Abstract
Recent research updates and advances have shown an upsurge of antibiotic resistance reports amongst bacterial species with increasing spread/distribution especially in the water nexus. Such has been the trend among Vibrio cholerae strain as it is observed to be emerging with diverse resistant determinants. The current study presents the occurrence of carbapenemase producing NAG Vibrio cholerae (NAG-CPV.c) in aquatic environment before the recommendation of Carbapenem antibiotics. It focused on carbapenem resistant phenotype/genotype among environmental and somatic antigen nonagglutinating V. cholerae (SANAG-Vc) strains recovered from water sources, applying standard microbiological, serological and molecular biology techniques. Domestic water samples were collected for isolation of V. cholerae strains in Eastern Cape Province, South Africa. Carbapenem and specific β-lactamase inhibitors were employed for antibiotic susceptibility testing using K-B disc diffusion technique in addition to the Modified-Hodge-Test (MHT). Our results revealed 61 strains of environmental and SANAG-Vc serogroups. Amongst these confirmed SANAG-Vc strains recovered, 25 possess carbapenemase phenotype or NDM-1 phenotype (40.89%), whereas 24 (39.34%) were MHT positive phenotype. Further gene-based detection revealed 20 (32.79%) PCR confirmed as NDM-1 resistant gene positive strains. It is important to note that the carbapenem members of antibiotics are not readily employed in the therapeutic control of cholera cases as recommended by CLSI. Observing such resistant phenotypes/genotypes indicates a possible transfer/dissemination and emergence of such resistant determinants in the environment as the coastal water serves as a potential breeding hub for such resistant genes among potential pathogens. This is a serious threat to environmental wellness/public health especially those in the sub-urban and poor living localities, who source the analyzed water as their major source of water for domestic activities. The need for adroit and continuous monitoring of released water effluents of domestic and clinical sources remains a control strategy for environmental wellness and water bodies safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cholera is described as an enteric disease associated with acute watery diarrhea which results from ingestion of Vibrio cholerae contaminated food or water [1, 2]. Other unhygienic and poor sanitary practices of human are also individuals associated driving factors for spread of cholera infection. The potential pathogen is a Gram negative bacteria that thrives and/or inhabit estuaries and coastal water as free living and colonizer of diverse locale [3, 4]. The case fatality reports of V. cholerae infections (cholera/acute watery diarrhea), variation in pathogenic determinants and changing antibacterial susceptibility profile indicate that the potential pathogen has a tendency to cause disturbing outbreaks if not controlled [1]. This microbial strain as well as other related group members and potential pathogens are said to inhabit estuary/coastal water were the estuary is reported as a black box were pathogens breed resistant genes by some investigators [2, 5,6,7,8,9]. In addition, the environmental water nexus is shown to have been fortified with numerous waste released chemical, disinfecting, cell-free nucleic acids and bactericidal agents [2, 6] which are potential contributing factors to emergence of multiple antibiotic resistance. The distribution of such multiple antibiotic resistant potential pathogens was recently reported in South Africa [2, 5, 7, 10, 11] environment and the possibility for the dissemination of such resistant phenotypes/genotypes further substantiate research interest.
Current area of growing interest has become assessment and report of antibiotic resistance to common and uncommon antibiotics used in the management/control of cholera. However, according to the Clinical Laboratory Standard Institute guidelines [12, 13], important and common antibiotics used for cholera treatment are tetracycline, doxycycline, ampicillin, chloramphenicol, trimethoprime–sulfamethoxazole, azithromycin and ciprofloxacin. Recent reports from various investigators depicts that the potential pathogen is expressing resistance and low susceptibility to these commonly applied antibiotics [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], arousing interest in the study. The study reports the occurrence of carbapenemase producing non-serogroup agglutinating V. cholerae (CP-NAG-V.c) in the environment were the carbapenem antibiotics have not been recommended.
2 Materials, methods and experimental details
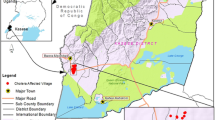
Various final effluents waste water treatment plants (feWWTPs) and domestic water sources which are located within three municipalities in Eastern Cape Province (S 31° 54.548′, E 026° 50.715ʹ, S 31° 54.548′, E 026° 50.715ʹ, S 31° 54.548′, E 026° 50.715ʹ) were sampled for the study. Water samples from 20 mapped and linked sampling points (including feWWTPs, rivers, receiving water shed, dam, Irrigation-canals and tap water) were retrieved and analyzed since they have similar receiving water shed/water body. Triplicate water samples were collected following standards as recommended [30, 31] into ice cooler boxes and taken for analysis within 8 h at Applied and Environmental Microbiology Research Group (AEMREG) Laboratory at the University of Fort Hare, Alice, South Africa.
