Abstract
Probiotics are living microbes that promote consumer health when taken in significant quantities with food. The aim of this research was identifying the probiotic properties (in vitro) of lactic acid bacteria (LAB) isolated from traditional fermented dairy products from Egypt (Kareish cheese, Rayeb milk, local yoghurt and buttermilk). Only 13 isolates were characterizes as Gram-positive, endospore-negative, non-motile and catalase-negative with small round colonies. All 13 isolates were examined for its probiotic properties (antibacterial activity, antibiotic susceptibility, acidity and bile salt tolerance). All isolates showed antimicrobial effect against bacterial pathogens tested. LAB-6 had a significantly larger inhibition zone followed by LAB-2 and LAB-3. Moreover, LAB-6 was the most resistant to all antibiotics tested. Seven of thirteen isolates were Penicillin-resistant. Only LAB-3 exhibited strong chlorophenicol resistance. The isolates that affect pathogens and resistant to antibiotics were found acid-and-bile salt-tolerant in varying degrees. All bile and acid tolerant isolates revealed variable antibiotic sensitivity. Regarding to previous results, only three selected isolates (LAB-2, LAB-3, and LAB-6) had their 16SrRNA gene sequences compared to Gene-Bank. Isolate LAB-2 was found in the genus Lactobacillus, with 98.7% similarity to L. rhamnosus OP268116 strain GCM20300. LAB-3 was Lactobacillus, with 98.9% similarity to L. rhamnosus OP268117 strain 6481. Comparative genomic research demonstrated very minimal changes between isolate LAB-6 and Enterococcus durans OP268118 strain CAU5334. Thus, these isolates could be used as food biopreservatives, starter cultures in the fermented dairy products and cheese industry, or novel strategies to combat the rising number of antibiotic-resistant pathogens in human infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Functional and probiotic foods, such as fermented dairy products, may be thought to give extra health advantages or protection against diseases caused by ingesting contaminated food, in addition to being a source of nutrients. Food-borne illness prevention and treatment necessitate interdisciplinary methods that utilize beneficial living microorganisms (probiotics) to battle food-borne pathogens and the accompanying health hazards. Food-borne diseases pose several difficulties. Several studies have shown that probiotic LAB inhibits the growth of food-borne infections. Consuming a considerable number of probiotic living microorganisms with food has been shown to benefit consumers' health [1,2,3]. Because of customer desire for fewer chemical preservatives, the importance of employing helpful microbes to preserve food has grown. Furthermore, animals have been given preventative antibiotic doses for decades. The potential for people and cattle to promote cross-resistance and multiple antibiotic resistance in pathogenic microorganisms, as well as the harmful impact of encountering drug residues, are emerging concerns. One strategy for decreasing such difficulties is to use growth enhancers, such as probiotics, that have a good influence on host health and growth efficacy [4,5,6].
The term "lactic acid bacteria" (LAB) refers to a collection of gram-positive, acid-tolerant, nonsporulating, microaerophilic rods or cocci that have been classified based on their similar physiological and metabolic features. There are roughly 20 distinct genera within the phylum Firmicutes, with Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, Enterococcus, Pediococcus, Aerococcus, Oenococcus, Tetragenococcus, Carnobacterium, Vagococcus, and Weissella being the main LAB. These microbes are found in nature in fermented foods such as cheese and yogurt. They improve the flavor and consistency of food while also inhibiting the growth of bacteria that cause degradation and sickness [7,8,9,10,11]. Recent research has demonstrated that some LAB isolates isolated from fermented foods exhibit desirable characteristics for probiotic cultures. Seven probiotic strains have been isolated from fermented foods including tiger nut milk, bovine milk [12] and natural whey starter used in cheese manufacture [13].
Egyptian traditionally fermented dairy products are consumed without heat treatment, making them good carriers for probiotic bacteria. Upper Egypt's probiotic sources include yogurt, Karish cheese (skim milk cheese), Rayeb milk (concentrated sour milk), and Kishk (wheat-based fermented milk). These things are used in large quantities [6, 14,15,16]. The probiotic strains isolated from these fermented foods and drinks show desirable functional properties for use as probiotics against food-borne illnesses, and they might be employed as a starting culture for the mass manufacture of the traditional product. Some LAB are probiotics, while others have the potential to be probiotics or are just fermentation cultures found in nature and used in the food industry. Both of these LAB groups have the potential to be advantageous [17].
