GARP (Golgi-associated retrograde protein) and COG (conserved oligomeric Golgi) complexes are essential for the viral infectious cycle to be completed [40]. The GARP complex, which is responsible for retrograde endosomal transport, is made up of four vacuolar protein sorting genes: VPS51, VPS52, VPS53, and VPS54, all of which were found to be enriched in both CA and WA strains (with the exception of VPS53, which is only found in the CA strain) [49]. Infected with MPXV, VPS52 and VPS54 mutant cells showed a significant decrease in EV yield, demonstrating their important role in virus egress and cell-to-cell spread [49]. The COG complex is necessary for the maintenance of Golgi structure and the regulation of intra-Golgi traffic.
The differences in virulence between the CA and WA strains are thought to be due to changes in the gene orthologs BR-203 (virulence protein), BR-209 (IL-1 binding protein), and COP-C3L (inhibitor of complement enzymes) [22, 50]. Other possible gene orthologs include the WA strain-specific COP-A49R (unknown function) and COP-A52R (bifunctional Toll-IL-1-receptor protein). Candidates for CA strain-specific orthologs include BR-19 and BR-20 (unknown function) [23]. Another key gene responsible for strain differences in virulence is the D14R gene, which codes for the inhibitor of complement-binding protein (MOPICE), a critical anti-inflammatory component that is missing in the MPXV WA strain [22, 51, 52]. However, these genes are not the only ones involved in virulence as many other candidates have not been identified.
Transmission dynamics
MPXV is primarily transmitted by close contact, although it can as well be spread through droplets or aerosols [15, 27, 53]. It is a zoonotic illness since it is mostly a wild animal disease, but it can also be passed from one individual to another [5, 18, 54,55,56]. The cell surface-binding protein E8L plays a central role in the transmission of the virus. It is located on MPV virion membranes and it enhances attachment of virion to host cells, rendering it a potential vaccine target [17, 57]. Since 2003, outbreaks have occurred as a result of import and travel related spread outside of Africa [58]. Frequent interactions with infected animals or persons are other risk factors associated with the acquisition of MPXV [15, 58].
Human-to-human transmission occurs via respiratory droplets, bodily fluids, contact with lesioned or contaminated surfaces, or other materials such as bedding and clothing [53, 59]. The following behaviors increase the likelihood of developing MPXV infection: sharing beddings and sharing of utensils with the primary patient [60]. Close contact during close sexual intimacy, such as oral, anal, and vaginal sex, hugging, massage, mutual masturbation, kissing, and embracing, can result in viral transmission between humans. Other means of transmission include handling textiles and objects used by MPXV-infected individuals during intercourse [25].
Majority of studies suggests that MPXV enters the human population through contact with infected wildlife, most commonly through eating or handling infected meat [7]. Similar investigations have indicated that the primary mode of transmission is via cutaneous, mucocutaneous, or airborne droplets [61,62,63]. Each year, a few thousand cases are reported throughout Africa, particularly in the continent’s western and central regions. However, outside of Africa, cases were previously limited to a few that were linked to travel to Africa or the importation of infected animals [57].
Unlike SARS-CoV-2, which spreads by tiny airborne droplets known as aerosols, MPXV is suspected to spread via direct contact with human fluids such as saliva. That indicates that someone infected with MPXV is capable of infecting fewer close contacts as compared to someone infected with SARS-CoV-2 [15, 57]. However, both viruses can have the potential of inducing influenza-like symptoms, but MPXV causes swollen lymph nodes as well as unique fluid-filled sores on the hands, face, and feet. Without treatment, most individuals recover from MPXV within a few weeks [57]. In contrast to SARS-CoV-2, MPXV rarely goes unreported when it infects a person, owing to the skin sores it creates. It would be especially concerning if MPXV could spread asymptomatically, as this would make the virus more difficult to track and report [57].
Interestingly, MPXV is not easily transmitted between persons. Close contact with infectious material from an infected person’s skin lesions, respiratory droplets in prolonged face-to-face contact, and fomites all contribute to human-to-human transmission. The outbreak’s prevalence of identified human MPX cases among homosexuals, as well as the pattern of the presenting lesions in some instances, suggest that transmission took place during sexual intercourse [26]. Recently, MPX was detected in a dog most likely as a result of human to animal transmission following close direct contact with its owners who were symptomatic with the disease. This was the first documented case of human to animal transmission of the virus [64].