2.1 Presumptive bacterial identification
Briefly, yellow/green 18–24 h old bacteria colonies on Thiosulphate-Citrate-Bile-Salts and Sucrose (TCBS/S) agar (Merck KGaA, Darmstadt, Germany) were purified on nutrient agar and stored in fresh aliquot tubes containing 40% glycerol in 2 ml of sterile Luria–Bertani liquid media on sterile 2 ml calibrated and Cryo-skirted-preservation tubes (Starlabs, Milton Keynes, UK)}. Colonial features/characteristic and cultural morphology observed were recorded as Gram negative rods, oxidase positive, motility positive, Voges–Proskauer test positive and d-mannitol catabolism (Table 1). Other in vitro presumptive virulence test including string test, protein hydrolyzing enzyme test, lipid hydrolyzing enzyme test, lecithin hydrolyzase test and cholera red production [2, 32] were also conducted and recorded for the selected Isolates.
2.2 Serological detection of isolates
The MAS-AGGL-M11004 Ogawa antisera, MAS-AGGL-M11003 Inaba antisera and MAS-AGGL-M15001 O139 Bengal antisera were commercially obtained from the Mast-Davis diagnostics {Gauteng, South Africa}. An 18–24 h old purified single presumptive colony was smear on clean grease-free slide containing three drops of sterile normal saline. Three drops of each obtained antisera was added and homogenised within one minutes as described in manufacturer instructions. Observation of clumps or agglutination was recorded as positive report depending on the applied antisera (Table 1).
2.3 Antibiotic susceptibility testing of positive/confirmed strains
Identified isolates were grouped based on their sources (feWWTPs, rivers, receiving water shed, dam, and irrigation canal) and subjected to antibiotic sensitivity testing following the guidelines documented by Clinical laboratory Standard Institute guideline [5, 8, 12]. Antibiotic susceptibility testing (AST) was conducted using the disc diffusion method as recommended by Clinical laboratory Standard Institute guideline and other investigators [5, 8, 12]. Antibiotics employed were chloramphenicol, doxycyclin, ampicillin, ceftazidime, cefotaxime, cefazolin, cefuroxime, cefepime, meropenem, doripenem, imipenem and ertapenem. However, based on the nature of the study which focused on carbapenem antibiotic group, only related antibiotics were selectively reported.
2.3.1 Carbapenemase phenotypic and genotypic detection
The study describes the antibiotics used and other methods employed including phenotyping and genotyping of carbapenemase determinants. Test strains with phenotypic resistance profile revealing resistant markers/determinants to the carbapenem antibiotic group; Doripenem (Dor-10 μg), Ertapenem (ETP-10 μg), imipenem (Imi-30 μg), meropenem (Mem-10 μg), and other β-lactam (cephalosporin) antibiotics including ceftriaxone (CRO-30 μg), cefotaxime (CTX-30 μg) and ceftazidime (CAZ-30 μg) were presumptively chosen as carbapenemase producers. The EDTA-Ertapenem synergy investigation was employed to determine presumptive or phenotypic carbapenemase production on previously confirmed nonagglutinating V. cholerae strains [8]. Such strains with positive carbapenemase phenotypic characteristic were confirmed employing the Modified Hodge Test (MHT). Their resistant genotype (New Delhi Metallo-β-lactamase-1 (NDM-1 gene) was further detected using target specific oligonucleotide primer pairs (Sect. 2.4).
2.3.2 EDTA-antibiotic disk diffusion synergy test
This was conducted by first standardizing the test strains on sterile saline suspension broth using 0.5 McFarland standards while the control strains (E. coli ATCC 25922) were treated as test strains in appropriately labeled pre-prepared test tubes. Pre-prepared Mueller–Hinton agar plates (MHAP) were inoculated with standardized suspension and allowed to stand for 10 min. Thereafter, an EDTA-disc which contains 10 µl of pre-prepared 0.5 M-EDTA (approxly; 1.5 mg/disc), Doripenem (Dor-10 μg), Ertapenem (ETP-10 μg) and/or Imipenem (Imi-30 μg) were positioned about 20 mm apart (centre to centre) on pre-prepared agar plates. A negative control test plate which was not fortified with EDTA-disc was also done. Both preparations were incubated at 37 °C for 18–24 h. A positive presumptive carbapenemase producing strains was reported when greater-than 4 mm zone of inhibition was observed on any EDTA fortified disc agar plate were compared to the non-fortified agar plates [5, 8, 9, 12, 33,34,35].
