Abstract
This study focuses on the environmental conditions of the Mahanadi Estuary near Paradeep Harbor and the adjacent sea. Data collected from May 2013 to April 2020 from 32 GPS fixed stations was analyzed to assess the water quality in different zones (estuarine, mixed zone, mixed zone south, and mixed zone north) of study area. Parameters such as pH, SST, TSS, nitrite, phosphate, silicate, TOC, chlorophyll, fecal coliform, and heavy metals were used to estimate the Water Quality Index (WQI) for each zone. The study found a deterioration (> 30%) in the overall water quality of the Mahanadi Estuary from 2013 to 2020, potentially attributed to river inflows, port activities, and industrial outflows in to the coastal ecosystem. Seasonal variations in temperature, salinity, turbidity, nitrite, nitrate, and ammonia were observed. The water quality showed a deteriorating trend in estuarine, mixed zone, mixed zone south, and mixed zone north. Based on the water quality indices, the ecosystem shows moderate levels of stress. The degraded water quality highlights the need for a targeted mitigation plan to reduce external pressures and enhance the overall ecosystem quality.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Climate change has presented significant challenges to oceans and estuaries, intricate systems with complex dynamic interactions. Ecosystem collapse can occur when these components are damaged or fail at different levels [1]. A healthy ecosystem operates successfully within the bounds of natural variability and exhibits resistance to stress up to a certain threshold [2,3,4]. Environmental factors such as pollution, changes in productivity, oceanic carbon dioxide (CO2) levels, and biodiversity patterns contribute to the system's complexity [5,6,7]. Various sources of pollution, including polluted river tributaries, urban sewage, agricultural and aquaculture runoff, port activities, and oil spills, contribute to increased marine pollution [8], exacerbating decomposition processes and adversely affecting coastal populations and ecology [9,10,11]. The degradation of estuarine ecosystems often manifests as a simultaneous decline in multiple structures and functions, necessitating comprehensive assessments prior to implementing restoration techniques [12, 13]. Regular assessment and monitoring are crucial to evaluate potential environmental damage resulting from anthropogenic activities that can cause large-scale release of harmful compounds, non-toxic organic matter, and nutrients, leading to marine life mortality due to local nutrient accumulation and hypoxia [14,15,16,17,18,19].
Risks associated with the transportation of various chemicals, fertilizers, petroleum products, coal, and iron ore, resulting in significant pollutant discharges during loading and unloading operations [20,21,22,23]. The presence of major industries, and urban in the catchment, contributes to potential discharge of pollutants into the Mahanadi River, which eventually flows into the Bay of Bengal at Paradeep [24, 25]. Additionally, agricultural runoff [15, 16], fisheries [26], flooding [27], recreational activities, and sewage inflows [28] also contribute to marine pollution in the Paradeep area. Continuous strategic monitoring is therefore essential to manage the cumulative impacts of these activities over time.
This study focuses on the temporal and spatial variability influenced by tides and various anthropogenic activities in the Mahanadi Estuary near Paradeep Harbour. The Water Quality Index (WQI) is used to assess the pollution status of Paradeep Port related to port activities. This approach aims to provide a comprehensive, long-term assessment of ecosystem health, valuable information on the Paradeep coastal area (estuarine and sea), and raise awareness among stakeholders and decision-makers.
2 Materials and methods
2.1 Description of study area
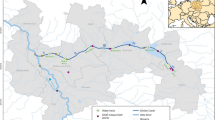
Paradeep, situated at the confluence of the Mahanadi River and the Bay of Bengal, holds significant strategic importance as a port. It is positioned approximately 210 nautical miles south of Kolkata and 260 nautical miles north of Visakhapatnam, marked by coordinates 20°15’55.44 north latitude and 86°40’34.62 east longitude. The study area covers a coastal stretch of about 45 km, extending from the mouth of the Jatadhari River to Hukitola Bay (Fig. 1 & Table1). This region boasts remarkable biodiversity, showcasing diverse mangrove and aquatic life. The ecosystem of Paradeep faces various challenges, largely driven by anthropogenic activities that exert substantial pressure on the environment. This study aims to assess the fundamental characteristics of ecosystem health and their impact on the Paradeep coastal area. To facilitate the assessment, the study area has been systematically divided into four zones based on the inflow from the Mahanadi River to the sea. These zones include the Estuary, the Mixed Zone, the southbound downstream of the Mixed Zone, and the northbound upstream of the Mixed Zone. Each of these designated zones serves as a sampling site for data collection and analysis.
The area is characterized by large industrial clusters, including oil refineries (IOCL), iron and steel plants (ESSAR), ports (PPT), steel and thermal power facilities (ESSAR), and fertilizer and brewery establishments (IFFCO, PPL, and SKOL) (Fig. 1). These activities generate a significant amount of wastewater, with a capacity of 32.72 million liters per day (MLD), resulting in potential anomalies within the estuarine ecosystem. Furthermore, the river system contributes to the pollution load through agricultural runoff, industrial leaching, mine discharges, and municipal effluents from the catchment area. The Mahanadi Basin houses 15 major industries, including aluminum and thermal power plants in Hirakud and Jharsuguda, chromium and power plants in Chowdwar, paper mills in Jagatpur, and fertilizer plants in Paradeep. These industrial units discharge liquid waste directly into the Mahanadi Basin, with larger units in Sambalpur, Cuttack, and Paradeep accounting for effluent volumes of 736 thousand liters per day (KLD), 2780 KLD, and 5280 KLD, respectively. Additionally, numerous medium and small industries in the Mahanadi Basin release approximately 100,000 cubic meters of wastewater daily, while 12 coal mines discharge 14,000 cubic meters of mine water per day during non-monsoon months [29,30,31].