The purpose of this study is to look at the in vitro probiotic properties of LAB isolated from three traditional Egyptian fermented dairy products (yogurt, Rayeb milk, buttermilk, and Kariesh cheese) in terms of their potential probiotic activity against different pathogens.
2 Materials and methods
2.1 Dairy samples
Forty fermented dairy samples were collected from numerous Fayoum governorate, Egypt, markets. Rayeb milk, yoghurt, Karish cheese, and buttermilk were included. Samples were put in clean containers and refrigerated until transport to the lab. When they entered the lab, they were separated.
2.2 Isolation of LAB
Each sample of dairy products was diluted in a saline solution with 0.85% sodium chloride before plating on MRS agar. The colonies were separated after incubation (37 °C/48 h). Randomly selected colonies (based on color, shape, elevation, and size) were striated on MRS agar-coated plates for future investigation. To retain culture, the initial colony was subcultured. Before being sealed, the isolates were inspected under a microscope. Gram-positive, catalase-negative cocci or bacilli are lactic acid bacteria. The bacteria were suspended in glycerol and kept at − 20 °C for further investigation.
2.3 Confirmation tests of isolates
Gram staining and morphology Using a light microscope with oil immersion lenses, fresh cultures of the isolated organisms were wet-mounted on microscopic transparencies. Cellular morphology involved cell shape and arrangement of the cells.
Catalase activity A single, uncontaminated colony was provided with a drop of 3% hydrogen peroxide solution to determine catalase activity.
Spore staining Gram-positive and catalase-negative isolates were examined for endospore formulation under a light microscope with oil immersion objectives after staining according to Prescott [15]. Therefore, isolates without endospores were studied.
Motility test The motility test was performed using the wet mount technique [18]. Non-motile microorganisms were chosen.
2.4 The probiotic properties LAB isolates
Antibacterial activity against pathogens According to Wolf and Wirth [19], The selected isolates were tested against food-borne pathogens Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enteritidis, and Escherichia coli using the agar well diffusion assay. Inhibition zones were detected and measured.
Antibiotic susceptibility Antibiotic effectiveness was measured by LAB's zone of inhibition diameter (mm). [20]. In vitro diagnostics use Flummox, Ampiclox, Chlorophenicol, Penicillin, and Tarivid. These antibiotics were chosen since they have worked against several infections in the past. Most of them broadcast.
Screening for acidity and bile salt tolerance of viable cells The stress tolerance trials were modified from Parente et al. [21]. The OD600 readings of overnight cultures of thirteen distinct LAB isolates were reduced to approximately 0.6 and then normalised to 1. 1 N HCl was used to create MRS broth with pH values of 2.0, 3.0, and 4.0 (for acid stress) and 0.1, 0.3, and 0.5% bile salt (for bile salt stress). Bacteria were re-cultured in MRS broth at 37 °C for 48 h after treatment. Viability % calculation:
2.5 Molecular identification (isolation of genomic and amplification of 16S rRNA)
LAB isolates were identified using morphological, phenotypic and biochemical characteristics. 16S rDNA sequencing was conducted to further confirm the identity of the isolates. All bacteria have the 16S rRNA gene, allowing relationship measurements so; 16S rRNA gene sequences identify strains by genus, species, and subspecies in all major bacterial phyla. Multiple well-known species with identical or similar 16S rRNA gene sequences are identified [22]. ABT DNA micro extraction reagent (Applied Biotechnology Co., Ltd., Egypt) extracted nucleic acid according to the manufacturer's instructions. Three isolates' 16S rRNA genes were amplified by PCR using universal primers (GAGTTTGATCCTGGCTCAG) and (GGTTACCTTGTTACGACT) to determine their species [23]. Solgent Co Ltd. (South Korea) gel-purified and sequenced PCR-amplified materials. Geneious (Biomatters) assembled the sequences. BLAST (GenBank) found shorter sequences. Phylogenetic analysis: MAFFT matched GenBank nucleotide sequences with identified sequences [24]. Neighbour joining method and Tamura-Nei were used to generate phylogenetic trees [25].