In the same manner, a 6 mm EDTA-disc (containing approxly; 1.5 mg/disc) was applied on Imipenem (IMI-10 µg) disc, placed 10 mm apart and incubated at 37 °C. Positive result is reported with observation of an enlarged clear inhibition zone [5, 8, 9, 12, 33,34,35].
2.3.3 MHT confirmation
The Modified Hodge Test (MHT) was applied as confirmatory test using Meropenem (MEM-10 μg) antibiotic disc on the centre of Mueller–Hinton agar plates while control strain (E. coli ATCC 25922) was treated as test strains. Standardized (0.5 McFarland) test strains were streaked in a straight line lawn across antibiotic disc and allowed to acclimatize for 3–5 min and thereafter incubated at 35 °C ± 2 °C in ambient air overnight. Any record of a clover leaf-type groove at culture intercept was interpreted as positive New Delhi Metallotype-β-lactamase-1 (NDM-1 gene) test.
2.4 Nucleic acid extraction
Nucleic acid (genomic DNA) was extracted from pure isolates using heat-to-boiling technique as previously described by various investigators [8, 36]. The gene-based characterization/detection of serologically positive V. cholerae was done using standard methods and reported elsewhere [8, 9]. Carbapenemase genes were targeted and detected by PCR using target specific primer sequences [37].
2.4.1 Genetic confirmation of blaNDM-1Resistance amongst NAG-V. cholerae
The various presumptive carbapenem enzyme producing strains or β-Metallotype lactamase producers were further confirmed using gene-based PCR detection of blaNDM-1gene. Briefly a 25 µl final volume reaction mixtures was obtained by mixing 15 µl GoTaqPCR master mix reagents (www.promega.com), 0.5 µl of 0.4 µM concentration of each target primer pair {(blaND-MβL-1-F: 5ʹ GGG CAG TCG CTT CCAACG GT 3ʹ and blaND-MβL-1-R: 5ʹ GTA GTG CTC AGT GTC GGC AT 3ʹ)}, 3 µL of extracted template DNA, and 7.0 µl of nuclease-free water was added into sterile 200 µl microfuge tube and loaded into a PCR machine (Bio-Rad T100™ thermal cycler, www.lasec.co.za, SA). The applied cycling condition include: initial denaturation of 94 °C for 2 min; 35 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and final extension: 72 °C for 10 min and 4 °C post-PCR storage. PCR products were electrophoresed on 1.5% (wt/vol) pre-prepared agarose gel stained with 4 μl/100 mL of 0.5 mg/l ethidium bromide. The observation of an amplicon size of 475 base pair was reported positive for blaNDM-1.
2.5 The study limitations
Following the specification of Clinical laboratory Standard Institute guideline [12] and other standard guidelines for the susceptibility testing of Carbapenem antibiotics, both the disc diffusion test (the Kirby–Bauer method), the micro broth dilution method and/or the agar well diffusion method {as recommended by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) [12, 13, 33, 34]} are needed for the appropriate determination of MIC and susceptibility testing. However, during the study, the micro broth dilution method and/or the agar well diffusion method were not applied for determination of antibiotic susceptibility testing. This was informed by resources available during study. In addition, horizontal transfer of extra-chromosomal DNA (Plasmid) among resistant strains as well as Plasmid profile of such environmental isolates was not also conducted. These areas of the study are currently ongoing in our lab. Furthermore, the whole-genomic and/or partial-genomic sequencing of carbapenem positive strains was not also conducted due to availability of funds. We are currently seeking for research funding (Grant) to cover unattended area of the study and do hope that the reports will be presented/published simultaneously in the future.
2.6 Statistical analysis
The epidemiological statistical tool was applied to determine V. cholerae mean-cell density/count as obtained from experimental water samples, while pearsons correlation was used to determine level of statistical significances in relation to sites (reported elsewhere [8].
3 Results
The results of the study (based on the analysis conducted) are presented as shown below in tables, figures and percentages occurrences. The Table 1 show the cultural, morphological and biochemical characteristics of the bacteria strains recovered during the study. The study showed that among the 943 samples collected, 756 presumptive strains were recovered.
3.1 Serological detection of isolates
The result of various determinate serological analysis conducted on tested isolates were also shown which revealed that among the 756 presumptive isolates recovered, 61 were confirmed as V. cholerae isolates, while 56 (90.2%) were positive to the Ogawa antisera whereas 6 (8.2%) were positive to the inaba antisera. One amongst the strains (1.6%) showed positive agglutination to the Bengal antisera employed during the study. Such observation shows that the bengal strains seldom occur in study environment however this was not the focus of our study.
