Abstract
Background
Lung cancer is a major cause of health loss internationally, and in Australia. Most of that loss is inequitably concentrated among vulnerable or disadvantaged people and amenable to prevention and earlier detection. In response, best practice lung cancer care considers peoples’ background, circumstances and care needs. Comprehensive, person level descriptions of demographic, health and discrete socio-economic disadvantage related factors are therefore required to inform best practice. We examine population wide correlations of demographic, health and socioeconomic characteristics with lung cancer diagnosis for use in cancer control programs, including screening.
Methods
A study of 5,504,777 (89.9%) adults living in New South Wales and participating in Australia’s Census in August 2016 with subsequent follow-up to the end of 2018. The Australian Bureau of Statistics’ (ABS) person-level integrated data asset linked census records with the NSW population cancer registry which includes primary site. Our study compared census participants who did not experience cancer in the follow-up period with those diagnosed with lung cancer, (n = 6160 and ICD10 C33-34). Outcomes are expressed as the adjusted relative odds (aOR) of incident lung cancer among adults in the community and measured using multi-variable logistic regression models. Validated ABS methods informed categorisation of social and economic variables.
Results
Multivariable comparison of those with lung cancer and those without a first cancer diagnosis (3276 lung cancers among 2,484,145 males; 2884 lung cancers among 2,944,148 females) showed associations with increasing age, varying ancestry, living alone (aOR = 1.30 95% CI 1.19–1.42 males; 1.24 95% CI 1.14–1.35 females), number of health conditions medicated, less than Year 12 education (aOR = 1.40 95% CI 1.30–1.51 males; 1.37 95% CI 1.27–1.48 females) and housing authority rental (aOR = 1.69 95% CI 1.48–1.94 males; 1.85 95% CI 1.63–2.11 females). Additional associations occurred among males with low income, disabilities before age 70, those unemployed and labouring occupations. As numbers of characteristics increased, so did the likelihood of lung cancer.
Conclusion
We provided a population wide description of characteristics relevant to lung cancer diagnosis. Deeper knowledge of these characteristics inform continuing development of lung cancer programs in prevention (e.g. tobacco control) and detection (e.g. lung cancer screening), then help prioritise targeted delivery of those programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Lung cancer is one of the top five contributors to disease burden globally [1] and in Australia [2]. This burden is mainly due to early death [1, 2] as 5-year survival is relatively poor whether compared against all cancers combined (19% versus 70% in 2012–2016) or the wider population [3].
Most health loss from lung cancer is amenable to change by prevention and early detection [4, 5] as incident cases are often attributable to risk exposure from tobacco use [6, 7], chemicals in the workplace or wider environment [7], inadequate diet and physical inactivity [4, 8]. As with health risks generally [9, 10], exposure to lung cancer related risks are unequally, and often inequitably, distributed. Risks cluster among groups within the population [4] and lead to disparities in lung cancer incidence and outcomes [11]. Put another way, people with higher likelihood of lung cancer are also more likely to share particular demographic characteristics, and health and socio-economic disadvantages. Demographic correlates include sex [7, 12,13,14], ancestry, ethnicity [13, 14] and Aboriginal status [3], living in remote locations [4] and living alone [15]. Pre-existing physical health conditions are common because of the strong associations with tobacco smoking and ageing [16,17,18,19]. Socio-economic disadvantage is strongly associated with tobacco use [20, 21] and, in turn, heavily contributes to lung cancer incidence [10, 22,23,24]. In Australia, relative disadvantage at area levels is estimated using 5-yearly census records [25] and matching with population cancer registry records allows for monitoring ecological cancer trends. Person level descriptions of some demographic, most health characteristics and the discrete factors contributing to socio-economic disadvantage in Australia are not routinely available and rely on infrequent survey samples [14, 26] or dedicated epidemiological studies. Other jurisdictions face similar limitations, for example in the US where lung cancer screening has been recommended for over a decade, information on eligibility and uptake of screening rely heavily on National Health Survey samples [27, 28].
A comprehensive, systematic approach toward person-centred information in cancer control is warranted. Information describing the characteristics of people diagnosed with lung cancer diagnosis can help meet that need [29]. The knowledge gained will assist with targeting interventions [30] to address health inequities across areas of prevention, screening and early detection, treatment and palliation while making best use of the limited resources available [31].
This study examines associations between demographic, health conditions and socio-economic characteristics of adults in New South Wales and the subsequent diagnosis of lung cancer. The purpose in doing so is to indicate people groups at elevated risk of lung cancer who may require additional attention in service planning and delivery [32].