The region also hosts over 60 coastal aquaculture firms operating on land areas exceeding 36 hectares for multiple years. Therefore, the present study aims to assess the fundamental characteristics related to the health of the ecosystem and investigate the resulting impacts on the bio-system in the coastal area of Paradeep. This region is subjected to various activities that potentially stress the coastal ecosystem, thus posing a significant threat. The overall condition of the study area plays a crucial role in shaping both the structural and functional aspects of the ecosystem, and conversely, these aspects have an influence on the overall condition. This approach adopts a holistic perspective to evaluate the ecosystem health of the Paradeep Coastal Area, distinguishing it from previous studies [24, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] (Table 2).
Intensive studies have been accomplished by different researchers to recognize the dynamics of sea current circulation in Bay of Bengal (BOB). It is compelled through southwest monsoon, with an anti-cyclonic gyre pattern in summer, and also influenced by the north eastern East Indian Coast Current (EICC) [48, 49]. The southwest monsoon current, develops in the summer season near east of Sri Lanka from local wind [50] have also impact on the coast. The northeast monsoon created in winter, dominates the entire BOB and generates a cyclonic motion, whereas the EICC flows southwest along the western boundary [51, 52]. The Paradeep coast is turbulent, experiencing with the highly unstable East Indian Coast current (EICC) along the coast. The major as well as high intra seasonal eddy kinetic energy centers are also part of Odisha coast [53]. The eddy current, EICC, and local turbulence with Ekman pump Combinely may play a contributory role in distribution of pollutant in the study area.
To facilitate the analysis, the study area has been systematically divided into four zones based on the inflow from the Mahanadi River to the sea. These zones include (1) the Estuary, (2) the Mixed Zone, (3) the Downstream of the Mixed Zone towards the South, and (4) the Upstream of the Mixed Zone towards the North. Appropriate sampling sites for the Estuary, the Mixed Zone, the Mixed Zone towards the north, and the Mixed Zone towards the south were considered based on the confluence point of the Mahanadi River and the salinity gradient (Fig. 1).
2.2 Sample collection and data analysis
The study covered 32 fixed GPS locations (Fig. 1) spanning the Mahanadi River estuary, including the estuarine and mixing zones, as well as the adjacent sea with mixing zones both upstream and downstream, along the Paradeep coast in the Bay of Bengal. These locations were strategically selected to capture the influential factors identified in Table 1.
Surface water samples were systematically collected at all sites between 2013 and 2020 during the pre-monsoon, monsoon, and post-monsoon periods. A Niskin vertical sampler was used to collect samples at depths ranging from 0.5 to 1.5 m. The physical and biochemical parameters were determined following the methods outlined by the American Public Health Association [54].
On-site measurements were conducted using a portable multiprobe (Make,model YSI-556) to assess parameters such as pH, sea surface temperature (SST), and salinity. Total suspended solids (TSS) were quantified gravimetrically using GF/C glass microfiber filter paper with a diameter of 47 mm. Turbidity was assessed through nephelometric method outlined by APHA in 2010 (Systronics, Model 135), while dissolved oxygen (DO) levels were determined using the Winkler's method with iodometric titration. Biological oxygen demand (BOD) was determined by incubating samples for 3 days at 27 °C, following the Winkler's method. The concentration of phosphate (PO4) and silicate (SiO4) were analyzed calorimetrically using an auto analyzer (Manufacturer: Skalar, Model: San++, Germany). Nitrite (NO2) levels were measured spectrophotometrically using a Perkin Elmer-Lambda 45 UV/VIS spectrophotometer. Total organic carbon (TOC) was estimated following the APHA 2010 guidelines using a TOC analyzer (Manufacturer: OI Analytical Xylem, model: 1030D, USA) based on NDIR principles.
Heavy metals (Fe, Mn, Cd, Pb) were classified according to the method developed by Satyanarayan and his research group [55] and analyzed by ICP-MS (Make: Perkin Elmer, Model: NexION 300X). Mercury (Hg) levels were assessed using the cold vapor atomic absorption spectrophotometry method outlined by APHA in 2010 [54], employing a mercury analyzer (Make: ECIL brand, Model: MA5840). Chlorophyll (Chl-a) concentrations were determined spectrophotometrically, following the methodology described by Parson and his coworkers [56]. Fecal coliforms (FC) were enumerated using the most probable number (MPN) method outlined in the APHA 2010 guidelines [54].
The water quality of the study area has been assessed according to Coastal Water Quality Standards [57,58,59] through a comprehensive analysis of 17 physico-biochemical parameters. The investigation revealed distinct patterns that are commonly observed in subtropical marine environments (Table 3). Indicators such as temperature, pH, dissolved oxygen (DO), biochemical oxygen demand (BOD), total suspended solids (TSS), turbidity, total organic carbon (TOC), nitrite, phosphate, silicate, chlorophyll-a, fecal coliform (FC), iron (Fe), manganese (Mn), mercury (Hg), lead (Pb), and cadmium (Cd) were compared to appropriate thresholds.
In this study, all collected data underwent analysis to calculate the Water Quality Index (WQI) using the aforementioned parameters. The calculation was performed in accordance with the Coastal Water Quality Standards established by the Central Pollution Control Board (CPCB) in 2010 [59], the Australian and New Zealand Environment and Conservation Council (ANZECC) in 1992 and 2000 [57, 58], and the methods outlined by the Canadian Council of Ministers of the Environment (CCME) in 2001 [60].