3.2 Antibiotic susceptibility testing of positive/confirmed strains
The antibiotic susceptibility testing employed three basic strategies which include the usual multidisc antibiotic susceptibility test, EDTA-antibiotic disk diffusion synergy test and MHT Confirmation test. These tests were applied during the study as basic standard and practicable test for carbapenem antibiotic susceptibility testing. Other tests which may have been applied were not conducted due to available funds during study. The Table 2 shows the selected usual multiple disc antibiotic susceptibility testing results while Figs. 1, 2, 3 show the phenotypic resistance and susceptibility profile of isolates with isolates distribution dynamics in the studied water nexus. There was a notable resistance to doripenem as 18% of isolates were resistant to the carbapenem antibiotic member with an intermediate susceptibility report on 23% of isolates. It was also observed that 19.7% of isolates were resistant to imipenem with an intermediate result on 32% of isolates. Furthermore, 34% and 45.9% of isolates had an intermediate susceptibility report to the Ertapenem and Meropenem respectively, while 85.2% of test isolates were resistant to ampicillin. Other detection strategy applied consist a disc of Imipenem, Imipenem-EDTA-disc, Ertapenem and Doripenem with EDTA-disc test, Erterpenem and EDTA-disc test samples as A,B,C,D,E,F are representative photo-microgram of Disk Diffusion Synergy Test. It may be reported that Carbapenemase production and phenotypic detection is shown by a clove expression by strain. It is important to note that only selected antibiotics that address the objective of the study were presented in this report.
3.3 Genetic confirmation of blaNDM-1 Resistance amongst NAG-V. cholerae
The results of genetic confirmation/genotyping of blaNDM-1 resistance are shown in Fig. 4 as revealed by the photo-microgram of gel electrophoresis for positive detected somatic antigen nonagglutinating V. cholera strains (SA-NAGVc). The genotypic detection of isolates using target specific New Delhi Metallo-beta-lactamase 1 (ND-MβL-1), which is specific for carbapenemase detection showed representative base pair band size of 475 bp as expected of all isolates producing such resistance genotype which indicates that the tested NAG-V. cholerae strains were producers of carbapenemase and/or carbapenem resistant genotype. The Table 3 and histogram (Fig. 5) shows an analysis conducted to reveal the prevalence of tested strains, distribution dynamics of strains in the study environment and in sampled water nexus. It shows that amongst recovered and SANAG-Vc confirmed strains, 21 isolates were recovered/confirmed from final effluent of waste water treatment plants (feWWTPs), 11 isolates were recovered/confirmed from receiving water shed, 13 isolates were recovered/confirmed from rivers, 6 isolates were recovered/confirmed from dams and 10 isolates were recovered/confirmed from irrigation canal (Table 3, Fig. 5). This is an indication that there is a notable distribution of such strains in addition to resistant phenotype and genotype within the water nexus studied which necessitates the attention of various water wellness regulatory bodies in the studied area water system.
4 Discussion
Diverse determinants of antibiotic resistance have been reported to be present amongst isolates with increasing emergence of newer resistant determinants; one of such is the resistance to carbapenem antibiotic group. The current surge and impact of vibriosis as well as V. cholerae infections in various endemic regions has also added to the threat, as it is revealing emerging resistance virulent determinants. Although carbapenem group of antibiotics are not routinely sourced therapeutic regimen for the control/treatment of V. cholerae related infections, the antibiotic members are currently sourced in some localities that harbors resistant strains of V. cholerae and diverse Vibrio related infections as specified by the CLSI [12]. The current study presents the occurrence of carbapenemase producing NAG Vibrio cholerae (NAG-CPV.c) in aquatic environment before the recommendation of Carbapenem antibiotics. Presumptive Vibrio species recovered during the study were 756 isolates, 61 of these strains were further confirmed as non-agglutinating serogroup (nonO1/nonO139 V. cholerae) using both microbiological methods, serology and molecular biology techniques (Table 1). Amongst the recovered and SANAG-Vc confirmed strains, 21 isolates were recovered/confirmed from final effluent of waste water treatment plants (feWWTPs), 11 isolates were recovered/confirmed from receiving water shed, 13 isolates were recovered/confirmed from rivers, 6 isolates were recovered/confirmed from dams and 10 isolates were recovered/confirmed from irrigation canal (Table 3, Fig. 5). It is important to note that all confirmed/selected isolates were somatic antigen nonagglutinating V. cholerae 1/139 (SANAG-Vc) strains. The feWWTPs isolates were observed to have the highest number of recovered/confirmed isolates implicating the feWWTPs as a potential hub for the distribution of such strains in the water nexus. It is worthy to emphasize that prior to the gene-based confirmation and detection techniques applied, the study also employed both the use of biochemical and serological methods as previously suggested/reported by various related investigators [5, 8, 9, 38,39,40,41]. It was observed that both applied techniques present related positive reports on the strains revealing them as somatic antigen non-agglutinating 1/139 V. cholerae or nonO1/non-O139 V. cholerae strains.