2 Methods
2.1 Study design
A statewide, population-based study of adults aged 18 years or more in New South Wales (NSW) at Australia’s 2016 Census (August 2016). Participants were then observed over the contiguous period from September 2016 to December 2018 for a first registration of lung cancer.
2.2 Data sources
We used person level, unit records from three data sources: the Australian census, Australia’s universal Pharmaceutical Benefits Scheme (PBS) and the NSW Cancer Registry (NSWCR). Census informed on demographic characteristics of individual and household composition as well as the principal components of socio-economic disadvantage. PBS records covered prescription medications in the 12-months before census among people without lung cancer, or the 12-months before lung cancer diagnosis informed by the NSWCR.
Data were deterministically linked by the Australian Bureau of Statistics (ABS) within their Person-Level Integrated Data Asset (PLIDA) [33, 34] using a unique Person Linkage Spine (PLS) for people recorded in the Australian Medicare Consumer Directory, Centrelink or Taxation datasets.
Our study included linked PLIDA records with a PLS. Those without a PLS, or those with a first, invasive cancer diagnosis other than lung cancer occurring from September 2016 to December 2018 were excluded.
2.3 Variables
The primary outcome is a first diagnosis of lung cancer (ICD10 C33-34) from September 2016 to December 2018.
Demographic characteristics included: age at census arranged in categories (18–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85 years or more) for tabled results and as a continuous measure in multivariable models; sex; geographic remoteness using the Accessibility and Remoteness Index of Australia (ARIA) categorised as major city, inner regional, outer regional/remote; country of birth and Aboriginal self-identification are adopted as proxy measures of ethnicity and ancestry [35], and grouped as Aboriginal, other Australian; China; Germany; Greece; Italy; Lebanon; New Zealand; the Philippines; the United Kingdom; Vietnam; “other mainly English speaking” countries and “mainly non-English speaking” countries [36]; and, single occupant households.
PBS records included the Anatomical Therapeutic Classification (ATC) of prescribed medications. We mapped medications to a set of specific conditions in the Rx Risk comorbidity index using a method validated in Australian settings [37].
Socio-economic disadvantage characteristics included area Index of Relative Socio-Economic Disadvantage (IRSD) [25] quintiles based on Statistical Area 2 level geography. Each of the principal components within IRSD were dichotomized following ABS methods [37, 38]. Those variables included poor English language proficiency, low household income, core function limiting disability at age less than 70, employment status and occupation (as drivers and labourers), no education or noncompletion of Year 12 high school, households with children, resident parent numbers and rental through a housing authority. The latter provided a signal on the nature of housing in lieu of overcrowding which, along with information on household internet connection and motor car availability, was not available in PLIDA.
The potential for selection bias within the whole of enumerated population was examined by testing for PLS presence (or not) for each census variable. A difference in PLS presence for a given characteristics indicates a systematic difference in the population as a whole, and the cases and non-cases compared. For example, if younger adults in the population census are less likely to have an administrative health, social security or taxation record, they are less likely to have a PLS, and less likely to be included in analyses.
2.4 Statistical analysis
Analyses were stratified by sex because patterns of lung cancer incidence vary markedly for males and females and reflect historical differences in tobacco and other risk exposures [39]. We first compared characteristics of participants diagnosed/not diagnosed with lung cancer using cross-tabulations with odds ratios and 95% confidence intervals from multiple logistic regressions (Step 1). We repeated this for each demographic, health and socio-economic characteristic. The odds ratios reported are age-adjusted (aOR) as age was strongly associated with most characteristics. Step 2 focussed on multivariable analyses. Starting with all potential covariates, we purposefully removed the least-contributing variables (with Wald statistic p-values of > 0.2 as a guide), then refitted the model with remaining covariates until deriving a main effects model where each retained covariate substantially contributed [40].
We further summarised the multivariable model by counting the number of relevant demographic, health condition and socio-economic characteristics present for each person—awarding one “point” for each characteristic. That is, for males we totalled points for: relevant ancestries, living alone, having four or more medicated conditions, and each of the relevant socio-economic variables. Similarly, for females we totalled: relevant ancestries, living alone, having four or more medicated conditions, less than Year 12 education and public housing rental. We repeated cross-tabulations and multivariable analyses using the number of correlated characteristics in lieu of discrete variables, then estimated the marginal mean change in odds of lung cancer in the presence of 0, 1, or 2 or more characteristics. Behavioural characteristics such as tobacco use are not included in the available datasets and not open to direct inference.