3 Results and discussion
3.1 Isolation and identification of LABs
A total of 13 isolates (from Kareish cheese, Rayeb milk, local yoghurt and buttermilk) were selected based on colony and cell morphology, staining, and the milk coagulation test. All of the selected isolates were Gram-positive, non-motile, non-spore producing, and catalase-negative, and they all produced distinctive tiny, whitish-creamy colonies on MRS agar. These strains' ability to develop in anaerobic environments suggests that they are linked to lactic acid bacteria, and hence they have been designated LAB-1 to LAB-13. Using a microscope, the cell morphology of all isolates was seen to be rod or coccus shaped (Table 1). LABs are bacteria with rod or coccus morphologies, negative catalase, homo or hetero fermentation, and grow in low acid environments [26, 27]. These are gram-positive bacteria that generate lactic acid as their primary fermentation product and are usually regarded as safe [28]. The milk coagulation test revealed that all of the isolates can coagulate milk at 37°C. Lactic acid bacteria can ferment milk to produce lactic acid and coagulate milk as result [27, 29].
4 Probiotic properties of LAB isolates
4.1 Antibacterial activity against pathogens
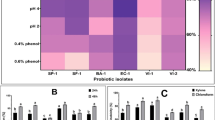
Antimicrobial activity against human pathogens is regarded as the most important characteristic of probiotic strains that promote gastrointestinal health. The antibacterial action of LAB may be attributable to the synthesis of numerous compounds during their growth and subsequent extracellular excretion. The LAB released several inhibitory compounds, including as organic acids, hydrogen peroxide, and bacteriocin, that are responsible for its antibacterial action [30, 31]. Hence, using the well diffusion technique, the antibacterial activity of the cell-free supernatant (CFS) of LAB isolates was evaluated. The results demonstrated that the CFS of all LAB isolates has an antibacterial action against selected pathogens, and the diameters of the inhibition zones differed as shown in Table 2. The diameter of inhibition zones demonstrated that crude extracts from each isolate exhibited significant (p < 0.001) antimicrobial action against each tested pathogen except LAB-10 which had no effect on B. cereus. Isolate LAB-3 had the greatest antagonistic activity against Staph. aureus, as measured by the inhibition zone's diameter of 23.33 mm. Similar results were reported in another studies [30, 32].
Whereas, the main effect of several LAB isolates and tested pathogens was statistically examined (Table 3). LAB-6 had a significantly larger inhibition zone of 18.40 mm, followed by LAB-2 (18.07 mm). LAB-10 had the least antibacterial activity, resulting in a zone measuring 10.53 mm. Many investigations supported these findings by demonstrating that certain LAB strains may generate antimicrobial compounds that, through various methods, exert a potent inhibitory effect on numerous bacteria, including pathogens. Antimicrobial agents generated by LAB include organic acids, hydrogen peroxide, ethanol, diacetyl, acetaldehyde, acetoin, carbon dioxide, and bacteriocins. LAB-produced organic acid resulted in low pH numbers and hydrogen peroxide activity. Organic acids and other compounds demonstrate antibacterial action against several Gram-negative pathogens [3, 33,34,35,36,37]. In a recent investigation, four different strains of LAB isolated from colostrum were shown to have effective antibacterial properties against harmful microorganisms [38]. Moreover, The zone of inhibition produced by eight different lactobacilli isolates against various pathogens varied from 4.66 mm to 27.66 mm [39].
4.2 Antibiotic susceptibility
Due to the rising prevalence of multidrug-resistant bacterial isolates, antibiotic resistance is a global health problem. Given that LAB might operate as a reservoir of transferable antibiotic resistance genes, LAB strains used as food additives of dairy starters should be evaluated for its antibiotic susceptibility. So, the susceptibility of the thirteen isolates to five different antibiotics is displayed in Table 4. Tarivid and Flummox were both effective against every isolate. Whereas seven of thirteen isolates were Penicillin-resistant. Only LAB-3 exhibited strong chlorophenicol resistance. Moreover, LAB-6, LAB-4, and LAB-13 exhibited Ampiclox resistance. Other antimicrobials have a range of effects on isolates, from sensitivity to resistance. According to Table 5's main effect data, LAB-6 was the most resistant to all antibiotics tested, with the smallest inhibition zone diameter of 14.40 mm, followed by LAB-4 (14.80 mm). The most susceptible isolate was LAB-12, which exhibited the largest zone of inhibition (27.26 mm).