In other circumstances, transmission between individuals happens mostly via large respiratory droplets, implying prolonged physical contact. However, the virus can also be transmitted by bodily fluids. Some of the transmission incidents have primarily involved men who have sex with men (MSM) [30]. Although MPXV has not previously been identified as a sexually transmitted infection, the UK Health Security Agency reported that it can be transferred through direct contact during sexual activity [30]. According to Mary Walker’s research, direct contact was most frequently reported as the most likely transmission method for MPXV. Direct contact induces MPXV spillover from zoonotic hosts to humans, according to approximately 83.33% of papers including a comment concerning transmission possibilities [1].
Another investigation on the transmission and pathogenesis of MPXV in prairie dogs discovered that both respiratory and direct mucocutaneous exposures are potentially relevant routes of MPXV transmission between rodents and humans. MPXV was detected in two prairie dogs using PCR, and pathologic examination indicated necrotizing bronchopneumonia, conjunctivitis, and tongue ulcers. In addition, immunohistochemistry studies for orthopoxviruses revealed numerous viral antigens in surface epithelial cells of conjunctival and tongue lesions, with smaller quantities in surrounding macrophages, fibroblasts, and connective tissues. Viral antigens were prevalent in bronchial epithelial cells, macrophages, and fibroblasts in the lung. Virus isolation and electron microscopy revealed that the virus was actively replicating in the lungs and tongue [28]. The incubation period for MPXV is usually between 6 and 13 days, but it can range between 5 and 21 days, and the duration of symptoms and signs is estimated to be 2 to 5 weeks [2, 5, 7, 24].
Clinical presentation
Historically, patients with MPX present with a distinctive rash preceded by mild to severe prodromal symptoms (fevers, malaise, generalized lymphadenopathy). Lesions are confined, deep entrenched, and frequently have umbilication. These lesions are frequently comparable in size and evolution on a single place of the body (pustules in face or vesicles in legs). Before the rash, the virus causes fever (malaise, headache, sore throat, cough, widespread lymphadenopathies) [16, 65, 66]. This is accompanied by lesions in the mouth (enanthem), torso (especially the palms and soles), and feet (exanthem: macule, papule, vesicles, pustules, scab). The sores are uncomfortable until they heal and become crusty. The patient is no longer contagious once the rash has healed and the scabs have gone off [15, 67]. When a suspected or confirmed new case is identified, proper isolation with supportive care and quarantine procedures should be prioritized. It is evident that the discontinuation of smallpox immunization in 1980, as well as diminishing immunity among the population and a growing number of unprotected individuals, resulted in an increase in MPXV incidence [36].
Interestingly, atypical presentations of MPXV are possible including cases with very few cutaneous lesions or without detectable cutaneous lesions. For instance, in a study conducted in Genoa, Italy, it was found that one of the patients had an atypical cutaneous manifestation as well as perianal superficial ulcers and erythematous vesicles. In addition, the patient developed a faint urticarial eruption on the legs and trunk [68]. A separate study conducted in Milan, Italy, found two cases of human MPX with a single cutaneous lesion and no evidence of systemic involvement. Patient 1 was a 35-year-old Italian MSM living with HIV who was on antiretroviral medication and had an undetectable viral load, whereas patient 2 was an HIV-negative Italian MSM aged 29. Both presented to Milan’s STI Centre with complaints of a single genital lesion. Both had a history of syphilis, and patient 2 reported having previously been treated for gonorrhea [69].
Figure 2 Shows the maculo-popular vesicular-pustular lesions of varying sizes on the face [15].