In each step, we assessed potential collinearity among covariates using variance inflation factors. Data preparation and analyses using Stata 17 were conducted within the ABS DataLab.
3 Results
3.1 Participants
Of 6,120,982 adults enumerated in the 2016 Census, 89.9% were assigned a PLS and became available to analysis (Fig. 1). Linkage was less likely among the youngest and oldest adults (11.9% aged 18 to 23 years and 13.1% aged 85 years or more were not linked), those in remote areas (15.5%), and those born in unspecified, non-English speaking countries (29.1%). After excluding a further 76,484 adults with a first diagnosis of cancer other than lung cancer in the period September 2016 to the end of 2018, a group of 6160 with lung cancer and 5,422,133 community members without a first cancer diagnosis in the period were available to analysis.
Comparatively more males than females were diagnosed with lung cancer (3276 or 0.13% versus 2884 or 0.10% respectively) (Table 1). Participants diagnosed with lung cancer were substantially older than those not diagnosed. For example, 63% of males and 64% of females diagnosed with lung cancer were aged between 50 and 74 years against 35% of males and females community members without cancer diagnosis.
3.2 Males
Distributions of demographic, health and socio-economic disadvantage characteristics among males are described in Table 2.
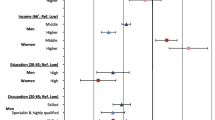
Age-adjusted odds of lung cancer among males increased in the presence of several demographic characteristics. For example, as area remoteness increased, so did the odds of lung cancer diagnosis. Compared with non-Aboriginal Australian born males, odds of lung cancer were twice as high among Aboriginal males (aOR = 2.19 95% CI 1.75,2.75) with elevated odds also observed among males born in Greece, Italy, Lebanon, New Zealand, the United Kingdom and other, non-specified non-English speaking countries, and, those living alone, aOR = 1.41 (95% CI 1.29,1.53). Among the discrete, disadvantage related variables, increased odds of lung cancer were evident among males with poor English proficiency, living in low-income households, with disability, being unemployed or having labourer occupations, less education, or renting through a housing authority.
In a combined, multivariable relationship of male characteristics with lung cancer diagnosis demographic characteristics of increasing age, living outside of major cities and specific backgrounds continued to show a substantial correlation. Compared to other Australian born males, Aboriginal males and those born in China, Greece, New Zealand, the United Kingdom and other predominately non-English speaking countries had increased odds of lung cancer, as did those who lived alone (aOR = 1.31 95% CI 1.20, 1.43). Similarly, as the number of medicated health conditions increased so too did the odds of lung cancer. Discrete socio-economic characteristics rather than the area index showed substantive relationships with lung cancer among males. Specifically, living in a low-income household, being younger while living with disability, unemployment or labouring occupations, having less than Year 12 education and renting through a public housing authority were each related with lung cancer diagnosis.
3.3 Females
Table 3 summarises distributions of demographic, health and socio-economic disadvantage characteristics with lung cancer among females.
Adjusting for age among females showed the odds of lung cancer increased across several demographic characteristics for example in outer regional areas (aOR = 1.20 95% CI (1.05,1.37)). Aboriginal females had more than two-fold higher odds of lung cancer (aOR = 2.43 95% CI 1.96,3.01) with heightened odds also observed among those born in New Zealand and the United Kingdom, those living alone, aOR = 1.36 (95% CI 1.25,1.47) and those with higher numbers of health conditions medicated. The odds of lung cancer increased along with area disadvantage with aOR = 1.48 (95% CI 1.32,1.66) in most versus least disadvantage. Among discrete disadvantage variables, increased odds of lung cancer were apparent among females in low-income households, living with disability, with less than Year 12 education and, those renting through a housing authority.
The multivariable relationships among characteristics and lung cancer diagnosis for females also featured increased age, being Aboriginal and birth in New Zealand, the United Kingdom or other mainly English-speaking countries, along with those living alone (aOR = 1.22 95% CI 1.12, 1.33). An increased number of health conditions again accompanied higher odds of lung cancer as did the discrete, socio-economic characteristics of less than Year 12 education and renting through a public housing authority.
Conversely, several characteristics lowered the odds of lung cancer diagnosis among females and these include having been born in Greece, Italy and Lebanon, or reporting a lower proficiency with English (aOR = 0.65 95% CI 0.55,0.81).