In recent study, Sixty-five LAB strains were used as probiotic additives or silage inoculants, were determined for susceptibility to eight clinically relevant antibiotics. Nineteen LAB strains showed phenotypic resistance to one antibiotic, while 15 bacteria were resistant to more than one antibiotic in the genera Lactobacillus and Pediococcus [40]. In another research by Nunziata et al., strains of Lactobacillus, Streptococcus, and Bifidobacterium that are now utilised for industrial dairy products are typically resistant to gentamycin, kanamycin, chloramphenicol, and tetracycline [41]. Also, eight isolates were examined for antibiotic sensitivity, including Lactobacillus plantarum, L. rhamnosus, L. acidophilus, L. salivarius, and L. paracasei, as well as Bifidobacterium longum, B. breve, and B. adolescentis. All of the lactobacilli strains were Vancomycin resistant, and all of them had intermediate Clindamycin resistance. Penicillin, Tetracycline, Ampicillin, Erythromycin, Streptomycin, Florfenicol, Chloramphenicol and Trimethoprim were all effective against all isolates [6].
Some Lactobacillus strains were evaluated for antibiotic susceptibility using disc diffusion [39]. These strains were sensitive to five different antibiotics, while L. plantarum GV69 was resistant to ampicillin and cefixime. One cell wall and protein-preventing antibiotic worked against the strains. Fonseca et al. [42] observed that L. brevis CCMA1284 was resistant to lincomycin, azithromycin, and penicillin, whereas two L. paracasei strains were mildly sensitive or susceptible. Also, some strains of Lactococcus lactis, Enterococcus spp., and Lactobacillus spp. were resistant to antibiotics, such as chloramphenicol, erythromycin, clindamycin, or tetracycline [43]. Numerous studies have documented a significant level of penicillin resistance in lactobacilli of fermented food origin. Resistance to penicillin and ampicillin varied by species. Strains of L. paracasei, L. casei, L. brevis, and L. plantarum have also been reported to be resistant to penicillin antibiotics at a high level [44,45,46,47].
4.3 Resistance to acid and bile salt
For a bacterial isolate to be considered a potential probiotic, it must be able to thrive under gastro-intestinal conditions and possess acid and bile tolerance, antimicrobial against pathogens, antibiotic susceptibility, and other characteristics. Acidity's effects on LAB isolates' viability are shown in Table 6. LAB-8 was the only isolate with a 100% viability at pH 4 and a 50% viability at pH 2, while being resistant to both of those conditions. Of the studied isolates, LAB-13 was the most acid-sensitive, dying out at pH 4, 3, and 2. This suggests that this isolate is particularly sensitive to the acidic environment of the intestines. Most isolates' viability (in percentage terms) reduced significantly (p 0.01) when pH fell.
In addition, every single isolate were tested for its tolerance to bile salt. The percentage of isolates that survived exposure to bile salts is shown in Table 6. The capacity of different cultures to thrive in MRS broth spiked with bile salts differed significantly (p > 0.01). It was discovered that LAB-8 was the strain least tolerant to bile, whereas LAB-5 was the most resistant. There was a statistically significant difference in the rate of growth between the sensitive and non-sensitive isolates (p > 0.05).
Probiotics must survive the stomach before accessing the small intestine so, tolerance to high bile salt levels and low pH are desired. In recent study, it was reported that 12 isolated LAB survived the stomach's acidic environment and high bile [48].
In two similar studies, some Lactobacillus spp. isolates' stomach pH and bile salt tolerance indicated their potential. Both studies found 0.3% bile salt resistance. However, Lactobacillus isolares were highly sensitive to stomach pH [49, 50]. Despite being lactic acid bacteria, fermented milks have high acidity at the end of production and during shelf life, which may explain their tolerance to low pH [51]. Mandal et al. found no significant difference in viable cell count for free and encapsulated L. casei cells at low pH and high bile salt concentration [52]. Moreover, L. brevis LB32 and L. pentosus LP05 could survive bile salt concentrations from 0.5 to 3%, however their coefficients of inhibition varied. Both isolates hydrolyzed bile well at pH 3, 4, and 5 [53].