Twenty-five isolates among these confirmed strains were shown to possess carbapenemase phenotype or NDM-1 phenotype (40.89%), (Figs. 1, 2) whereas 24 (39.34%) were observe to produce the MHT positive phenotype (Fig. 3). The application of a gene-based detection strategy for the observed resistant phenotype revealed that 20 (32.79%) were PCR confirmed as NDM-1 resistant gene positive strains (Fig. 4). The observation of such high resistance phenotype/genotype is of potential clinical relevance especially as the carbapenem antibiotic members have not been recommended as a therapeutic regimen for the treatment of V. cholerae infection and other vibriosis within the study region. Furthermore, observing such carbapenem resistant phenotype/genotype in the studied nexus is an indication that there is an existing and ongoing spread as well as sharing of the phenotype/genotype even before the use of the antibiotic group for treatment purpose. Suffice it to say that carbapenemase phenotype/genotype is one amongst the various resistant dynamics that is basically harbored by extra-chromosomal nucleic acids (plasmids) and distributed via horizontal genetic transfer (HGT). Additionally, there is also possibility that any ongoing spread and/or sharing of resistant genes (markers) with relevant resistant phenotype may influence therapeutic management/control of other related bacterial infections. This was the position of some related investigators who opined that because of the increasing heave of carbapenem resistance determinants, there is a significant increase in health concern which have resulted in delayed treatment of related Vibrio infection or other related bacteria cases and a major challenge to treatment [5, 9, 39] of disease cases. Similar reports were published by Shah, his group and other researchers that such observed increase in resistant phenotypes (especially carbapenem which spreads mainly via extra-chromosomal DNA) may result potential increase in the volume and/or incidence of intensive care unit (ICU) cases [9, 42,43,44].
In addition, the PCR detection of such resistant gene (NDM-β-1) among environmental strains of SANAG V. cholerae in water nexus also indicates a potential risk to human, animal, plants, and microbial subjects that applies the water as a source for livelihood. The observed resistant gene also implies that the environmental strains of SANAG- V. cholerae, are emerging with diverse resistant determinants (eg: NDM-1) which also necessitates adroit implementation of appropriate hygienic practice in handling water sources. It has also revealed that there is need for appropriate reporting and documentation of the emerging nature of such resistance determinants especially in water nexus for epidemiological reference in endemic area.
Most recently, the study of diverse investigators [42, 45] did report the occurrence of NDM-1 resistance gene among potential pathogens in water bodies especially amongst Gram-negative strains. NDM-1 resistance genotype and phenotype was also reported among Klebsiella species in patients (hospitalised) in Pakistan and Nepal region with various lineage, plasmid characterization and sequence types [46,47,48,49,50]. However, our study followed a different path where horizontal resistant gene transfer was not described by profiling and detecting resistant plasmid on strains. Although this study structure does not include describing the transferability of resistant genes, the previous reports of related investigators have shown that strains with such resistant gene are potential pathogens of environmental risk. It is therefore, noteworthy for various regulatory organizations and individuals in the study region to employ health related practices and implement novel research based strategies and report any risk related case about users of the water sources.
5 Conclusion
It is evident from the study that carbapenemase producing V. cholerae strains are emerging in the water bodies which necessitates a continuous monitoring and surveillance of the various water bodies using molecular typing technique in major endemic regions. Although the NDM-1 resistant genotypes and phenotypes has been reported to be encoded by plasmid as various related investigators have reported, our study has not confirmed such among the V. cholerae determined. We are hoping to confirm such in our future research as it is an important ongoing research in our lab. However we will not fail to mention that such observation, if true for these V. cholerae strains, would reveal the tendency of strains to disseminate their resistant genotypes among other environmental bacterial strains that co-habit in these water sources and may also contribute to thriving tendency and spread of strains in the estuaries. It is therefore recommended that wastewater regulatory organization as well as nationals should encourage both local and municipal individual wastewater treatment practitioners’ for sustainable management/control and compliance with standard of operations in the near future. Major microbial emerging contaminants of the wastewater final effluent have been described to pose public, clinical and eco-environmental health concerns. Such contaminants/release from non-compliance wastewater works has burdened water re-cycling and reuse as well as irrigation with possible impact on the agricultural production. Important note should be emphasized that any contact and accumulation of microbial contaminants with farm produce (vegetables) may produce and/or increase potential health implications. Consequently, such emerging microbial contaminants (EMC) in effluent release have also been previously reported by related investigators to be associated with high antibiotic resistance in bacteria (ARB) and resistant genes dissemination in the environment, since wastewater serves as a favourable medium/hub for proliferation of both antibiotic resistance and pathogens. Hence, intervention efforts towards controlling the indiscriminate use of antibiotics and unwanted release of effluent into the environment should also be periodically monitored/checked regularly. Furthermore, observing new bacteria implicated antibiotic resistant phenotypes/genotypes such as carbapenem resistance among V. cholerae (CRV.c) strains or New Delhi Metallo β-lactamase producing V. cholerae (NDM-1-V.c) strains and other emerging resistant phenotypes and genotypes observed in this study has revealed the need for another area of research. The need for design/development of potent and better antimicrobial agents (via drug design research) or alternative chemotherapeutics is both imminent and paramount with a view to combat the cholera menace in endemic localities.