The maculo-popular vesicular-pustular lesions of varying sizes on the face [15]
Diagnostic perspectives
Globally, there has been a rise in the incidence of sexually transmitted infections (STIs) following MPXV outbreak in May 2022. Therefore, it is recommended that patients with MPX undergo screening for sexually transmitted infections (STIs) in order to diagnose other sexually transmitted diseases (STDs), particularly in patients with rectal involvement. In a study conducted in Madrid, Spain, 36 pathogens were detected in 30 patients ranging from Chlamydia trachomatis (13.8%), herpes simplex virus (33.3%), Neisseria gonorrhoeae (25%), Treponema pallidum (11.1%), Chlamydia trachomatis-lymphogranuloma venereum (8.3%), and Mycoplasma genitalium (8.3%) [70]. Moreover, a study of 16 MPX-infected men in Genoa, Italy, found a significant prevalence of STIs such as gonococcal/nongonococcal proctitis and anal high-risk human papillomavirus infections [68]. To curb this, all healthcare providers, not only those serving STI patients, should receive specific training to recognize and manage MPX infection and to recommend vaccination for the prevention of MPX disease in vulnerable populations.
Various assays can be employed in the diagnosis of MPXV. These tests include PCR, viral culture/isolation, immunohistochemistry test, electron microscopy, anti-orthopoxvirus IgG test, Tetracore Orthopox Bio threat alert test, and anti-orthopoxvirus IgM tests [5, 25, 53, 66]. These tests check for the presence of several biomarkers. All of these tests must be carried out in a large laboratory by well-trained and competent laboratory technologists [15, 16]. For instance, MPXV-specific DNA fingerprints are detected by PCR [5, 71]. Using lesion material collected from a patient, the PCR method can be utilized to diagnose an active case. The assay employs viral DNA, which is usually stable when stored in a dark, cool environment. The assay is highly specific, but the cost of the reagents, consumables, and equipment required is significantly high [16, 25].
The second technique involves the detection of orthopoxvirus IgG antibodies using anti-orthopoxvirus IgG testing. The assay determines prior exposure to an orthopoxvirus, including pathogens, or smallpox immunization. The obstacles associated with this assay include the need for blood collection and subsequent cold chain transit. Furthermore, past smallpox immunization has an influence on the results generated. Moreover, the duration of the response is frequently variable [16, 25]. Thirdly, in viral culture technique, a live culture is often generated from a patient specimen. This technique can produce a pure, live viral culture for species identification. In this assay, orthopoxviruses leave characteristic “pocks” on the chorioallantoic membrane. The assay, however, takes a couple of days to complete. Furthermore, patient specimens may have bacteria, making culture attempts difficult. Again, further characterization is required for definitive viral isolation [25].
The fourth assay is tetracore orthopox Bio Threat Alert which detects the presence of orthopoxvirus antigens and can quickly diagnose an active case using patient lesion material. It can be performed at room temperature by a trained, competent laboratory technologist. The assay, however, is less sensitive than PCR and is not selective for MPX [25]. In contrast, anti-orthopoxvirus IgM antibodies are used to detect the existence of orthopoxvirus antibodies. This technique can determine a recent exposure to an orthopoxvirus. However, the assay has some limitations including the need for blood collection and subsequent cold chain transit [16, 25].
Other techniques employed in the MPXV diagnosis include electron microscopy in which negative staining gives a distinct appearance of a brick-shaped particle in an electron microscopy test, enabling for visual classification of a poxvirus other than parapoxvirus. The assay can detect viral particles in a biopsy sample, scab material, vesicular fluid, or viral culture. The assay easily distinguishes orthopoxvirus from Herpesviridae. The sole flaw is that orthopoxviruses are morphologically indistinguishable from one another [25, 61]. Lastly, the immunohistochemistry assay checks for orthopoxvirus-specific antigens. This method can rule out or identify additional suspicious agents [15, 25].
Therapeutic perspectives
Majority of the treatment is symptomatic and supportive, including the prevention, treatment, and management of bacterial infections. Smallpox vaccination is often recommended for post-exposure prophylaxis of close contacts at high risk of severe disease; however, a rigorous benefit/risk evaluation for the exposed individuals should be undertaken. Furthermore, antivirals may be used as a therapeutic option in extreme situations [26]. According to data from African outbreaks, previous smallpox vaccination provides 85% protection against MPXV. Immunization efficacy was found to be sustained, with protection observed even a couple of years after vaccination, and the occurrence of sequelae was significantly reduced [5].