Figure 2 summarises the estimated change in probability of lung cancer diagnosis as age increases and as the number of characteristics increased among males and females (detailed in the Supplementary File). On average, being younger with none of the identified characteristics minimised the likelihood of subsequent lung cancer diagnosis. Age increased the likelihood of diagnosis and the presence of any identified characteristics further increased that likelihood, and in a stepwise manner. For example, a 60 year old female with 1 of the identified characteristics had almost three times greater probability of lung cancer over the next two years than another with none of the characteristics (3 in 10,000 versus 9 in 10,000). The probability doubled again where two or more characteristics were present (20 in 10,000).
4 Discussion
Our population level analysis used a newly available digital platform to expand our knowledge of associations between lung cancer and a wide range of demographic, health and socio-economic characteristics. PLIDA’s extensive population coverage (90%) and person-centred construction allowed our analysis to move beyond ecological studies of socio-economic disadvantage onto individuals’ exposure to specific, disadvantage factors correlated with lung cancer. Having unpacked area level disadvantage into constituent parts at an individual person-level, we found some socio-economic characteristics were associated with lung cancer incidence in both males and females while other characteristics were associated for males rather than females and vice versa.
The likelihood of lung cancer increased among adults of older age, who lived alone, had more medicated health conditions and experienced economic disadvantage. More specifically, disadvantage in the form of not completing high school and renting through a housing authority correlated with lung cancer diagnosis. Aboriginal people along with those born in China, New Zealand and the UK had higher odds of lung cancer than other, non-Aboriginal Australians. So too did males born in Greece and Italy, yet the converse was observed among females of those countries and females reporting poor English proficiency more generally. This is consistent with cultural differences in tobacco use in those contexts [14]. Among males, several other characteristics also related to lung cancer and included: living outside major cities; unemployment or labouring roles; and, being younger while living with long-term disability.
4.1 Limitations
Our analysis was limited in four areas. First, having dichotomised predictor variables in line with a validated, disadvantage index producing method [25, 38], we acknowledge this purposefully focussed our examinations on the extreme end of distributions of income, education, employment and housing. As a result our analysis may have less statistical power and under-estimates [41] the correlation of disadvantage with lung cancer diagnosis. Second, smoking status is important in the aetiology of lung cancer but information on this and other behavioural risks are unavailable in census records [42] which limits statistical modelling in this analysis presented. Given the strong association of tobacco exposure with socioeconomic disadvantage, we feel that it is reasonable to infer that this remains a likely key risk factor for lung cancer among cases. Third, several existing, disadvantage measures of internet access, private motor vehicle access and household over-crowding were unavailable to our first use of the data platform [25]. However, we partially offset this limitation in housing characteristics at least, by including low-rental housing through a public housing authority. Finally, the administrative data sourced for describing health condition burden were limited to the fact of prescription filled medications, rather than severity, of conditions experienced [43].
4.2 Comparison and interpretation
Our results align with earlier studies in confirming correlations of lung cancer diagnosis across Aboriginal status and/or country of birth by sex [13, 14], among males in remote areas [44] in line with known patterns of tobacco use [14] and reduced contact with general practitioners in rural areas [45]. Results also confirm that one in nine adults lived alone [46, 47] which had a strong association with lung cancer diagnosis [15]. Living alone is a poor proxy for social support but nevertheless our findings reinforce the importance of social connectedness in maintaining positive health practices such as attending primary care for health checks, particularly where that care is less readily accessed in rural areas [44].
Physical comorbidities assessed using hospital records often pre-exist cancer diagnosis, including lung cancer [16,17,18,19]. Our results are consistent with those findings while using medicated conditions as a wider, population alternative to hospital records. That is, lung cancer was likely preceded by conditions that were not necessarily serious enough to involve hospitalisation.
Area socio-economic disadvantage is strongly related to lung cancer incidence in NSW and Australia more widely [7, 11, 22, 36]. Our study disaggregated area disadvantage into constituent parts. As expected highly prevalent, person-level characteristics of low income, low educational attainment, unemployment and particular occupations related strongly with lung cancer diagnosis. Two less prevalent characteristics with similar strength of relationship to lung cancer diagnosis became apparent in being younger with disability and renting through a public housing authority. We know tobacco use is up to two times more prevalent among adults living with disabilities in Australia [48] so the potential for lung cancer disparities in the presence or absence of disabilities exists but is little understood [49, 50]. One response to our findings is the further examination of particular limitations in mobility, sensory, learning, or cognition [50] in relationship with cancer diagnosis. Housing is more widely acknowledged as an environmental health risk [10] and exposure to sub-optimal housing tenure contributes to accumulating adversity. Suffice to say, our results may help sensitise, then orient preventive efforts toward previously under-recognised groups of people with potential to benefit from health related information and proportionately greater attention to their health, social and economic needs [10, 51].
4.3 Generalisability
Precision medicine asserts each tumour is different and should be treated with specific regard to its distinctive characteristics [52]. Precision prevention might similarly point to individual differences among people at risk of lung cancer and the need to move past a one size fits all approach [53] by “treating” people according to their distinctive characteristics [54]. Developing those interventions will benefit from knowing about the characteristics described in this paper and how they vary (or not) by sex. In the meantime, results provide a base for regional profiles used by local health authorities engaging with their communities. Predicted lung cancer distribution can be validated against observations within each local health district. If the model performs acceptably, the distribution of specific characteristics associated with lung cancer can be mapped to smaller areas within each local district. This could initially inform community collaborations on which characteristics are most prevalent. Secondly, it informs thinking about how cancer control initiatives might have regard for the nature and distribution of those characteristics [55] in prioritising higher-risk individuals in clinically effective, cost-effective and equitable programs [11, 56,57,58].
For example, current recommendations to Australian Government advocate the inclusion of adults aged 50 to 70 in screening “to support the early detection of lung cancer in asymptomatic high-risk individuals” [59]. High risk individuals are initially described in terms of current or past smoking history—information that is not routinely available or recorded outside of some clinical environments. Our results follow from information that is more routinely available and focus on characteristics relevant to lung cancer diagnosis, characteristics which are also shared among current smokers [14]. Geoffrey Rose identified several strengths in prioritising approaches toward smaller numbers of higher-risk people [60]. Here, prioritisation means applying more effort to greater capacity to benefit where the ability to readily participate may be lower. Such an approach may: reduce interfering with those at lower risk; offer more cost-effective resource use; and, use selectivity to improve case finding. A key advantage though, is focussing on issues appropriate to an individual. Knowing the characteristics of a person at heightened risk is an important step. Engaging with the person and learning about their context and perceived need is a next step. The person may become a candidate for lung cancer screening and/or perhaps a candidate for a tobacco control intervention. A lot depends on the flexibility of approach to deliver a fixed program, versus responding to a person’s need and their readiness and ability to participate [61]. None of this precludes other eligible candidates in the population from participating in screening or other assistance seeking activities. The example simply describes the opportunity to apply a vertical equity principle, by making a relevant program available to a wide audience or population while more actively seeking to offer assistance to a smaller group of individuals where assistance is most needed [51].
4.4 Further data enhancement and research
Our analysis improves Australian knowledge of social determinants on health inequity in lung cancer [62]. Further research using PLIDA will build on this study. For example, examination of potential interactions between living alone and/or the presence of disability prior to lung cancer diagnosis is warranted. Better understanding the nature and severity of disability will add to understanding disability’s aetiological role through increased tobacco use [63, 64] for example, and the potential for disability to mask or facilitate cancer diagnosis as seen with morbid health conditions generally [43]. Additional research may address our paper’s limitations by examining gradients within disadvantage related characteristics and lung cancer. For example, rather than dichotomising income, census records include 15 household income levels [65] making it possible to investigate incremental change across income level and determine income thresholds correlating with risk and/or protection against lung cancer. Further enhancement of PLIDA data could extend the look-back period used. This would support a deeper understanding of the longitudinal consequences of earlier life exposure to disadvantage characteristics and how those characteristics relate to health generally [10] and lung cancer specifically.
In the first instance, an easy data enhancement on the current platform is to add cause of death, then hospital and other health administration data. These data will further person-centred analyses along the cancer care pathway into treatment exposure and eventually the quantity and quality of life after lung cancer diagnosis. Taking account of the characteristics reported in this paper may enhance our understanding of how models of care and treatment pathways are influenced by each person’s circumstances and location.
More broadly, our correlational findings are not necessarily causal. For example, we noted an association between males living in more remote areas and increased risk of lung cancer. Living remotely can contribute to inequitable experiences of illness and cancer diagnosis. Conversely, individuals may also experience illness, reduced income as a result, and subsequently need to move out of major cities to more affordable housing because of their health.