According to a number of studies, acid and biliary resistance is an essential indicator in choosing probiotic strains. The most beneficial probiotic bacteria survive pH 3.0 for two hours according to Garriga et al. [54]. Consistent with this finding was the acid resistance of the selected isolates from our investigation. Some isolates were not resistant to pH levels below 2.0, possibly because probiotic bacteria are protected by food and other carrier matrix molecules in the stomach [55]. Moreover, 12 lactobacilli isolates including L. plantarum, L. sakei and L. bulgaricus have an excellent survival rate in bile salts ranging from 45 to 94% [56]. Since these microorganisms are naturally resistant to bile salts, their high resistance may also be explained by their pH tolerance.
5 Sequence analysis of 16S rRNA gene and phylogenetic analysis
Total genomic DNA was extracted from each of the three isolates LAB-2, LAB-3, and LAB-6 with the highest probiotic potential in order to identify their genotypic characteristics. This extraction was performed in order to identify the isolates via amplification of the bacterial-specific 16S rRNA gene sequencing which permits identification of bacteria into to the genus and species level as stated by Sadrani et al. [57]. The 16SrRNA gene sequences of selected isolates were compared with sequences in the Gene-Bank database. A phylogenetic tree based on 16SrRNA sequences (Fig. 1) revealed affiliation of isolate LAB 2 to the genus Lactobacillus, comprising 98.7% identity to L. rhamnosus OP268116 strain GCM20300, which is most closely related to L. rhamnosus. LAB 3 was revealed to genus Lactobacillus, comprising 98.9% identity to L. rhamnosus OP268117 strain 6481. While revealed affiliation of isolate LAB 6 to the genus Enterococcus, comprising 94.9% identity to Enterococcus durans OP268118 strain CAU5334; Comparative genomic research indicated minimal variations between this strain and Enterococcus durans.
Using phylogenetic estimation of 16S rDNA genes, other recent studies have identified at the strain level diverse lactic acid bacteria with potent probiotic properties isolated from fermented food products [2, 3]. Comparative 16S rRNA gene analysis conducted by Shokryazdan et al. [58] revealed that LAB belonged to L. fermentum, L. acidophilus, L. casei and L. buchneri. Using 16S rRNA gene analysis, Cho, Lee, and Hahm [59] have identified Lactobacillus strains with putative probiotic properties. In another recent study, two of the 60 isolates found in fermented foods showed the best probiotic results and were identified as Lactobacillus acidophilus CM1 and Lactobacillus delbrueckii OS1 where there are numerous Lactobacillus species in fermented foods, including yoghurt and kefir [60]. Seven lactobacilli isolates from human milk were identified by 16S rRNA gene analysis as Lacticaseibacillus paracasei and Lactobacillus gasseri. Overall, each strain demonstrated superior probiotic and technological properties for use in lactic fermentation [61]. Moreover, Coulibaly et al. [62] identified 12 LAB strains via 16S rDNA gene sequence homology analysis to be found from two genera, Pediococcus (P. acidilactici and P. pentosaceus) and Lactobacillus (L. plantarum) which selected considering to probiotics, functional, storage, and safety factors.
Our investigation led to the detection of three probiotic organisms, which were later described and genotypically confirmed to be members of the Lactobacillus and Enterococcus genera. Due to their unique properties, these indigenous probiotic strains may be beneficial to the food industries. This study demonstrates the importance of oral and dietary sources of new probiotics with desirable functional properties.
6 Conclusions
Due to their numerous health benefits, the study of LAB and their potential as probiotics has garnered significant attention in recent years. Lactobacillus species can be found in fermented foods. In the present research, probiotic properties of LAB isolated from fermented dairy products were evaluated. The thirteen selected isolates from the preliminary screening test met multiple requirements for use as probiotic microorganisms, including broad-spectrum antibacterial activity, susceptibility to antibiotics, and resistance to gastric pH and bile salts. The isolates LAB-2, LAB-3, and LAB-6 were identified to be L. rhamnosus OP268116, L. rhamnosus OP268117, and Ent. durans OP268118. Thus, these isolates can be considered potential probiotic strains with a beneficial role in maintaining good intestinal tract health in humans, and they could be used in novel strategies against the rising number of antibiotic-resistant pathogens in human infections, or even as food biopreservatives or a starter culture in the fermented dairy products and cheese industry. To complete the safety evaluation and probiotic properties of these newly characterized LAB strains, however, in vivo and clinical trials, additional in vitro studies, and genomic characterization are necessary.
Availability of data and materials
All data generated or analyzed during the process of this investigation is included in this article.
References
Hill C, Guarner F, Reid G et al (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514
Dowarah R, Verma AK, Agarwal N, Singh P, Singh BR (2018) Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. Plos One 13(3):e0192978
Mulaw G, Tessema ST, Muleta D, Tesfaye A (2019) In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int J Microbiol 25:7179514
Awaisheh SS, Ibrahim SA (2009) Screening of antibacterial activity of lactic acid bacteria against different pathogens found in vacuum-packaged meat products. Foodborne Pathog Dis 6(9):1125–1132
Dowarah R, Verma AK, Agarwal N (2017) The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: a review. Anim Nutr 3(1):1–6
Shehata A, Zayed G, Saad OAO, Gharib S (2020) Antimicrobial activity and probiotic properties of lactic acid bacteria isolated from traditional fermented dairy products’. J Mod Res 2(2):40–48
Schrezenmeir J, De Vrese M (2001) Probiotics, prebiotics and synbiotics—approaching a definition. Am J Clin Nutr 73:361–364
Axelsson L (2004) Lactic acid bacteria: classification and physiology. In: Salminen S, Wright A, Ouwehand A (eds) Lactic acid bacteria. Microbiological and functional aspects. Marcel Dekker Inc., New York, pp 1–66
Akkoc N, Ghamat A, Akcelik M (2011) Optimisation of bacteriocin production of Lactococcus lactis subsp. lactis MA23, a strain isolated from Boza. Int J Dairy Technol 64(3):425–432
Gao G, Ma T, Zhang T, Jin H, Li Y, Kwok LY, Zhang H, Sun Z (2021) Adjunctive probiotic Lactobacillus rhamnosus Probio-M9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol 14(12):772532
Moh LG, Etienne PT, Jules-Roger K (2021) Seasonal diversity of lactic acid bacteria in artisanal yoghurt and their antibiotic susceptibility pattern. Int J Food Sci 12:6674644
Srinivash M, Krishnamoorthi R, Mahalingam PU, Malaikozhundan B, Keerthivasan M (2023) Probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from homemade fermented food products. J Agric Food Res 11:100517
Lozano J, González Revello Á, Hirigoyen D, Martínez M, Scorza C, Zunino P (2022) Probiotic potential of GABA-producing lactobacilli isolated from Uruguayan artisanal cheese starter cultures. J Appl Microbiol 133(3):1610–1619
Kalam Saleena LA, Phing P, Gan R, Al-Nabulsi A, Osaili T, Kamal-Eldin A, Ayyash M (2023) Fermented dairy products from Middle Eastern and Northern African (MENA) countries: insight on production and physiochemical characteristics. Int Dairy J 141:105614
Magdoub MNI, Hassan ZMR, Effat BAM, Sadek ZIM, Tawfik NF, Mabrouk AMM (2015) Probiotic properties of some lactic acid bacteria isolated from Egyptian dairy products. Int J Curr Microbiol Appl Sci 4(12):758–766
Ali FS, Zayed G, Saad OAO, Gharib SAH (2018) Molecular identification and properties of some probiotic bacteria isolated from fermented dairy products. Minia J Agric Res 36(3):447–457
Ricci A, Cirlini M, Maoloni A, Del Rio D, Calani L, Bernini V, Galaverna G, Neviani E, Lazzi C (2019) Use of dairy and plant-derived Lactobacilli as starters for cherry juice fermentation. Nutrients 11(2):213
Prescott H (2002) Laboratory exercises in microbiology. 5th Edn, The McGraw-Hill Companies.
Caprette DR (2012) How to prepare a Wet mount (Vaseline mount). Available at: http://www.ruf.rice.edu/~bioslabs/methods/microscopy/wetmount.html. B, VIII
Wolf GA, Wirth SJ (1996) J Microbiol Methods 25:337–342
Kumar S, Tripathi VR, Garg SK (2013) Antibiotic resistance and genetic diversity in water-borne Enterobacteriaceae isolates from recreational and drinking water sources. Int J Environ Sci Technol 10:789–798
Parente E, Ciocia F, Ricciardi A, Zotta T, Felis G, Torriani S (2010) Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: a multivariate screening study. Int J Food Microbiol 144:270–279
Clarridge JE (2004) Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17(4):840–862
White BA (1993) PCR protocols: current methods and applications. Humana Press, Totowa
Katoh K, Standley DM (2014) MAFFT: iterative refinement and additional methods. Methods Mol Biol 1079:131–146
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Holzapfel EH, Haberer P, Geisen R, Björkroth J, Schillinger U (2001) Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr 73(2):365S-373S
Sowmya NS, Nandini K, Earanna N, Sajeevan RS, Nataraja K (2016) Molecular identification and genetic diversity of Lactobacillus species isolated from different edible sources. J Pure Appl Microbiol 10:3155–3162
Konings WN, Kok J, Kuipers OP, Poolman B (2000) Lactic acid bacteria: the bugs of the new millennium. Curr Opin Microbiol 3(3):276–282
Hoque MZ, Akter F, Hossain KM, Rahman MSM, Billah MM, Islam KMD (2010) Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World J Dairy Food Sci 5(1):39–46
Hor YY, Liong MT (2014) Use of extracellular extracts of lactic acid bacteria and bifidobacteria for the inhibition of dermatological pathogen Staphylococcus aureus. Dermatol Sin 32:141–147
Ha TQ, Hoa TM (2016) Selection of lactic acid bacteria producing bacteriocin. J Viet Environ 8:271–276
Mohamad NI, Manan MA, Sani NA (2022) The antibacterial activity of lactic acid bacteria from pickled Spondias dulcis (Ambarella) against foodborne pathogens. Trends Sci 19:2896–2896
Ponce A, Moreira M, Del Valle C, Roura S (2008) Preliminary characterization of bacteriocin-like substances from lactic acid bacteria isolated from organic leafy vegetables. LWT-Food Sci Technol 41(3):432–441
Maragkoudakis PA, Mountzouris KC, Psyrras D, Cremonese S, Fischer J, Cantor MD, Tsakalidou E (2009) Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. I J Food Microbiol 130(3):219–226
Šušković J, Kos B, Novak J, Pavunc A, Habjanič K, Matoć S (2010) Antimicrobial activity—the most important property of probiotic and starter lactic acid bacteria. Food Technol Biotechnol 48:296–307
Al-Gamal MS, Ibrahim GA, Sharaf OM, Radwan AA, Dabiza NM, Youssef AM, El-Ssayad MF (2019) The protective potential of selected lactic acid bacteria against the most common contaminants in various types of cheese in Egypt. Heliyon 5(3):e01362
Dbeibia A, Mahdhi A, Jdidi S, Altammar KA, Zmanter T, Mzoughi R, Jabeur C (2023) Probiotic potential of lactic acid bacteria isolated from colostrum of 3 different mammals. Food Biotechnol 37(2):166–190
Vasudha M, Prashantkumar CS, Bellurkar M, Kaveeshwar V, Gayathri D (2023) Probiotic potential of β-galactosidase-producing lactic acid bacteria from fermented milk and their molecular characterization. Biomed Rep 18:23
Refay RM, Abushady HM, Amer SA, Mailam MA (2020) Determination of bacteriocin-encoding genes of lactic acid bacteria isolated from traditional dairy products of Luxor Province, Egypt. Future J Pharm Sci 6(1):1–14
Stefanska I, Kwiecie E, Józwiak-Piasecka K, Garbowska M, Binek M, Rzewuska M (2021) Antimicrobial susceptibility of lactic acid bacteria strains of potential use as feed additives—the basic safety and usefulness criterion. Front Vet Sci 8:687071
Fonseca HC, de Sousa MD, Ramos CL, Dias DR, Schwan RF (2021) Probiotic properties of Lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiot Antimicrob Proteins 13:102–112
Flórez AB, Delgado S, Mayo B (2011) Antimicrobial susceptibility of lactic acid bacteria isolated from a cheese environment. Can J Microbiol 51(1):51–58
Duche RT, Singh A, Wandhare AG et al (2023) Antibiotic resistance in potential probiotic lactic acid bacteria of fermented foods and human origin from Nigeria. BMC Microbiol 23:142
Wang Y, Dong J, Wang J, Chi W, Zhou W, Tian Q, Hong Y, Zhou X, Ye H, Tian X, Hu R, Wong A (2022) Assessing the drug resistance profiles of oral probiotic lozenges. J Oral Microbiol 14(1):2019992
Osaro-Matthew RC, Nweke OG (2021) Antibiotic resistant profile of lactic acid bacteria isolated from swine and poultry faeces in Umuahia Metropolis. J Adv Biol Biotechnol 24(5):21–25
Nunziata L, Brasca M, Morandi S, Silvetti T (2022) Antibiotic resistance in wild and commercial non-enterococcal lactic acid bacteria and bifidobacteria strains of dairy origin: an update. Food Microbiol 103999
Anandharaj M, Sivasankari B (2014) Isolation of potential probiotic Lactobacillus oris HMI68 from mother’s milk with cholesterol-reducing property. J Biosci Bioeng 118(2):153–159
Corsetti A, Caldini G, Mastrangelo M et al (2008) Raw milk traditional Italian ewe cheeses as a source of Lactobacillus casei strains with acid-bile resistance and antigenotoxic properties. Int J Food Microbiol 125:330–335
Urnau D, Cirolini A, Terra NN et al (2012) Isolamento, identificação e caracterização quanto à resistência ao pH ácido e presença de sais biliares de cepas probióticas de leites fermentados comerciais. Rev Inst Latic Can Tost 67:5–10
Cunha AF, Acurcio LB, Assis BS, Oliveira DLS, Leite MO, Cerqueira MM, Souza MR (2013) In vitro probiotic potential of Lactobacillus spp. isolated from fermented milks. Arq Bras Med Ve Zootec 65(6):1876–1882
Mandal S, Puniya AK, Singh K (2006) Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int Dairy J 16(10):1190–1195
Mojgani N, Hussaini F, Vaseji N (2015) Characterization of indigenous Lactobacillus strains for probiotic properties. Jundishapur J Microbiol 8(2):e17523
Garriga M, Pascual M, Monfort JM, Hugas M (1995) Selection of Lactobacilli for chicken probiotic adjuncts. J Appl Microbiol 84(1):125–132
Prasad J, Gill HS, Smart J, Gopal PK (1998) Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int Dairy J 8(12):993–1002
Hoxha R, Evstatieva Y, Nikolova D (2023) New lactic acid bacterial strains from traditional fermented foods—bioprotective and probiotic potential. J Chem Technol Metall 58(2):252–269
Sadrani H, Dave J, Vyas BRM (2014) Screening of potential probiotic Lactobacillus strains isolated from fermented foods, fruits and of human origin. Asian J Pharm Clin Res 7:216–225
Shokryazdan P, Sieo CC, Kalavathy R et al (2014) Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res Int 2014:927268
Cho IJ, Lee NK, Hahm YT (2009) Characterization of Lactobacillus spp. isolated from the feces of breast-feeding piglets. J Biosci Bioeng 108(3):194–198
Khushboo KA, Malik T (2023) Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front Microbiol 5(14):1170725
Pires V et al (2023) Probiotic, technological, and health-related characteristics of lactobacilli isolated from breast milk. J Appl Microbiol 134(6):122
Coulibaly WH, Kouadio NR, Camara F et al (2023) Functional properties of lactic acid bacteria isolated from Tilapia (Oreochromis niloticus) in Ivory Coast. BMC Microbiol 23:152
Funding
There was no funding, grants, or other support received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this research, the execution of the experiments, the interpretation of the results, and the writing and editing of the Manuscript. The content of the manuscript has not been previously published, is not currently being reviewed for publication, and is not being submitted elsewhere at the same time.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nasr, N.M., Abd-Alhalim, L.R. Characterization and identification of Lactobacillus rhamnosus and Enterococcus durans as probiotic potential isolated from selected dairy products in Egypt. J.Umm Al-Qura Univ. Appll. Sci. 10, 168–177 (2024). https://doi.org/10.1007/s43994-023-00090-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00090-1