In general, MPXV cases are mild, and individuals usually feel better within a few weeks. However, the death rate is variable based on variant type. According to reports, the WA clade, the kind seen so far in Europe, has a case fatality rate of roughly 3.6%. Children, young adults, and immunocompromised people are more likely to die [30, 66]. Although no particular treatments exist for MPXV, the smallpox vaccine, which has been demonstrated to be approximately 85% effective in preventing MPX, as well as the antivirals cidofovir and tecovirimat, can be employed to suppress outbreaks. According to reports, the UK government purchased thousands of vaccine doses and distributed them to affected people’s close contacts [30].
There are several drugs available, including tecovirimat, vaccinia immune globulin intravenous (VIGIV), brincidofovir, and cidofovir [71]. Tecovirimat is an antiviral medicine licensed by the Food and Drug Administration (FDA) to treat human smallpox illness in adults and children weighing 3 kg and above. TPOXX or ST-246 are other names for the antiviral medication [16, 72]. On the other hand, the FDA approved VIGIV is used for the treatment of vaccinia vaccination problems such as severe generalized vaccinia, eczema vaccinatum, progressive vaccinia, vaccinia infections in people with skin disorders, and aberrant infections caused by vaccinia virus [15].
On November 3, 2021, the Advisory Committee on Immunization Practices (ACIP) recommended the immunization of certain individuals at risk of occupational exposure to orthopoxviruses. These included research laboratory staff, clinical laboratory personnel performing orthopoxvirus diagnostic tests, and designated emergence response members who were at risk of occupational exposure to the viruses [9]. In addition, healthcare staff administering ACAM2000 or care for patients infected with replication competent orthopoxviruses based on shared clinical decision-making were included in the category [15].
Currently, two vaccines are recommended for use worldwide. These are ACAM2000 and JYNNEOS [21, 53, 55]. JYNNEOS is a live vaccine derived from the strain modified vaccinia Ankara-Bavarian Nordic (MVA-BN), an attenuated, non-replicating orthopoxvirus [16]. This vaccine, also known as IMVAMUNE, IMVANEX, and MVA, was approved by the FDA in September 2019. The vaccine is approved for the prevention of smallpox and MPX disease in individuals 18 years and above who have been assessed to be highly susceptible to smallpox or MPX infection [15]. It is given as two-dose subcutaneous injectable series 28 days apart [9].
Following immunization with JYNEOS, there is no substantial cutaneous reaction, often known as a “take” and hence no risk of unintentional inoculation or autoinoculation. The JYNNEOS immunogenicity in clinical investigations and efficacy data from animal challenge experiments were used to determine its effectiveness. Because JYNNEOS is a replication-deficient viral vaccine, serious adverse effects are unlikely. However, because the mechanism for myopericarditis after ACAM2000 administration is thought to be immune-mediated, it is unknown if the antigen or antigens that precipitate autoantibodies are also detectable in JYNNEOS [9]. ACAM2000, on the other hand, is a live vaccinia virus vaccine that was approved by the FDA in August 2007. This vaccine replaced Dryvax, whose producer revoked its license and destroyed the leftover vaccine. The ACAM2000 vaccine is recommended for active vaccination against smallpox infection in people who have been assessed to be highly susceptible to infection [15].
In 2015, ACIP recommended the use of ACAM2000, the only orthopoxvirus vaccine available in the US at the time [73]. From 2020 to 2021, ACIP considered evidence for employing JYNNEOS, a replication-deficient vaccinia virus vaccine, as an alternative to ACAM2000. In November 2021, ACIP unanimously authorized JYNNEOS as an alternative to ACAM2000 for initial vaccination and booster doses. With new JYNNEOS use recommendations, ACAM2000 and JYNNEOS were recommended for preexposure prophylaxis against orthopoxvirus infection in people who are at risk of such exposures [9]. Because ACAM2000 is replication-competent, there is a danger of serious side effects including progressive vaccinia and dermatitis vaccinatum. Furthermore, incidences of myopericarditis were documented, with an estimated rate of 5.7 per 1000 primary vaccinees based on clinical trial data [74].