2.3 Statistical calculation
The assessment followed the guidelines of the Canadian Council of Ministers of the Environment Water Quality Index (CCME) [60], which compares to baseline conditions on the basis of severity (F1: proportion of indicators missing one or more targets) and frequency (F2: Proportion) included all comparisons in which the targets were not met) and amplitude (F3: normalized degree of target exceeding). These indicators have been integrated to calculate an overall health index, represented by a score and a corresponding color code. Using these values, a Geographical Information System (GIS) was used to create a colour-coded representation of the Health Index.
Brown and his research group developed an assessment to determine the level of pollution in the sea water (Table 3) [61]. Table 4 provides a high-level overview of seventeen parameters included in this methodology, along with the corresponding thresholds assigned to each parameter. Equation (1) represents the WQI equation that quantifies overall water quality by assigning a numerical value based on various water quality parameters [60, 62, 63]. The Water Quality Index (WQI) is a dimensionless value that summarizes multiple water quality factors into a single number by normalizing their values using subjective rating curves [64]. It serves as an appropriate and specific indicator for a broader assessment of the ecological health of ecosystems [65, 66]. In addition, the WQI provides a standard metric for assessing the effectiveness of water quality management strategies [67, 68]. The choice of factors to include in a WQI model depends on the specific water use and local preferences. This model condenses large amounts of water quality data into simplified terms (e.g., “excellent,” “good,” or “poor”), facilitating consistent reporting for managers and the public [69, 70].
Index score in CCME WQI was calculated by the relation
where, Scope (F1), frequency (F2) and amplitude (F3) was calculated as.
F1 =\(\left(\frac{\text{number of failed variables}}{\text{total number of variables}}\right)\) × 100.
F2 = \(\left(\frac{\text{number of failed tests}}{\text{total number of tests}}\right)\) × 100.
F3 is calculated in three steps:
-
1.
“Excursion” is the number of times by which an individual concentration is greater than the objective.When the test value must not exceed the objectives
-
When the test value must not exceed the objectives
-
-
a)
\({excursion}_{i}=\left(\frac{{Failedtestvalues}_{i}}{{Objectives}_{j}}\right)\)
-
When the test value must not fall below the objective,
-
-
b)
\({excursion}_{i}=\left(\frac{{Objectives}_{j}}{{Failedtestvalues}_{i}}\right)\)
-
2.
Normalized sum of excursion (nse) is calculated as:
-
3.
\(\mathrm{F}3\hspace{0.17em}=\hspace{0.17em}\frac{nse}{0.01nse+0.01}\)
The WQI score obtained is compared with grading scale, which is broken down into the five classifications listed in Table 5. Water quality is considered improved when WQI values increase from 0 to 100. Based on the value obtained, the WQI rating was compared with the general conditions: Excellent (A): 80–100, Good (B): 60–80, Fair (C): 40–60, Poor (D): 20–40 and Very Poor (F): 0–20 as indicated [71, 72].
The application of various multivariate statistical techniques such as cluster analysis (CA), principal components analysis (PCA) and factor analysis (FA) allows the identification of significant components or factors responsible for most of the variances in a system [73, 74]. These techniques aim to reduce the number of variables to a small set of indices while preserving the relationships present in the original data [75]. To assess the variability of the parameters, multidimensional scaling (MDS) and hierarchical cluster analysis were conducted using IBM SPSS (Version: 22) and Minitab 17 software packages. The MDS and hierarchical cluster analysis aimed to determine the similarity index between different sampling stations based on the measured parameters, as well as the similarity index among the parameters themselves. The dissimilarity between the stations and parameters was calculated using the Euclidean distance metric. Moreover, a cluster analysis (CA) was carried out to evaluate the similarity index, considering the Euclidean distance, among all stations with respect to the measured parameters. Similarly, the similarity index among all parameters was determined using the same cluster analysis approach. To present the seasonal variations, boxplots were generated using Origin 8.5 software, incorporating the seasonal data. Principal Component Analysis (PCA) is a robust statistical technique widely used to analyse the variance present in a large dataset consisting of inter-correlated variables, thereby reducing the number of independent variables employed [76]. The PCA process involves optimizing various aspects, such as selecting relevant variables and determining optimal weights to categorize the index variables [77].
Qualitative examination of the association between principal components and manifest variables, measured by stress (i.e., the correlation between principal component values and manifest variables), can contribute to the interpretation of a principal component. The PCA technique involves extracting eigenvalues and eigenvectors from the covariance matrix of the original variables. The principal components (PCs) are uncorrelated (orthogonal) variables obtained by multiplying the original correlated variables by the eigenvector, which represents a set of coefficients (loadings or weights). As a result, the PCs are weighted linear combinations of the original variables. PCs provide valuable information about the most significant parameters that describe the entire dataset, enabling data reduction with minimal loss of original information [78, 79].
The first principal component corresponds to the linear combination of the original variables that contributes the most to their overall variance, while the second principal component, uncorrelated with the first, contributes the most to the remaining variance. This process continues iteratively until the entire variance is analysed. It is important to note that the suitability of this method relies on all variables being measured in the same units due to its dependence on the total variance of the original variables [80, 81].