Data availability
Data generated and used in the study were original data of authors from laboratory experiments within the stated period of the study in the region which may be made available by corresponding author via specific request.
References
World Health Organization. Fourth annual meeting of the Global Task Force on Cholera Control: 21–22 June 2017, Cape Town, South Africa (No. WHO/WHE/IHM/EMI/2018.2). World Health Organization; 2018.
Igere BE, Okoh AI, Nwodo UU. Wastewater treatment plants and release: the vase of Odin for emerging bacterial contaminants, resistance and determinant of environmental wellness. Emerg Contam. 2020a. https://doi.org/10.1016/j.emcon.2020.05.003.
Cabral JP. Water microbiology. Bacterial pathogens and water. Int J Environ Res Public Health. 2010;7(10):3657–703.
Hossain ZZ, Farhana I, Tulsiani SM, Begum A, Jensen PK. Transmission and toxigenic potential of Vibrio cholerae in hilsha fish (Tenualosa ilisha) for human consumption in Bangladesh. Front Microbiol. 2018;9:222.
Igere BE, Okoh AI, Nwodo UU. Antibiotic susceptibility testing (AST) Reports: a basis for environmental/epidemiological surveillance and infection control amongst environmental Vibrio cholerae. Int J Environ Res Public Health. 2020;17:5685. https://doi.org/10.3390/ijerph17165685.
Manaia CM, Rocha J, Scaccia N, Marano R, Radu E, Biancullo F, Cerqueira F, Fortunato G, Iakovides IC, Zammit I, Kampouris I. Antibiotic resistance in wastewater treatment plants: tackling the black box. Environ Int. 2018;115:312–24.
Igere BE, Okoh AI, Nwodo UU. Non-serogroup O1/O139 agglutinable Vibrio cholerae: a phylogenetically and genealogically neglected yet emerging potential pathogen of clinical relevance. Arch Microbiol. 2022;204(6):1–28.
Igere BE, Okoh AI, Nwodo UU. Lethality of resistant/virulent environmental Vibrio cholerae in wastewater release: an evidence of emerging virulent/antibiotic-resistant-bacteria contaminants of public health concern. Environ Challenges. 2022;1(7): 100504.
Igere BE, Okoh AI, Nwodo UU. Atypical and dual biotypes variant of virulent SA-NAG-Vibrio cholerae: an evidence of emerging/evolving patho-significant strain in municipal domestic water sources. Ann Microbiol. 2022;72(1):1–13.
Perovic O, Ismail H, Schalkwyk EV. Antimicrobial resistance surveillance in the South African public sector. South Afr J Infect Dis. 2018;33(4):118–29.
Igere BE, Onohuean H, Nwodo UU. Modern knowledge-scape possess petite influence on the factual persistence of resistance determinants (ARGs/MGEs): a map and assessment of discharged wastewater and water bodies. Heliyon. 2022;8: e12253.
CLSI. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-fifth information supplement M100-S25. CLSI, 2015 Wayne, PA USA
Matuschek E, Brown DFJ, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20:O255–66. https://doi.org/10.1111/1469-0691.12373.
Fluit AC, Schmitz FJ. Resistance integrons and super-integrons. Clin Microbiol Infect. 2004;10(4):272–88.
Vaseeharan B, Ramasamy P, Murugan T, Chen JC. In vitro susceptibility of antibiotics against Vibrio spp. and Aeromonas spp. isolated from Penaeus monodon hatcheries and ponds. Int J Antimicrob Agents. 2005;26(4):285–91.
Dabanch PJ, Herrero CD, Pavez AC, Veas PN, Braun JS, Porte TL. Vibrio parahaemolyticus bacteremia: case report and literature review. Revista Chilena de Infectologia: Organo Oficial de la Sociedad Chilena de Infectologia. 2009;26(4):360–2.
Quilici ML, Massenet D, Gake B, Bwalki B, Olson DM. Vibrio cholerae O1 variant with reduced susceptibility to ciprofloxacin, Western Africa. Emerg Infect Dis. 2010;16(11):1804.
Wang R, Lou J, Liu J, Zhang L, Li J, Kan B. Antibiotic resistance of Vibrio cholerae O1 El Tor strains from the seventh pandemic in China, 1961–2010. Int J Antimicrob Agents. 2012;40(4):361–4.
Yu L, Zhou Y, Wang R, Lou J, Zhang L, Li J, Bi Z, Kan B. Multiple antibiotic resistance of Vibrio cholerae serogroup O139 in China from 1993 to 2009. PLoS ONE. 2012;7(6): e38633.
Po KHL, Wong MHY, Chen S. Identification and characterisation of a novel plasmid-mediated quinolone resistance gene, qnrVC7, in Vibrio cholerae of seafood origin. Int J Antimicrob Agents. 2015;45(6):667–8.
Ceccarelli D, Chen A, Hasan NA, Rashed SM, Huq A, Colwell RR. Non-O1/non-O139 Vibrio cholerae carrying multiple virulence factors and V. cholerae O1 in the Chesapeake Bay, Maryland. Appl Environ Microbiol. 2015;81(6):1909–18.
Thapa Shrestha U, Adhikari N, Maharjan R, Banjara MR, Rijal KR, Basnyat SR, Agrawal VP. Multidrug resistant Vibrio cholerae O1 from clinical and environmental samples in Kathmandu city. BMC Infect Dis. 2015;15(1):1–7.
Carraro N, Rivard N, Ceccarelli D, Colwell RR, Burrus V. IncA/C conjugative plasmids mobilize a new family of multidrug resistance islands in clinical Vibrio cholerae non-O1/non-O139 isolates from Haiti. MBio. 2016;7(4):10–1128.
Wang R, Yu D, Yue J, Kan B. Variations in SXT elements in epidemic Vibrio cholerae O1 El Tor strains in China. Sci Rep. 2016;6(1):22733.
Cross R, Ling C, Day NP, McGready R, Paris DH. Revisiting doxycycline in pregnancy and early childhood–time to rebuild its reputation? Expert Opin Drug Saf. 2016;15(3):367–82.
Dengo-Baloi LC, Semá-Baltazar CA, Manhique LV, Chitio JE, Inguane DL, Langa JP. Antibiotics resistance in El Tor Vibrio cholerae 01 isolated during cholera outbreaks in Mozambique from 2012 to 2015. PLoS ONE. 2017;12(8): e0181496.
Narendrakumar L, Thomas S. Vibrio cholerae O1 gaining reduced susceptibility to doxycycline, India. J Glob Antimicrob Resist. 2018;12:141–2.
Ottaviani D, Medici L, Talevi G, Napoleoni M, Serratore P, Zavatta E, Bignami G, Masini L, Chierichetti S, Fisichella S, Leoni F. Molecular characterization and drug susceptibility of non-O1/O139 V. cholerae strains of seafood, environmental and clinical origin, Italy. Food Microbiol. 2018;72:82–8.
Igere BE, Onohuean H, Iwu CD, Igbinosa EO. Polymyxin sensitivity/resistance cosmopolitan status, epidemiology and prevalence among O1/O139 and non-O1/non-O139 Vibrio cholerae: a meta-analysis. Infect Med. 2023;2:283–93.
Igbinosa EO, Okoh AI. Toxigenic Vibrio cholerae strains and their associated malaises. Afr J Microbiol Res. 2009;3(5):200–11.
Igere BE, Onohuean H, Gxalo O. Occurrence of New Delhi metallo-beta-lactamase 1 producing Enterococcus species in Oghara Water Nexus: an emerging environmental implications of resistance dynamics. ogy Insights. 2022;15:11786361221133732.
Huq A, Haley BJ, Taviani E, Chen A, Hasan NA, Colwell RR. Detection, isolation, and identification of Vibrio cholerae from the environment. Curr Protoc Microbiol. 2012;26(1):6A-A5.
CLSI. 2017 Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI M100-S26; Clinical and Laboratory Standards Institute: Wayne, PA, USA; 2017.
CLSI. 2019. Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI M100-S26; Clinical and Laboratory Standards Institute: Wayne, PA, USA; 2020.
Tejashree A, Deepashree R, Devananda D, ArchanaHegde M. Detection of CTX-M and NDM-1 gene in clinical isolates of E. coli. Indian J Res. 2017;6(8):5–10.
Maugeri TL, Carbone M, Fera MT, Gugliandolo C. Detection and differentiation of Vibrio vulnificus in seawater and plankton of a coastal zone of the Mediterranean Sea. Res Microbiol. 2006;157(2):194–200.
Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11(5):355–62.
Aberkane S, Compain F, Barraud O, Ouédraogo AS, Bouzinbi N, Vittecoq M, Jean-Pierre H, Decré D, Godreuil S. A non-O1/non-O139 Vibrio cholerae avian isolate co-carrying the blaVIM-1 and blaVIM-4 genes, France. Antimicrob Agents Chemother. 2015;59:6594–6.
Aires-de-Sousa M, Ortiz de la Rosa JM, Goncalves ML, Costa A, Nordmann P, Poirel L. Occurrence of NDM-1-producing Morganella morganii and Proteus mirabilis in a single patient in Portugal: probable in vivo transfer by conjugation. J Antimicrob Chemother. 2020;75(4):903–6.
Baron S, Larvor E, Chevalier S, Jouy E, Kempf I, Granier SA, Lesne J. Antimicrobial susceptibility among urban wastewater and wild shellfish isolates of Non-O1/Non-O139 Vibrio cholerae from La Rance estuary (Brittany, France). Front Microbiol. 2017;8:1637.
Uddin ME, Akter T, Sultana P, Sultana P, Hasan MI, Lubna MA, Al Monem H, Parvez MAK, Nahar S, Khan MS. Isolation, identification and antimicrobial susceptibility profile analysis of Vibrio cholerae O1 from stool samples of Bangladesh. Adv Microbiol. 2018;8(03):188.
Khan S, Mustafa A. Antibiotic-resistant bacteria and bla NDM-1 genes in the drinking tap water of a megacity. Water Environ J. 2021;5:1313–24.
Shah KJ, Cherabuddi K, Shultz J, Borgert S, Ramphal R, Klinker KP. Ampicillin for the treatment of complicated urinary tract infections caused by vancomycin–resistant Enterococcus spp. (VRE): a single-center university hospital experience. Int J Antimicrob Agents. 2018;51(1):57–61. https://doi.org/10.1016/j.ijantimicag.2017.06.008.
Shanthi M, Sekar UMA, Arunagiri K, Bramhne HG. OXA-181 beta lactamase is not a major mediator of carbapenem resistance in Enterobacteriaceae. J Clin Diagn Res JCDR. 2013;7(9):1986.
Ranjan R, Thatikonda S. β-lactam resistance gene NDM-1 in the aquatic environment: a review. Curr Microbiol. 2021;78(10):3634–43.
Eva H, Ejaz H, Scott JB, Wang N, Gujaran S, Pickard D, Wilksch J, Cao H, Haq I, Dougan G, Strugnel RA. Resistance mechanisms and population structure of highly drug resistant Klebsiella in Pakistan during the introduction of the carbapenemase NDM-1. Sci Rep. 2019;9:2392. https://doi.org/10.1038/s41598-019-38943-7.
Diep TT, Nguyen NTN, Nguyen TNC, An HK, Nguyen TQ, Nguyen VH, Nguyen TV, Nguyen TNA, Izumiya H, Ohnishi M, Yamashiro T. Isolation of New Delhi metallo-β-lactamase 1-producing Vibrio cholerae non-O1, non-O139 strain carrying ctxA, st and hly genes in southern Vietnam. Microbiol Immunol. 2015;59(5):262–7.
Van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae (CPE). Virulence. 2017;8(4):460–9.
Wei WJ, Yang HF, Ye Y, Li JB. New Delhi metallo-β-lactamase-mediated carbapenem resistance: origin, diagnosis, treatment and public health concern. Chin Med J. 2015;128(14):1969.
Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Clevel Clin J Med. 2013;80(4):225.
Acknowledgements
Authors are grateful to the Patho-Biocatalysis Group (PBG) Laboratory at the University of Fort Hare, Alice, South Africa and the Africa German Network of Excellence in Science for their support
Author information
Authors and Affiliations
Contributions
IBE: Conceptualised, investigated, validate analysis, formal analysis, data curation, writing original draft, editing, review. OJO: validated, formal analysis, editing. UUN; conceptualized, validation, resources, data curation, supervised, review/editing, visualization. All authors have read and agreed on the current version.
Corresponding author
Ethics declarations
Competing interests
Authors declared no competing interest either financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Igere, B.E., Onojafe, J.O. & Nwodo, U.U. Occurrence of carbapenemase producing NAG Vibrio cholerae (NAG-CPV.c) in aquatic environment before the recommendation of carbapenem antibiotics. Discov Water 4, 64 (2024). https://doi.org/10.1007/s43832-024-00098-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-024-00098-6