Scientifically, either infection with an orthopoxvirus or immunization with an orthopoxvirus vaccine offers immunologic cross-protection against other viruses in the same genus [2, 73]. Reports indicate that until 1971, children in the US received orthopoxvirus vaccine as part of their mandatory childhood immunizations. However, after the World Health Organization (WHO) declared the elimination of smallpox in 1980, immunization was discontinued [9]. With an efficiency rate of 85%, smallpox vaccination has been shown to be protective against MPXV [2, 7, 53]. In this regard, the discontinuation of smallpox vaccination as a result of smallpox eradication in 1980 has been postulated as one of the likely causes of the rapid rise in cases in Africa and outbreaks outside the continent [24, 75].
Conclusion
MPXV outbreaks in Europe, Australia, and US generated serious concerns. The fact that it expanded to 19 nations reveals that it is a disease of medical importance. As part of the lessons learnt from the COVID-19 pandemic, nations must prioritize early detection, monitoring, contact mapping, and reporting of MPX cases. Moreover, contact tracing methods should be updated and the effectiveness of diagnostic and therapeutic technologies assessed. Additionally, proactive risk communication and community involvement efforts should be implemented to raise awareness, provide updates, and support to persons at high risk and the wider public. Because of the risk posed by human-to-animal transmission, intensive intersectoral collaboration between human and veterinary public health agencies operating from a “One Health” viewpoint is required to manage exposed pets and curb disease transmission in wildlife. Moreover, future scientific research must concentrate on integrative methodologies that combine human, animal, and environmental efforts to better understand the various components of this disease system and give suitable remedies to protect public health.
Availability of data and materials
Not applicable.
Abbreviations
- ACIP:
-
Advisory Committee on Immunization Practices
- CA:
-
Central African
- COG:
-
Conserved oligomeric golgi
- DRC:
-
Democratic Republic of Congo
- EV:
-
Extracellular enveloped virions
- FDA:
-
Food and Drug Administration
- GARP:
-
Golgi-associated retrograde protein
- MPV:
-
Monkeypox
- MPXV:
-
Monkeypox virus
- MSM:
-
Men who have sex with men
- MV:
-
Intracellular mature virions
- MVA-BN:
-
Modified vaccinia ankara-bavarian nordic
- PCR:
-
Polymerase chain reaction
- UK:
-
United Kingdom
- US:
-
United States
- VIGIV:
-
Vaccinia immune globulin intravenous
- WA:
-
West African
- WHO:
-
World Health Organization
References
Walker M. Monkeypox Virus Hosts and Transmission Routes. A systematic Review of a Zoonotic Pathogen. Biol Sci Undergrad Honor Theses. 2022. Retrieved from https://scholarworks.uark.edu/biscuht/69.
Ihekweazu, C., & Zumla, A. (n.d.). Human monkeypox – epidemiological and clinical characteristics, diagnosis and prevention. Eskild Petersen , Anu Kantele , Marion Koopmans , Danny Asogun , Adesola Institutional affiliations : Mailing Addresses : Keywords : Monkeypox , smallpox, W. 1–24.
Lansiaux, E. (2022). The virology of human monkeypox virus (hMPXV ): a brief overview. (June). https://doi.org/10.13140/RG.2.2.30021.73442
Parigger, L., Krassnigg, A., Grabuschnig, S., Resch, V., Gruber, K., & Gruber, C. C. (2022). Preliminary structural proteome of the monkeypox virus causing a multi-country outbreak in May 2022. (May), 1–9.
Pal M, Mengstie F, Kandi V. Epidemiology, diagnosis, and control of monkeypox disease: a comprehensive review. Am J Infect Dis Microbiol. 2017;5(2):94–9. https://doi.org/10.12691/ajidm-5-2-4.
Bernstein AS, Ando AW, Loch-Temzelides T, Vale MM, Li BV, Li H, et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci Adv. 2022;8(5):1–14. https://doi.org/10.1126/sciadv.abl4183.
Cabanillas B, Valdelvira R, Akdis CA. Monkeypox outbreak in Europe, UK, North America, and Australia: a changing trend of a zoonotic disease. Allergy. 2022;77:1. https://doi.org/10.1111/all.15393.