3 Result and discussion
3.1 Seasonal and temporal variation
The present study reveals significant temporal and spatial differences in the physicochemical and biological properties that are considered as key drivers of the marine environment. To allow for better comparison and analysis of the data, a categorization based on three-year phases (i.e. 2013–2015, 2015–2017 and 2017–2020) has been adopted. The parameter contribution was observed using a logarithmic transformation and the results are presented in Fig. 2 (a, b, c).
To assess the variability of each parameter over different years, the data were analysed using boxplots as shown in Fig. 3a–r. The box plots were labelled PR (pre-monsoon), MO (monsoon), PO (post-monsoon), and Y (year) for the respective years. The analysis showed that most parameters showed significant deviations in 2019 and 2020, with the exception of nitrite (Fig. 3i), salinity (Fig. 3g), faecal coliform bacteria (Fig. 3l), mercury (Fig. 3n) and chlorophyll (Fig. 3m). This observation is of concern and suggests that the discrepancies may be due to municipal and river discharges from land into the estuarine-sea ecosystem.
On the other hand, parameters such as total suspended solids (TSS) (Fig. 3e), turbidity (Fig. 3f), total organic carbon (TOC) (Fig. 3h), nitrite (Fig. 3i) and faecal coliform (Fig. 3l) showed maximum variability in 2014, 2015 and/or 2016 (Fig. 3). This variability is due to the Mahanadi River floods in 2014–2016. Furthermore, the boxplots comparing seasonal data (Fig. 3a–r) showed that in 2014, 2015, 2016 and 2019 pH (Fig. 3a) showed a typical trend of pre-monsoon < monsoon < post-monsoon while showing another trend in other years. Salinity (Fig. 3g) consistently showed lower values during the monsoon season throughout the study period. Nitrite (Fig. 3i) had unusually high levels during the 2015 and 2016 pre-monsoons compared to other years, which had a significant impact on the dataset. This anomaly can be attributed to uncontrolled discharges from fertilizer plants in those particular years, later regulated by the State Pollution Control Board (OSPCB). Phosphate (Fig. 3j) showed higher levels during the 2019 pre-monsoon, suggesting that special attention and limitations are needed due to possible contributions from fertilizer crops.
Chlorophyll-a (Fig. 3m) showed seasonal variations with a trend of pre-monsoon > monsoon > post-monsoon in 2013, 2015 and 2017, possibly due to upwelling and nutrient enrichment. However, in 2019 and 2020 the trend shifted towards pre-monsoon < monsoon < post-monsoon. The cadmium (Fig. 3r) concentrations did not show any significant seasonal fluctuations, but the concentration was significantly higher in the period 2019–2020 compared to the previous years (2013–2018). Whereas, iron (Fig. 3p), manganese (Fig. 3o), and lead (Fig. 3q) showed strong seasonal variation during 2019, 2020, 2016, 2020, and 2018, 2019, respectively. However, their concentrations were significantly higher in 2019–2020 compared to 2013–2018. In contrast, mercury (Fig. 3n) concentrations were minimal in 2019–2020 compared to 2013–2018. It showed strong seasonal variation during 2014, 2015, 2016, 2017, and 2018. The significant change in the chlorophyll-a trend during the pre-monsoon and post-monsoon periods in the first phase (2013–17) compared to the second phase (2019–20) can be attributed to physical phenomena such as upwelling processes. The term "upwelling" pertains to the vertical transport of nutrient-laden waters from the deep regions of the ocean to its surface, leading to heightened levels of primary productivity and chlorophyll-a concentration. The process of upwelling facilitates the upward movement of nutrient-laden waters from lower depths to the uppermost layers, thereby serving as a vital nutrient supply for the proliferation of phytoplankton. The increase in nutrient levels serves as a catalyst for the proliferation of phytoplankton, resulting in elevated concentrations of chlorophyll-a. Coastal regions are notably impacted by upwelling phenomena as a result of the presence of coastal upwelling systems. These systems facilitate the upwelling of nutrient-rich waters in coastal regions, resulting in elevated levels of chlorophyll-a and enhanced primary productivity. Numerous investigations [82,83,85] have been conducted to explore the relationship between the intensity of upwelling and the concentrations of chlorophyll-a in various geographical areas. These studies have yielded compelling evidence supporting the impact of upwelling on primary productivity and the dynamics of chlorophyll-a.
3.2 Tidal variation
Upon analysis, it was observed that some environmental parameters exhibited noteworthy variations when comparing HT and LT conditions (Fig. 4a–r). Notably, parameters such as pH, salinity, and TOC consistently showed higher values during high tide events. The increase TOC could be linked to enhanced organic matter input, possibly facilitated by the lower water volume evacuation during HT. The increase values may be attributed to the influx of seawater and associated factors during high tides, leading to higher concentrations of these parameters. Dissolved oxygen concentrations were also elevated during high tide cycles, could be due to reduced water movement during high tide, allowing for increased gas exchange at the water–air interface [99].
However, TSS, turbidity, BOD, NO2, PO4, SiO4, FC, Chlorophyll and Heavy metals levels were consistently higher during low tide events [86,87,88,89,90]. This observation might be due to increased sediment resuspension during lower tidal energy conditions, leading to a cloudier water column. Interestingly, pH and temperature did not exhibit significant differences between high and low tide conditions. This observation suggests that tidal fluctuations might not exert a substantial influence on these two parameters, likely owing to other prevailing factors that dominate their variation. The observed variations in environmental parameters during tidal cycles highlight the complex interactions between tidal dynamics and biogeochemical processes in coastal ecosystems. The findings underscore the need to consider the multifaceted nature of these systems when evaluating the impacts of tidal influences on water quality and ecosystem health.
Observation was made for higher-than-expected concentrations of nitrate and silicate, along with an elevation of phosphate levels in the specific region. The nutrient concentrations in the previous studied found to be greater than expected, corroborating previous researches carried out in the estuarine regions of the country (Table.3) [41, 91,92,93,94,95,96,97,98,99]. The unusual weather conditions, characterized by heavy rainfall (> 1638 mm) in 2019 and 2020 in comparison to previous years, could have led to runoff from the nearby phosphate fertilizer plants. This runoff might have carried phosphate compounds from the factories into local water bodies, thereby increasing the phosphate concentration. This phenomenon has ecological implications, emphasizing the need for further research and potential management strategies to mitigate adverse impacts on the aquatic ecosystem.
3.3 Water quality index (WQI)
To assess the overall water quality, the Water Quality Index (WQI) was calculated for different zones in different phases (2013–15, 2015–17 and 2017–20) as well as for the whole period. Figs. 5, 6 show the values of different zones in relation to the WQI. The total value of coastal waters fell below Class C in 2017–20, indicating the impact of river inflows, port activities and industrial discharges in and around Paradeep. This finding clearly indicates that the water quality of the Mahanadi River and its estuary falls below Class D. The WQI ratings for the estuary zone and mixed zone were 30 and 46, respectively, indicating the impact of loading in the estuary, which may have been diluted beyond the confluence.
In 2013–15, the WQI scores for the Estuarine Zone (E), Mixing Zone (MZ), Mixing Zone North (MZN), and Mixing Zone South (MZS) were 44, 47, 65, and 59, respectively. In the subsequent 2017–20 period, these scores dropped to 32, 39, 45, and 38. This decrease, particularly in the estuary zone (from 44 in 2013–15 to 30 in 2017–20), indicates a 32% deterioration in water quality. Although the mixed zone status declined from 47 in 2013–15 to 39 in 2015–17, it improved again to 46 in 2017–20. The mixed zone also showed a downward trend, with the WQI 65 in 2017 drop to 43 in 2020, a 32% decrease. The mixed zone south, influenced by the Jatadhari River and IOCL activities, saw a decline from a WQI score of 59 to 38 (36%) in 2015–17 and further to 41 (30%) in 2017–20. Rainwater, which carries sediment, nutrients and heavy metals, is a major contributor to the deterioration of the Mahanadi River estuary. In addition, the presence of port stockpiles with ore dumps, extensive storage sites for industrial waste (e.g. IFFCO and PPL gypsum), discharges from boats at the fishing dock, agricultural effluents and industrial discharges from Paradeep and the catchment area aggravate the decline in water quality in the estuary and in the mixed zone (class C or D). Therefore, appropriate mitigation plans are required to regulate the flow of pressures and improve ecosystem quality.
3.4 Trend analysis
A comprehensive analysis was performed considering multiple studies conducted earlier in the same area (Table 2). In the present study, 18 parameters were examined and various trends analyzed over different study periods. Among the parameters considered was TSS (Total Suspended Solids), turbidity, FC (faecal coliform bacteria), nitrite, phosphate, silicate, mercury and iron were found to be the most important factors for the route studied. These parameters showed significant differences during their respective phases from 2013 to 2020. However, despite their significant contributions, TSS and turbidity remained relatively stable throughout the study period (Fig. 2 a, b, c). During 2017–20 (Fig. 2c), the dominance of iron and mercury in the water samples can be attributed to under management of the Paradeep Harbor ore stock, which allowed storm water intrusion into the study area, thereby affecting water quality. The increase in nitrite and phosphate levels in the study area over time may be influenced by nearby fertilizer plants such as IFFCO and PPL.
Data from 32 stations monitored from 2013 to 2020 was considered to observe the trends of individual parameters at each station. The regression value (R2) was used to determine the increasing or decreasing trend of each parameter. The percentage of stations showing an increasing or decreasing trend was then calculated and expressed as a percentage of the total 32 stations as shown in Table 6. The aim of this analysis was to provide site-specific trends in pollution levels for important water quality parameters. It underscores the need for constant monitoring to predict pollutant properties, their presence, changes, movement or leakage in complex ecosystems where time is of the essence.
Observed trends indicate that TSS, BOD (Biochemical Oxygen Demand), turbidity and silica are of particular concern, with 44%, 36%, 28% and 28% of stations showing an upward trend respectively. Although high levels of nitrite were expected due to the presence of large fertilizer plants (IFCCO and PPL) nearby, 40% of the stations experienced a decrease. This decrease is due to strict regulations by the regulator on industrial discharges and modernization of household and ETP (Waste Water Treatment Plants) plants in industry.
3.5 Multidimensional scaling analysis (MDS)
To characterize the variability of the parameters, a multidimensional scaling analysis (MDS) was performed with IBM SPSS (version: 22). The data set from 2013 to 2020 was subjected to this analysis and revealed four different groups of parameters with similarities (Fig. 7). TSS and pH formed a separate group while nitrite, temperature and mercury merged into another group. Another group formed fecal coliforms, turbidity, silicates, phosphates and DO (dissolved oxygen). Chl-a (chlorophyll-a), BOD, TOC (Total Organic Carbon), salinity, iron (Fe) and cadmium (Cd) formed the third distinct group. Remarkably, metals such as manganese (Mn) and lead (Pb) did not belong to any group with a strong similarity. However, in the cluster analysis (Fig. 8) three distinct groups were observed with a similarity of 48.2%. Group one included temperature, salinity, Cd, Hg, BOD, TOC and Chl-a. Group two consisted of Mn, Fe and Pb. Group three included parameters such as pH, phosphate, turbidity, nitrate, TSS, silicate, FC and DO, which were partially consistent with MDS analysis. Mn and Pb, which were independent of each other in MDS, showed strong resemblance to Fe in cluster analysis and formed a group. At ~ 70% similarity level, a narrower micro group consisting of pH, PO4, turbidity and nitrate emerged, which showed differences to TSS, silicate, FC and DO within the third group. This clearly demonstrates the interdependence of parameters such as DO, PO4, silicate, TSS, turbidity and nitrite, which control ecosystem biological community parameters and consequently affect coastal health. Likewise, BOD and chlorophyll-a are interdependent and play a crucial role in the health of the coastal environment [17, 29, 100]. Hierarchical cluster analysis (Fig. 8) shows three distinct groups, with metals (Fe, Mn and Pb) forming a separate group [101]. Cd and Hg, which share similarities with salinity, are in a higher range of distinguishable similarity [29]. BOD, TOC and Chl-a are grouped together but differ significantly at a higher similarity range and are closely related [102]. Phosphate, DO, silicate, TOC, turbidity, FC and nitrite are also grouped together, indicating a higher degree of similarity compared to other groups [103].
In the multi-dimensional scaling (MDS) analysis (Fig. 9), six distinct groups were identified upon comparing various stations. The aforementioned groups are linked to four distinct zones, which are referred to as the Estuary (E), Mixing Zone (MZ), Mixing Zone South (MZS), and Mixing Zone North (MZN). It is worth mentioning that the estuary stations, ES1 to ES6, constituted a distinct group. Further, MZ9 of M zone and MZS7 of MS zone exhibited a close association with the E zone (Fig. 10). The mixing zone stations displayed a range of distinct characteristics and were classified into various categories. Within the set of Mixing Zone stations, MZ2 is associated with the MZN zone, MZ1 is affiliated with the MZS Zone, and MZ5 has formed a distinct new group with MZS4 of MZS zone. Upon analysis of the MN zones' stations, it was observed that MZN3, MZN4, MZN5, and MZN6, in addition to MZ2 of the Mixing Zone, exhibited a discernible cluster. Nevertheless, MZN1 and MZN2 exhibited a noticeable clustering pattern with MZ8 of the Mixing Zone. The stations within the Mixing Zone South, have been classified into four distinct categories. Specifically, MZS11, MZS3, and MZS9 have been grouped together, while MZS1, MZS6, MZS10, MZS2, MZS5, and MZS9 have been grouped with the mixing zone. Eventually, MZS4 of the Mixing Zone South zone and MZ5 of the Mixing Zone separated into their own distinct group.
3.6 Principal component analysis (PCA)
The principal component analyses were performed on the parameters of coastal water samples from 2013 to 2020. The results are presented in Table 7, which identifies the correlated variables (principal components) from the extensive data set. The table includes the loadings and eigenvalues for each component, as well as the percentage and cumulative percentage of variance explained by each parameter. The objective is to explain the maximum variance with the fewest number of principal components.
The analysis revealed that the first eight principal components account for over 90% of the total variance in the dataset. The first principal component accounts for 34.34%, the second for 17.92%, the third for 9.49%, the fourth for 7.83%, and the fifth for 7.02% of the total variance. The eigenvalues of the first five principal components can be used to assess the dominant physicochemical and biological processes of the coastal ecosystem.
In the first principal component (PC1), salinity, turbidity, BOD, TOC, phosphate, silicate, Chl-a, Mn, Fe, and Cd showed strong positive loadings (0.516–0.823), indicating an interdependence between phytoplankton growth, increased chlorophyll levels, and the rise in phosphate and silicate. This, in turn, contributes to the increase in BOD due to the death and decay of phytoplankton [104]. This finding supports the results of previous studies [105, 106]. Turbidity played a significant role with the highest loading in this study.
The second major component (PC2) exhibited high positive loadings for dissolved oxygen (DO) (0.722) and fecal coliform (FC) (0.691), along with negative loadings for pH (-0.747), total suspended solids (TSS) (-0.549), and salinity (-0.607). The inflow of the Mahanadi River into the study area brings surface water and industrial effluent, which may explain these associations. Salinity showed a negative correlation with DO and silicate, while DO solubility decreased with increasing salinity. Freshwater inflow from rivers is considered the primary source of silicate and phosphate in coastal waters [107]. The positive correlation between DO, phosphate, and silicate in this study could be attributed to the intrusion of freshwater with low salinity but high nutrient content (phosphate and silicate) into the coastal waters [108], thus confirming our findings.
The third (PC3) and fourth (PC4) principal components did not exhibit significant positive or negative loadings for any of the parameters. In PC5, BOD showed a negative loading (-0.649) without any notable evidence of positive loading.
The cumulative percentage of variance for the different components, presented in ascending order (34.38, 52.30, 61.8, 69.63, 76.64, 81.9, 86.4, 90.2, etc.), indicates that the first eight components account for over 90% of the total variance. The first five components explain over 70% of the deviations and are influenced by parameters such as salinity, turbidity, BOD, TOC, phosphate, silicate, Chl-a, Mn, Fe, and Cd, which likely play a crucial role in determining the characteristics of this coastal ecosystem.
4 Conclusion
The Paradeep shoreline has been under constant pressure for a long time, from both natural and human sources. The initial trophic level of a coastal environment is affected by variables such as biochemical oxygen demand (BOD), total organic carbon (TOC), turbidity, nitrite, silicate, and phosphate. Discharges from rivers are the primary entry point for pollutants into ecosystems. In particular, pollution from the Mahanadi River basins reaches the estuary carrying agricultural runoff, effluents from industry, mines, and urban settlements. The increasing complexities of the problems need thorough research in order to protect the environment. This study can be used as a resource to advocate for environmentally responsible management and call for more careful planning of the ecological and biological characteristics of the area.
Effective pollution management in the Mahanadi river basin requires regulatory measures as follows.
-
Strengthening the operability of the Effluent Treatment Plant (ETP) or establishing a Common Wastewater Treatment Plant (CWTP) can effectively treat point source pollutants discharged by industry, thereby restoring the health of the coastal ecosystem.
-
The impacts of non-point sources, particularly from agricultural fields to Mahanadi River and their subsequent intrusion into the study area requires a comprehensive plan of action to limit their impact.
-
It is important not to overlook the heavy metal contamination leaking from the Paradeep Port storage yard. The port's open courtyard serves as a storage facility for significant amounts of raw materials, including coal and iron ore, which must be remodelled and stored indoors to contain this source of contamination.
-
Raising awareness among stakeholders and decision-makers is crucial. Which must be adopted through
-
Communication campaigns: Using various channels to disseminate relative information on health of the ecosystem.
-
Stakeholder engagement: Involving stakeholders in decision-making processes in the study area.
-
Data dissemination: Providing access to relevant scientific information.
-
Policy advocacy: Advocating for supportive policies and regulations.
-
Monitoring and feedback: Evaluating effectiveness and gather input for improvement of the site is the need.
Data availability
All the data generated or collected for this study are included in this published articles.
References
Chen X, Gao H, Yao X, Chen Z, Fang H, Ye S. Ecosystem health assessment in the pearl river estuary of china by considering ecosystem coordination. Journal Plos One. 2013;8(7):70547.
Panda BS, Mahanta SK. Spatio seasonal discrepancies in the physico-chemical parameters along the surface water of gahirmatha estuary, east coast of India in the Bay of Bengal. Adv Environ Technol. 2019;5(3):171–83. https://doi.org/10.22104/aet.2020.4306.1216.
Murugesan K, Mallick N, Pati SS, Sharma SD, Panda BS, Mishra S, Nayak S, Sarangi B, Mohanty SK. A Pilot Study on Beach Litter Pollution Paradeep (2019–20), CMC, ICZMP, SPCB, Odisha. 2022;pp: 1–82.
Meyer SE, Callaham MA, Stewart JE, Warren SD. Invasive species response to natural and anthropogenic disturbance. In: Poland TM, Patel-Weynand T, Finch DM, Miniat CF, Hayes DC, Lopez VM, editors. Invasive Species in Forests and Rangelands of the United States. Cham: Springer; 2021. https://doi.org/10.1007/978-3-030-45367-1_5.
Malhi Y, Franklin J, Seddon N, Solan M, Turner MG, Field CB, Knowlton N. Climate change and ecosystems: threats, opportunities and solutions. Philos Trans R Soc B Biol Sci. 2020;375(1794):20190104. https://doi.org/10.1098/rstb.2019.0104.
Shivanna KR. Climate change and its impact on biodiversity and human welfare. Proc Indian Natl Sci Acad. 2022;88(2):160–71. https://doi.org/10.1007/s43538-022-00073-6.
Zhang L, Xu M, Chen H, Li Y, Chen S. Globalization, green economy and environmental challenges: state of the art review for practical implications. Front Environ Sci. 2022;10:870271. https://doi.org/10.3389/fenvs.2022.870271.
Elliott JE, Elliott KH. Tracking marine pollution. Science. 2013;340(6132):556–8. https://doi.org/10.1126/science.1235197.
Newton A, Icely J, Cristina S, Perillo GME, Turner RE, Ashan D, Kuenzer C. Anthropogenic, direct pressures on coastal wetlands. Front Ecol Evol. 2020. https://doi.org/10.3389/fevo.2020.00144.
Alam MdW. Land-based marine pollution: an emerging threat to bangladesh. IntechOpen. 2023. https://doi.org/10.5772/intechopen.107957.
Panda BS, Nayak S, Pati SS, Mallick N, Mishra S, Mahala SS. Seasonal variation of heavy metals in the surface water of gahirmatha estuary, Kendrapara, Odisha. Int J Sci Eng Res. 2021;12(2):243–53.
Wu L, Ouyang Y, Cai L, Dai J, Wu Y. Ecological restoration approaches for degraded muddy coasts: Recommendations and practice. Ecol Ind. 2023;149:110182. https://doi.org/10.1016/j.ecolind.2023.110182.
Coleman MA, Wood G, Filbee-Dexter K, Minne AJP, Goold HD, Vergés A, Marzinelli EM, Steinberg PD, Wernberg T. Restore or redefine: future trajectories for restoration. Front Mar Sci. 2020;7:237. https://doi.org/10.3389/fmars.2020.00237.
Panda BS, Mahanta SSK. Determination of herbicides and pesticides in estuary and sea water of Mahanadi, near Paradeep. Int J Res Anal Rev. 2020;7(3):683–97.
Banoo S, Achary C, Behera RR. Phytoplankton population dynamics in relation to environmental variables at paradip port. East Coast of India Thalassas. 2022;38:1135–53. https://doi.org/10.1007/s41208-022-00443-3.
Panda BS, Mahanta SSK. Quantification of poly aromatic hydrocarbons in waters of mahanadi estuary at Paradeep, Odisha. Int Res J Eng Technol. 2020;07(06):6971–81.
Murugesan K, Mallick N, Pati SS, Mishra S, Nayak S, Behera R, Swain S, Sharma SD, Mahapatro D, Mahala SS, Panda BS, Patnaik S, Pati S, Mallik MR, Ratha D, Mahanta B, Mohanty SK, Patnaik A, Sarangi B. Status and Trends of Coastal Parameters: Paradeep-GahirmathaDhamra Coastal Environment (2013–2022). 1st ed. State Pollution Control Board, Odisha, 2023
Panda BS, Nayak S, Pati SS, Mahala SS, Mishra S. Seasonal assessment of heavy metals along the Estuarian region of Dhamra, East coast of India: a special approach through distribution maps and correlation matrix. J Global Ecol Environ. 2022;14(3):39–65.
Panda BS, Mahanta SSK, Ahmed SN. A review on micro plastics and few strategies for their reduction in marine environment. Int J Curr Res. 2021;13(03):16666–73.
Grote M, Mazurek N, Grabsch C, Zeilinger J, Le Floch S, Wahrendorf D-S, Hofer T. Dry bulk cargo shipping—An overlooked threat to the marine environment. Mar Pollut Bull. 2016;110(1):511–9. https://doi.org/10.1016/j.marpolbul.2016.05.066.
Barberi S, Sambito M, Neduzha L, Severino A. Pollutant Emissions in Ports: a comprehensive review. Infrastructures. 2021;6(8):114. https://doi.org/10.3390/infrastructures6080114.
Maurya P, Kumari R. Toxic metals distribution, seasonal variations and environmental risk assessment in surficial sediment and mangrove plants (A marina), Gulf of Kachchh (India). J Hazard Materials. 2021;413:125345. https://doi.org/10.1016/j.jhazmat.2021.125345.
Song D, Panayides P. Maritime logistics: a complete guide to effective shipping and port management. India: Kogan; 2012.
Behera R, Mahapatro D, Mishra S, Pati SS, Nayak S, Mohanty SK, Sharma SD, Ojha A, Behera A, Mallick N, Murugesan K. Distribution of Macrobenthos in Relation to Physico Chemical Parameters in Mahanadi Estuary and Near Shore Area of Paradeep, Odisha, East Coast of India. In: Current Research in Life Sciences, AkiNik Publication, India. 2021; pp 23–46.
Behera RR, Satapathy DR, Majhi A, Panda CR. Spatiotemporal variation of atmospheric pollution and its plausible sources in an industrial populated city, Bay of Bengal, Paradip. India Urban Climate. 2021;37:100860. https://doi.org/10.1016/j.uclim.2021.100860.
Thiemann T. Microplastic in the Marine Environment of the Indian Ocean. J Environ Prot. 2023;14:297–359. https://doi.org/10.4236/jep.2023.144020.
Ramesh R, Purvaja R, Lakshmi A, Newton A, Kremer HH, Weichselgartner J. South Asia Basins: LOICZ Global Change Assessment and Synthesis of River Catchment - Coastal Sea Interaction and Human Dimensions. LOICZ Research & Studies No. 32. GKSS Research Center, Geesthacht. 2009; pp 121.
Acharyya T, Sudatta BP, Srichandan S. Deciphering long-term seasonal and tidal water quality trends in the Mahanadi estuary. J Coast Conserv. 2021;25:56. https://doi.org/10.1007/s11852-021-00843-2.
Mishra S, Nayak S, Pati SS, Nanda SN, Mohanty S, Behera A. Spatio temporal variation of phytoplankton in relation to physicochemical parameters along mahanadi estuary & inshore area of paradeep coast, north east coast of India in Bay of Bengal. Indian J Geo Mar Sci. 2018;47(7):1502–17.
Jena M. Pollution in the Mahanadi: urban sewage, industrial effluents and biomedical waste. Econ Pol Wkly. 2008;43(20):88–93.
Ratha KC. Growing industrialization and its cumulative impacts: insights from the Mahanadi River Basin. Soc Chang. 2019;49(3):519–30. https://doi.org/10.1177/0049085719863904.
Pathak V, Shrivastava NP, Chakraborty PK, Das AK. Ecological status and production dynamics of river Mahanadi. Indian Counc Agric Res Bull. 2007;149:1–40.
Samantray P, Mishra BK, Panda CR, Rout SP. Assessment of water quality index in mahanadi and atharabanki rivers and taldanda canal in paradip area. India J Hum Ecol. 2009;26(3):153–61. https://doi.org/10.1080/09709274.2009.11906177.
Khadanga MK, Das S, Sahu BK. Seasonal variation of the water quality parameters and its influences in the mahanadi estuary and near coastal environment, east coast of India. World Appl Sci J. 2012;17(6):797–801.
Dixit PR, Kar B, Chattopadhyay P, Panda CR. Seasonal variation of the physicochemical properties of water samples in mahanadi estuary, east coast of India. J Environ Prot. 2013;4:843–8. https://doi.org/10.4236/jep.2013.48098.
Dixit P, Kar B, Chattopadhyay P, Panda CR. Seasonal and Spatial Distribution of the Hydro-chemical properties of Mahanadi estuary and influence near the Mahanadi and Paradip coastal environment, East coast of India. Bull Environ Pharmacol Life Sci. 2013;12(12):13–20.
Mohapatra RK, Panda CR. