Abstract
Neurofibromatosis type 1, resulting from dominantly inherited mutations affecting neurofibromin, is among the most common human genetic syndromes associated with many neurocutaneous manifestations. Neoplasms, neurogenic and non-neurogenic, are frequent, as are the gastrointestinal manifestations. Gastrointestinal tumors and vasculopathy are well-reported in individuals with neurofibromatosis type 1. A combination of somatostatioma and gastrointestinal stromal tumor is considered pathognomonic. We here report a case of neurofibromatosis type 1 with a triad of distinct neoplasms: gastrointestinal stromal tumor, neuroendoscrine tumor, and intra-ampullary papillary-tubular neoplasm. The trilogy of these neoplasms is unique and, to our knowledge, has never been reported in the literature. The report also emphasizes the role of advanced immunochemical staining in day-to-day practice, which has improved diagnostic accuracy and yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type 1 (NF1) is one of the most common monogenic disorders inherited dominantly from mutations in the NF1 gene. The most common manifestations include multiple cutaneous neurofibromas and café au lait spots. A total of 10–25% of individuals with NF1 experience gastrointestinal lesions, like tumors or vasculopathy. However, the actual extent of gastrointestinal involvement may be underreported, as some of these lesions may remain undiagnosed or overlooked. Neurogenic tumors, neurofibromas, and ganglioneuromatosis are the most frequent gastrointesional tumors in NF1. Gastrointestinal stromal tumors (GIST) are the most frequent non-neurogenic tumors; others include neuroendocrine tumors (NET) and, less commonly, adenocarcinomas [1].
Case report
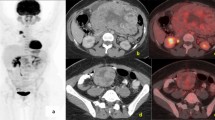
A 41-year-old female with NF1 presented with upper abdominal nonspecific pain, bloating, belching, and weight loss of 1-month duration. There was no history of dysphagia, vomiting, altered bowel habits, or overt gastrointestinal bleeding. She has a family history of neurofibromatosis in her siblings and father, with multiple cutaneous neurofibromas and café-au lait spots, but has no history of gastrointestinal neoplasms. Clinical examination revealed multiple and extensive cutaneous neurofibromas (more than 100) and café-au-lait spots of varying sizes scattered all over the body, axillary freckling, and conjunctival pallor. A hemogram revealed iron deficiency anemia. Hepatic biochemistry revealed normal bilirubin and protein levels, alkaline phosphatase 282 IU/ml (normal, 40–150 IU/ml), aspartate aminotransferase 48 IU/ml (normal, 5–34 IU/ml), and alanine aminotransferase 71 IU/ml (normal, 0–55 IU/ml). Dilation of the common bile duct, 18 mm (normal, < 6 mm), bilateral intrahepatic biliary radicles, and pancreatic duct, 7 mm (normal < 4 mm), were demonstrable in ultrasound. Contrast-enhanced computed tomography, in addition to confirming the sonological findings, revealed an enhancing mass lesion at the second part of the duodenum involving the ampulla, narrowing of the common bile duct and the main pancreatic duct, with upstream dilation and another non-obstructing enhancing polypoidal lesion involving the jejunum (Fig. 1). The serum chromogranin-A was elevated, 1388 ng/L (normal < 43 ng/L), while insulin, somatostatin, gastrin, and vasoactive intestinal peptide levels were within normal limits. The upper gastrointestinal endoscopy revealed a bulky ampulla, and biopsies revealed only unremarkable duodenal epithelium. The patient underwent pylorus-preserving pancreatoduodenectomy. Gross analysis of the resection specimen revealed three lesions, two in the periampullary region adjacent to each other and a third in the proximal jejunum (Fig. 2). Biopsy confirmed the three histologically discrete lesions.
-
Lesion 1: Duodenal well-differentiated, grade 2, NET, positive for synaptophysin and chromogranin, with submucosal and lymphovascular invasion and a Ki-67 index of 6% (Fig. 3)
-
Lesion 2: Intra-ampullary papillary-tubular neoplasm (Fig. 3)
-
Lesion 3: Jejunal spindle cell type, GIST, positive for CD 117, DOG1, and negative for S100, desmin (Fig. 3)
Five of the 27 lymph nodes in the resection specimen revealed NET deposits. The patient had an uneventful inhospital course and was discharged on the fifth postoperative day. An oncology consult was obtained, and she is being followed up on an outpatient basis, remains asymptomatic, and can tolerate oral fluid and feeds.
Discussion
NF1, also known as Von Recklinghausens disease, is a frequently encountered genetic disorder. It is known to be associated with many tumors or neoplasms of neural and extra-neural origin; hence, it is considered an inherited tumor syndrome [2]. Gastrointestinal manifestations include tumors and vasculopathy. Tumors may arise from the neurons or ganglia of the autonomic nervous system or the interstitial cells of Cajal [2]. Neurofibromas and ganglioneuroma are the more frequent neural lesions, while paragangliomas and schwannomas are also reported. GISTs are increasingly reported; typically small and asymptomatic, they are most frequently reported in the duodenum [2, 3]. Due to unknown reasons, the periampullary duodenum and ampulla of Vater have a high propensity for tumors in NF1; tumors involving these regions are hence considered a characteristic feature. Periampullary somatostatinomas and GIST constitute most of these lesions. A coexistence of periampullary somatostatinoma or gastrointestinal nerve tumors with GIST is almost unique and pathognomonic of NF1 [3]. Gastrointestinal adenocarcinomas are more frequently reported in the setting of NF1, but whether there is a true association remains controversial.
GIST is often regarded as the most common gastrointestinal tumor in NF1, with a 150-fold higher risk than the general population. NF1-associated GIST more frequently involves the small intestine and multifocal with lower malignant potential and often lacks KIT or PDGFRA mutations [4, 5]. The spindle cell variant is the most frequent subtype. Computed tomography (CT) and magnetic resonance imaging (MRI) help diagnose and confirm with histology, immunochemistry, and genetic tests. Immunochemical markers include CD117 and DOG1; the latter is considered more sensitive and specific [5].
Intra-ampullary papillary-tubular neoplasms are preinvasive neoplasms of the ampulla of Vater [6]. They are rare tumors, accounting for about 0.5% of gastrointestinal tumors [7]. They are mass-forming and obstructing, with an upstream dilatation of biliary and/or pancreatic ducts. The histological growth may follow a predominant papillary (villous) or tubular pattern or a mixed tubulopapillary pattern. Lesions may progress to invasive neoplasms, which may be detectable at initial histology. Compared to invasive neoplasms, the overall prognosis is better, even in the presence of invasive elements at the initial diagnosis [6].
Neuroendocrine tumors are well documented in individuals with NF1; they typically involve the ampulla and periampullary duodenum. Somatostatinomas are the most classical lesions. Concomitant GIST and somatostatinoma are almost pathognomonic of NF1. Pheochromocytomas are also reported in patients with NF1. Serum markers, chromogranin A or catecholamines, and urinary metabolite assays suggest the diagnosis. Cross-sectional imaging and endoscopic ultrasound help in localizing the lesion. Diagnosis is confirmed with a biopsy and immunohistochemical staining with synaptophysin or chromogranin A [8].
A population-based study published in 2013 reported 42 cases of colorectal cancer in an NF1 cohort of 8003 patients [9]. Also, a literature search reveals a handful of reported cases of gastrointestinal and pancreatic-biliary adenocarcinomas in NF1 [10, 11]. Hence, NF1 may be associated with an increased risk of adenocarcinomas; however, this association is controversial and can be casual.
We here report a case meeting of the clinical diagnostic criteria for NF1, with a triad of neoplasms of distinct cells of origin in the duodenum, ampulla, and jejunum. A handful of reports of synchronous gastrointestinal neoplasms in NF1 exist in published medical literature. Zhang et al. reported a case of coexistent small intestinal adenocarcinoma and GIST [12]. Park et al. reported three cases with synchronous ampullary NET and GIST, treated with pancreaticoduodenctomy [13]. Makita et al. reported a case with concurrent GIST and NET who underwent pancreaticoduodenectomy but succumbed to a massive bleed [14]. Even though neoplasms are frequently reported in NF1, simultaneous detection of three discrete tumors undergoing successful surgical resection is exceedingly rare. This case report emphasizes that NF1 is a preneoplastic condition, and the mutated neurofibromin predisposes to various neurogenic and non-neurogenic neoplasms. We were unable to perform molecular or genetic confirmatory tests for neurofibromatosis in this case, owing to a lack of facilities for the same, and the diagnosis of neurofibromatosis was made exclusively based on clinical criteria.
Conclusion
Neurofibromatosis, one of the most common inherited genetic syndromes, is established to be associated with a variety of neurogenic and non-neurogenic tumors. The gastrointestinal tract is no exception to neoplastic risk and can harbor a variety of tumors in Von Recklinghausen’s disease, the most common being GIST. A combination of GIST and NET, especially somatostatinoma, is considered a pathognomonic feature of this monogenic syndrome. Individuals with neurofibromatosis type 1 are hence at a higher likelihood of gastrointestinal neoplasms and should be promptly evaluated for those presenting with gastrointestinal symptoms. Immunochemical staining techniques will increase the diagnostic yield and accuracy, especially in the setting of uncommon neoplasms.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available as per institutional policies but are available from the corresponding author as well as the medical records departments of Ahlaia Hospital and Mietra Hospital on reasonable request.
References
Garrouche N, Ben Abdallah A, Arifa N, Hasni I, Ben Cheikh Y, Ben Farhat W, Ben Amor S, Jemni H. Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights Imaging. 2018;9(5):661–71. https://doi.org/10.1007/s13244-018-0648-8. Epub 2018 Sep 4. PMID: 30187267; PMCID: PMC6206377.
Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen’s disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5(9):852–62 Epub 2012 Oct 20. PMID: 23119102; PMCID: PMC3484498.
Zöller ME, Rembeck B, Odén A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer. 1997;79(11):2125–31 PMID: 9179058.
Andersson J, Sihto H, Meis-Kindblom JM, Joensuu H, Nupponen N, Kindblom LG. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol. 2005;29:1170–6. https://doi.org/10.1097/01.pas.0000159775.77912.15.
Abu-Abaa M, Abdulsahib A, Kananeh S, Aldookhi A. A rare association between gastrointestinal stromal tumor and neurofibromatosis type 1: a case report. Cureus. 2023;15(1):e34148. https://doi.org/10.7759/cureus.34148. PMID: 36843795; PMCID: PMC9949750.
Ohike N, Kim GE, Tajiri T, Krasinskas A, Basturk O, Coban I, Bandyopadhyay S, Morohoshi T, Goodman M, Kooby DA, Sarmiento JM, Adsay NV. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34(12):1731–48. https://doi.org/10.1097/PAS.0b013e3181f8ff05. PMID: 21084962; PMCID: PMC3168573.
Tejaswi S, Parikh M, Fananapazir G, Olson K, Gui D. Intra-ampullary papillary-tubular neoplasm. VideoGIE. 2023;8(7):277–82. https://doi.org/10.1016/j.vgie.2023.03.002. PMID: 37456221; PMCID: PMC10338961.
Dare AJ, Gupta AA, Thipphavong S, Miettinen M, Gladdy RA. Abdominal neoplastic manifestations of neurofibromatosis type 1. Neurooncol Adv. 2020;2(Suppl 1):i124–33. https://doi.org/10.1093/noajnl/vdaa032. PMID: 32642738; PMCID: PMC7317050.
Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108(1):193–8. https://doi.org/10.1038/bjc.2012.535. Epub 2012 Dec 20. PMID: 23257896; PMCID: PMC3553528.
Kim IY, Cho MY, Kim YW. Synchronous multiple colonic adenocarcinomas arising in patient with neurofibromatosis type 1. Ann Surg Treat Res. 2014;87(3):156–60. https://doi.org/10.4174/astr.2014.87.3.156. Epub 2014 Aug 26. PMID: 25247170; PMCID: PMC4170576.
Costi R, Caruana P, Sarli L, Violi V, Roncoroni L, Bordi C. Ampullary adenocarcinoma in neurofibromatosis type 1. Case report and literature review. Mod Pathol. 2001;14(11):1169–74. https://doi.org/10.1038/modpathol.3880454. PMID: 11706080.
Zhang W, Hu X, Chen Z, Lai C. Case report: neurofibromatosis type 1 gastrointestinal stromal tumor and small bowel adenocarcinoma with a novel germline NF1 frameshift mutation. Front Oncol. 2022;22(12):1052799. https://doi.org/10.3389/fonc.2022.1052799. PMID: 36620543; PMCID: PMC9815498.
Park EK, Kim HJ, Lee YH, Koh YS, Hur YH, Cho CK. Synchronous gastrointestinal stromal tumor and ampullary neuroendocrine tumor in association with neurofibromatosis type 1: a report of three cases. Korean J Gastroenterol. 2019;74(4):227–31. https://doi.org/10.4166/kjg.2019.74.4.227. PMID: 31650799.
Makita N, Kayahara M, Kano S, Munemoto M, Yagi Y, Onishi I, Kawashima A. A case of duodenal neuroendocrine tumor accompanied by gastrointestinal stromal tumors in type 1 neurofibromatosis complicated by life-threatening vascular lesions. Am J Case Rep. 2021;11(22):e927562. https://doi.org/10.12659/AJCR.927562. PMID: 33424018; PMCID: PMC7810287.
Acknowledgements
Nil.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Dr. G initially evaluated the patient. Dr. G and Dr. A performed the endoscopic evaluation. Dr. S and Dr. R performed surgery. Dr. C performed histopathological/immunochemical evaluations. Dr. G and Dr. A drafted the manuscript, which was proofread by Dr. S, Dr. R, and Dr. C. Dr. G and Dr. A performed literature searches and revisions in the manuscript. The final manuscript was read and acknowledged by all the authors, and it was unanimously decided that Dr. A would be the corresponding author for the manuscript for further clarifications and queries. None of the authors has received any funding for this publication. None of the authors has any conflicts of interest to declare.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for case presentation and publication was obtained from the Mietra Hospital Institutional Academic and Ethics Committees in October 2023.
Consent for publication
Written informed consent for the publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kakkat, S., Zacharia, G.S., Ravindran, R. et al. Neurofibromatosis type 1 with three synchronous tumors. J Rare Dis 3, 6 (2024). https://doi.org/10.1007/s44162-024-00030-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44162-024-00030-5