Mauldin MR, McCollum AM, Nakazawa YJ, Mandra A, Whitehouse ER, Davidson W, et al. Exportation of monkeypox virus from the African continent. J Infect Dis. 2022;225(8):1367–76. https://doi.org/10.1093/infdis/jiaa559.
Rao, A. K., Petersen, B. W., Whitehill, F., Razeq, J. H., Isaacs, S. N., & Merchlinsky, M. J. (2022). Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses : recommendations of the Advisory Committee on Immunization Practices — United States, 2. 71(22).
Atoui A, Jourdain F, Mouly D, Cordevant C, Chesnot T, Gassilloud B. A review on mpox (monkeypox) virus shedding in wastewater and its persistence evaluation in environmental samples. Case Stud Chem Environ Eng. 2023;7:100315.
Bonilla-Aldana DK, Rodriguez-Morales AJ. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet Q. 2022;42(1):148–50.
Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8(2):129–57. https://doi.org/10.2217/fvl.12.130.
Yu X, Shi H, Cheng G. Mpox virus: its molecular evolution and potential impact on viral epidemiology. Viruses. 2023;15(4):995.
Jahrling PB, Wahl-Jensen V. Orthopoxviruses. In: Biodefense Research Methodology and Animal Models. 2nd ed. 2012. p. 255–70. https://doi.org/10.1201/b11523.
Cortez KJ. Monkey Pox: Ignored or emerging pathogen. 2022. Retrieved from https://scholar.rochesterregional.org/grandrounds_ched/8.
Pittman, P. R., Martin, J. W., Kingebeni, P. M., Tamfum, J. M., Wan, Q., Reynolds, M. G., … Military, N. (2022). Clinical characterization of human monkeypox infections in the Democratic Republic of the Congo. Clinical characterization of human monkeypox infection.
Gao A, Gao S. In silico identification of non-cross-reactive epitopes for monkeypox cell surface-binding protein. Preprint at Research Square 2022. https://doi.org/10.21203/rs.3.rs-1693979/v1.
Yoo J. Once bitten, twice shy: our attitude towards monkeypox. J Korean Med Sci. 2022;37(22):6–8.
Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J Gen Virol. 2009;90(9):2266–71. https://doi.org/10.1099/vir.0.010207-0.
Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):1–20. https://doi.org/10.1371/journal.pntd.0010141.
Yang Z. Monkeypox: a potential global threat? J Med Virol. 2022; https://doi.org/10.1002/jmv.27884.
Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. https://doi.org/10.1016/j.virol.2005.05.030.
Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225(1):96–113. https://doi.org/10.1111/j.1600-065X.2008.00691.x.
Shanmugaraj B, Khorattanakulchai N, Phoolcharoen W. Emergence of monkeypox: another concern amidst COVID-19 crisis. Asian Pac J Trop Med. 2022;15(5):193. https://doi.org/10.4103/1995-7645.346081.
Makkar, D. (2022). The latest news for May 2022 all you need to know on monkeypox. (June). https://doi.org/10.20944/preprints202206.0036.v1
Centre for Disease Prevention, E. Monkeypox multi-country outbreak Key messages. European Centre for Disease Control and Outbreak. 2022. Available on https://www.ecdc.europa.eu/sites/default/files/documents/Monkeypox-multi-countryoutbreak.pdf.
Palmer M. Could current monkeypox outbreak be natural. 2022. Available at: https://doctors4covidethics.org/could-thecurrent-monkeypox-outbreak-be-natural/.
Guarner J, Johnson BJ, Paddock CD, Shieh WJ, Goldsmith CS, Reynolds MG, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis. 2004;10(3):426–31. https://doi.org/10.3201/eid1003.030878.
Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15(4):280–7. https://doi.org/10.1053/j.spid.2004.09.001.
Mahase E. Monkeypox: what do we know about the outbreaks in Europe and North America? Bmj. 2022;1274 https://doi.org/10.1136/bmj.o1274.
Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140(3–4):229–36. https://doi.org/10.1016/j.vetmic.2009.08.026.
Simpson K, Heymann D, Brown CS, Edmunds WJ, Elsgaard J, Fine P, et al. Human monkeypox – after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077–81. https://doi.org/10.1016/j.vaccine.2020.04.062.
Arita I, Gispen R, Kalter SS, Wah LT, Marennikova SS, Netter R, Tagaya I. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull World Health Organ. 1972;46(5):625–31.
Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Human monkeypox and other poxvirus infections of man. Smallpox Eradication. 1988;29:1287–319.
Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther. 2022;7(1):1–22. https://doi.org/10.1038/s41392-022-01215-4.
Sklenovská N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:1–12. https://doi.org/10.3389/fpubh.2018.00241.
Levine RS, Peterson AT, Yorita KL, Carroll D, Damon IK, Reynolds MG. Ecological niche and geographic distribution of human monkeypox in Africa. PLoS One. 2007;2(1):1–7. https://doi.org/10.1371/journal.pone.0000176.
Vol, E. R., Outbreak, M., States, N., Minhaj, F. S., Ogale, Y. P., Whitehill, F., … Foote, M. (2022). Monkeypox outbreak — nine states, May 2022 71(May), 1–6.
Mucker EM, Thiele-Suess C, Baumhof P, Hooper JW. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol Ther - Nucleic Acids. 2022;28(June):847–58. https://doi.org/10.1016/j.omtn.2022.05.025.
Realegeno S, Priyamvada L, Kumar A, Blackburn JB, Hartloge C, Puschnik AS, et al. Conserved oligomeric golgi (COG) complex proteins facilitate orthopoxvirus entry, fusion and spread. Viruses. 2020;12(7) https://doi.org/10.3390/v12070707.
Golden JW, Zaitseva M, Kapnick S, Fisher RW, Mikolajczyk MG, Ballantyne J, et al. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol J. 2011;8 https://doi.org/10.1186/1743-422X-8-441.
Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79(21):13454–62. https://doi.org/10.1128/jvi.79.21.13454-13462.2005.
Bengali Z, Townsley AC, Moss B. Vaccinia virus strain differences in cell attachment and entry. Virology. 2009;389(1–2):132–40. https://doi.org/10.1016/j.virol.2009.04.012.
Chiu W-L, Lin C-L, Yang M-H, Tzou D-LM, Chang W. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J Virol. 2007;81(5):2149–57. https://doi.org/10.1128/jvi.02302-06.
Chung C-S, Hsiao J-C, Chang Y-S, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72(2):1577–85. https://doi.org/10.1128/jvi.72.2.1577-1585.1998.
Lin C-L, Chung C-S, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol. 2000;74(7):3353–65. https://doi.org/10.1128/jvi.74.7.3353-3365.2000.
Law M, Carter GG, Roberts KL, Hollinshead M, Smith GL. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc Natl Acad Sci USA. 2006;103(15):5989–94. https://doi.org/10.1073/pnas.0601025103.
Roberts KL, Breiman A, Carter GC, Ewles HA, Hollinshead M, Law M, Smith GL. Acidic residues in the membrane-proximal stalk region of vaccinia virus protein B5 are required for glycosaminoglycan-mediated disruption of the extracellular enveloped virus outer membrane. J Gen Virol. 2009;90(7):1582–91. https://doi.org/10.1099/vir.0.009092-0.
Realegeno S, Puschnik AS, Kumar A, Goldsmith C, Burgado J, Sambhara S, et al. Monkeypox virus host factor screen using haploid cells identifies essential virus formation. J Virol. 2017;91(11):1–16.
Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661–72. https://doi.org/10.1099/vir.0.81215-0.
Liszewski MK, Leung MK, Hauhart R, Buller RML, Bertram P, Wang X, et al. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J Immunol. 2006;176(6):3725–34. https://doi.org/10.4049/jimmunol.176.6.3725.
Lopera JG, Falendysz EA, Rocke TE, Osorio JE. Attenuation of monkeypox virus by deletion of genomic regions. Virology. 2015;475:129–38. https://doi.org/10.1016/j.virol.2014.11.009.
Saxena SK, Ansari S, Maurya VK, Kumar S, Jain A, Paweska JT, et al. Re-emerging human monkeypox: a major public-health debacle. J Med Virol. 2022; https://doi.org/10.1002/jmv.27902.
Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12(11). https://doi.org/10.3390/v12111257.
Harris E. What to know about monkeypox. Jama. 2022;1–2 https://doi.org/10.1001/jama.2022.9499.
Wilson ME, Hughes JM, McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260–7. https://doi.org/10.1093/cid/cit703.
Kozlov M. Monkeypox goes global: why scientists are on alert. Nature. 2022;606(7912):15–6. https://doi.org/10.1038/d41586-022-01421-8.
Milbank C, Vira B. Wildmeat consumption and zoonotic spillover: contextualising disease emergence and policy responses. Lancet Planet Health. 2022;6(5):e439–48. https://doi.org/10.1016/S2542-5196(22)00064-X.
Petersen E, Abubakar I, Ihekweazu C, Heymann D, Ntoumi F, Blumberg L, et al. Monkeypox — enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78–84. https://doi.org/10.1016/j.ijid.2018.11.008.
Nolen LD, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, et al. Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the democratic republic of the congo. Am J Trop Med Hyg. 2015;93(2):410–5. https://doi.org/10.4269/ajtmh.15-0168.
Ahsan MM, Uddin MR, Farjana M, Sakib AN, Al Momin K, Luna SA. “Image Data collection and implementation of deep learning-based model in detecting Monkeypox disease using modified VGG16,” arXiv preprint. 2022;arXiv:2206.01862. Available on; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10304329/. Accessed 29 Mar 2023.
Beer EM, Bhargavi Rao V. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10):1–20. https://doi.org/10.1371/journal.pntd.0007791.
Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7(3):434–8. https://doi.org/10.3201/eid0703.017311.
Seang S, Burrel S, Todesco E, Leducq V, Monsel G, Le Pluart D, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400(10353):658–9. https://doi.org/10.1016/S0140-6736(22)01487-8.
Centre for Disease Prevention. Priotizing Case Investigation and Contact Tracing for COVID-19. 2022. Available at; https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracingplan/prioritization.html. Accessed 27 May 2022.
Kabuga AI, El Zowalaty ME. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J Med Virol. 2019;91(4):533–40. https://doi.org/10.1002/jmv.25348.
Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect. 2016;1(1):1–13. https://doi.org/10.3390/tropicalmed1010008.
Ciccarese G, Di Biagio A, Bruzzone B, Guadagno A, Taramasso L, Oddenino G, et al. Monkeypox outbreak in Genoa, Italy: clinical, laboratory, histopathologic features, management, and outcome of the infected patients. J Med Virol. 2023;95(2):1–12. https://doi.org/10.1002/jmv.28560.
Quattri E, Avallone G, Maronese CA, Cusini M, Carrera CG, Marzano AV, Ramoni S. Unilesional monkeypox: a report of two cases from Italy. Travel Med Infect Dis. 2022;49:102424.
Maldonado-Barrueco A, Sanz-González C, Gutiérrez-Arroyo A, Grandioso-Vas D, Roces-Álvarez P, Sendagorta-Cudos E, et al. Sexually transmitted infections and clinical features in monkeypox (mpox) patients in Madrid, Spain. Travel Med Infect Dis. 2023;52:102544. https://doi.org/10.1016/j.tmaid.2023.102544.
Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;3099(22):1–10. https://doi.org/10.1016/s1473-3099(22)00228-6.
Nakoune E, Olliaro P. Waking up to monkeypox. Bmj. 2022;1321 https://doi.org/10.1136/bmj.o1321.
Petersen BW, Harms TJ, Reynolds MG, Harrison LH. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses — recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65(10):257–62. https://doi.org/10.15585/mmwr.mm6510a2.
Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43(9):1503–10. https://doi.org/10.1016/j.jacc.2003.11.053.
Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973;37(1):1–18. https://doi.org/10.1128/mmbr.37.1.1-18.1973.
Acknowledgements
Not applicable
Funding
The authors declare that the research was conducted in the absence of any financial grants.
Author information
Authors and Affiliations
Contributions
Josephine Wambani, Tom Were, and Patrick Okoth contributed equally to this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wambani, J., Were, T. & Okoth, P. Monkeypox: genetic, clinical, molecular, diagnostic, and therapeutic perspectives. J Rare Dis 3, 18 (2024). https://doi.org/10.1007/s44162-024-00042-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44162-024-00042-1