5 Conclusion
Our analysis provided a population wide description of person-centred characteristics relevant to lung cancer diagnosis. Similarities and differences based on sex confirmed the varying relationships of high prevalence characteristics with disease attributes. The results also reveal less discussed but relevant characteristics of living alone, disability and housing. Improved knowledge of these characteristics in the community can inform the development of lung cancer prevention and detection programs, then guide the targeted delivery of those programs. The data platform and method applied are relevant not only to other cancer sites but other chronic diseases.
Data availability
The data that support the findings of this study are available from the Cancer Institute of New South Wales (CINSW), the Australia Bureau of Statistics (ABS) and the Australian Department of Health (ADH), but restrictions apply to the availability of these data, which were used under licence for the current study and so are not publicly available. The data are, however, available from the authors upon reasonable request and with the permission of the CINSW, ABS and ADH.
References
Global Burden of Disease Cancer C, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8:420–44.
Australian Institute of Health and Welfare: Australian Burden of Disease Study 2018: Impact and causes of illness and death in Australia. Canberra: AIHW; 2021.
Australian Institute of Health and Welfare: Cancer in Australia 2021. Canberra: AIHW; 2021.
Laaksonen MA, Canfell K, MacInnis R, Arriaga ME, Banks E, Magliano DJ, Giles GG, Cumming RG, Byles JE, Mitchell P, et al. The future burden of lung cancer attributable to current modifiable behaviours: a pooled study of seven Australian cohorts. Int J Epidemiol. 2018;47:1772–83.
World Health Organization. Cancer control: WHO guide for effective programmes. Geneva: WHO; 2006.
Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer. 2012;12:385.
Cancer Australia: Risk factors for lung cancer: an overview of the evidence. Surry Hills, NSW: Cancer Australia; 2014.
Australian Institute of Health and Welfare: Burden of cancer in Australia: Australian Burden of Disease Study 2011. Canberra: AIHW; 2017.
Diderichsen F, Evans T, Whitehead M. The social basis of disparities in health. In: Evans T, Whitehead M, Diderichsen F, Bhuiya A, Wirth M, editors. Challenging inequities in health: from ethics to action. Oxford: Oxford University Press; 2001. p. 12–23.
Marmot M, Allen J, Boyce T, Goldblatt P, Morrison J: Marmot review 10 years on. Institute of Health Equity; 2020.
Cancer Australia: Report on the Lung Cancer Screening Enquiry. Surry Hills, NSW: Cancer Australia; 2020.
Hellyer JA, Patel MI. Sex disparities in lung cancer incidence: validation of a long-observed trend. Transl Lung Cancer Res. 2019;8:543–5.
Jaradeh M, Vigneswaran WT. Epidemiology of lung cancer and the gender differences in risk. J Men’s Health. 2022;18:073.
Siahpush M, Borland R. Socio-demographic variations in smoking status among Australians aged ≥18: multivariate results from the 1995 National Health Survey. Aust N Z J Public Health. 2001;25:438–42.
Elovainio M, Lumme S, Arffman M, Manderbacka K, Pukkala E, Hakulinen C. Living alone as a risk factor for cancer incidence, case-fatality and all-cause mortality: a nationwide registry study. SSM Popul Health. 2021;15: 100826.
Gould MK, Munoz-Plaza CE, Hahn EE, Lee JS, Parry C, Shen E. Comorbidity profiles and their effect on treatment selection and survival among patients with lung cancer. Ann Am Thorac Soc. 2017;14:1571–80.
Boakye D, Günther K, Niedermaier T, Haug U, Ahrens W, Nagrani R. Associations between comorbidities and advanced stage diagnosis of lung, breast, colorectal, and prostate cancer: a systematic review and meta-analysis. Cancer Epidemiol. 2021;75: 102054.
Lin J, McGlynn KA, Nations JA, Shriver CD, Zhu K. Comorbidity and stage at diagnosis among lung cancer patients in the US military health system. Cancer Causes Control. 2020;31:255–61.
Ahn DH, Mehta N, Yorio JT, Xie Y, Yan J, Gerber DE. Influence of medical comorbidities on the presentation and outcomes of stage I-III non-small-cell lung cancer. Clin Lung Cancer. 2013;14:644–50.
Bilenduke E, Anderson S, Brenner A, Currier J, Eberth JM, King J, Land SR, Risendal BC, Shannon J, Siegel LN, et al. Equitable implementation of lung cancer screening: avoiding its potential to mirror existing inequities among people who use tobacco. Cancer Causes Control. 2023;34:209–16.
McRonald FE, Yadegarfar G, Baldwin DR, Devaraj A, Brain KE, Eisen T, Holemans JA, Ledson M, Screaton N, Rintoul RC, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res. 2014;7:362–71.
Cancer Institute New South Wales: Lung cancer pathways in NSW: Exploratory report. In Reporting for Better Cancer Outcomes, One edition; 2022.
Gupta A, Omeogu CH, Islam JY, Joshi AR, Akinyemiju TF. Association of area-level socioeconomic status and non-small cell lung cancer stage by race/ethnicity and health care-level factors: analysis of the National Cancer Database. Cancer. 2022;128:3099–108.
Warner ET. Race, place, and socioeconomic status: a path toward lung cancer early detection. Cancer. 2022;128:3016–8.
Australian Bureau of Statistics: Socio-Economic Indexes for Areas (SEIFA) - Technical Paper, 2016. 2018.
Health AIo, Welfare: Alcohol, tobacco & other drugs in Australia. Canberra: AIHW; 2023.
Fedewa SA, Kazerooni EA, Studts JL, Smith RA, Bandi P, Sauer AG, Cotter M, Sineshaw HM, Jemal A, Silvestri GA. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113:1044–52.
Pham D, Bhandari S, Pinkston C, Oechsli M, Kloecker G. Lung cancer screening registry reveals low-dose CT screening remains heavily underutilized. Clin Lung Cancer. 2020;21:e206–11.
Spencer K. Identifying the unseen and unmet; using data to target blind spots in cancer care. J Cancer Policy. 2023;35: 100409.
Couso-Viana S, Bentue-Martinez C, Delgado-Martin MV, Cabeza-Irigoyen E, Leon-Latre M, Concheiro-Guisan A, Rodriguez-Alvarez MX, Roman-Rodriguez M, Roca-Pardinas J, Zuniga-Anton M, et al. Analysis of the impact of social determinants and primary care morbidity on population health outcomes by combining big data: a research protocol. Front Med. 2022;9:1012437.
Begley CE, Lairson DR, Morgan RO, Rowan PJ, Balkrishan R. Evaluating the healthcare system: effectiveness, efficiency and equity. 4th ed. Chicago: HAP & AUPHA; 2013.
Roder D, Banham D, George J, Rushton S, O’Brien T. Demographic, health, and prognostic characteristics of Australians with liver cancer: a study of linked data in New South Wales to inform cancer control. BMC Public Health. 2023. https://doi.org/10.1186/s12889-023-16809-y.
Multi-Agency Data Integration Project (MADIP) [https://www.abs.gov.au/about/data-services/data-integration/integrated-data/multi-agency-data-integration-project-madip]
Supporting Analysis of The Life Course, the Life Course Centre Data for Policy Summit: Keynote address [https://www.abs.gov.au/about/our-organisation/australian-statistician/speeches/supporting-analysis-life-course#:~:text=MADIP%20is%20being%20renamed%20PLIDA]
Overview of Ancestry data in Census. https://www.abs.gov.au/statistics/detailed-methodology-information/information-papers/understanding-and-using-ancestry-data#overview-of-ancestry-data-in-census
Little A, Roder D, Zhao GW, Challam S, Malalasekera A, Currow D. Country of birth and non-small cell lung cancer incidence, treatment, and outcomes in New South Wales, Australia: a population-based linkage study. BMC Pulm Med. 2022;22:366.
Pratt NL, Kerr M, Barratt JD, Kemp-Casey A, Kalisch Ellett LM, Ramsay E, Roughead EE. The validity of the Rx-Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) Classification System. BMJ Open. 2018;8: e021122.
Australian Bureau of Statistics: Building on SEIFA: Finer Levels of Socio-Economic Summary Measures. Canberra: ABS; 2013.
Australian Institute of Health and Welfare, Cancer Australia: Lung cancer in Australia: an overview. Canberra: AIHW; 2011.
Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. New York: Wiley; 2013.
Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080.
Thomas DP, Scollo M. Should a smoking question be added to the Australian 2021 census? Aust N Z J Public Health. 2018;42:225–6.
Fleming ST, Sarfati D, Kimmick G, Schoenberg N, Cunningham R. Impact of comorbidity on cancer screening and diagnosis. In: Koczwara B, editor. Cancer and chronic conditions: addressing the problem of multimorbidity in cancer patients and survivors. Singapore: Springer; 2016. p. 105–30.
Cramb SM, Mengersen KL, Baade PD. Identification of area-level influences on regions of high cancer incidence in Queensland, Australia: a classification tree approach. BMC Cancer. 2011. https://doi.org/10.1186/1471-2407-11-311.
Australian Bureau of Statistics: Patient Experiences [Internet]. Canberra: ABS; 2021–22.
de Vaus D, Qu L: Demographics of living alone. Melbourne: Australian Institute of Family Studies; 2015.
Hughes ME, Waite LJ. Health in household context: living arrangements and health in late middle age. J Health Soc Behav. 2002;43:1–21.
Australian Institute of Health Welfare: People with disability in Australia. Canberra: AIHW; 2022.
Shin DW, Cho JH, Noh JM, Han H, Han K, Park SH, Kim SY, Park JH, Park JH, Kawachi I. Disparities in the diagnosis and treatment of lung cancer among people with disabilities. J Thorac Oncol. 2019;14:163–75.
Iezzoni LI. Cancer detection, diagnosis, and treatment for adults with disabilities. Lancet Oncol. 2022;23:e164–73.
Carey G, Crammond B, De Leeuw E. Towards health equity: a framework for the application of proportionate universalism. Int J Equity Health. 2015;14:81.
Re A, Nardella C, Quattrone A, Lunardi A. Editorial: precision medicine in oncology. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00479.
Brown A, Garvey G, Rankin NM, Nightingale C, Whop LJ. Lung cancer screening for Aboriginal and Torres Strait Islander peoples: an opportunity to address health inequities. Med J Aust. 2023;219:398–401.
Arnold C. Spurred by COVID, public health gets precise. Nature. 2022;601:18–20.
Australia C. Lung cancer framework: principles for best practice lung cancer care in Australia. Sydney: Cancer Australia; 2017.
Baldwin DR. Socioeconomic position and delays in lung cancer diagnosis: should we target the more deprived? Thorax. 2017;72:393–5.
Saab MM, Fitzgerald S, Noonan B, Kilty C, Collins A, Lyng Á, Kennedy U, O’Brien M, Hegarty J. Promoting lung cancer awareness, help-seeking and early detection: a systematic review of interventions. Health Promot Int. 2021;36:1656–71.
Duma N, Evans N, Mitchell E. Disparities in lung cancer. J Natl Med Assoc. 2023;115:S46–53.
Medical Services Advisory Committee: Public Summary Document Application No. 1699 – National Lung Cancer Screening Program Applicant: Date of MSAC consideration: Cancer Australia 28–29 July 2022 Canberra: MSAC; 2022.
Rose G, Khaw K-T, Marmot M. Rose’s strategy of preventive medicine. Oxford: Oxford University Press; 2008.
Miller WR, Rollnick S. Motivational interviewing: preparing people to change. New York: Guilford; 1991.
Flavel J, McKee M, Freeman T, Musolino C, van Eyk H, Tesfay FH, Baum F. The need for improved Australian data on social determinants of health inequities. Med J Aust. 2022;216:388–91.
Vourliotis T, Twyman L. Tobacco use by people with disabilities. Sydney: Cancer Council NSW; 2022.
Schulz JA, West JC, Hall JP, Villanti AC. Disparities in tobacco use by disability and type: findings from the 2019 National Health Interview Survey. Am J Prev Med. 2022;63:552–63.
Australian Bureau of Statistics: Census of Population and Housing: Census Dictionary Australia 2016. Canberra: ABS; 2016.
Acknowledgements
We acknowledge the Australian Bureau of Statistics for their technical and administrative support which was essential for successful completion of this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DB conducted the analyses and wrote the main manuscript text. DR, ES, SQ, SR and TO revised the manuscript critically for important intellectual content. All authors reviewed the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NSW Population and Health Services Research Ethics Committee (PHSREC 2019/ETH13324) and Aboriginal Health and Medical Research Committee (AH&MRC 1739/20) approved this project. The authors confirm the project was carried out in accordance with the relevant PHSREC guidelines and regulations. The project made secondary use of existing de-identified registry and administrative records. No experimental protocols were relevant or used in the project. Subsequently, obtaining consent to participate in this project was not applicable. The requirement for informed consent was waived by the NSW Population and Health Services Research Ethics Committee and Aboriginal Health and Medical Research Committee because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest in the research, authorship and publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banham, D., Roder, D., Stone, E. et al. Demographic, health and socioeconomic characteristics related to lung cancer diagnosis: a population analysis in New South Wales, Australia. Discov Soc Sci Health 4, 34 (2024). https://doi.org/10.1007/s44155-024-00095-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44155-024-00095-z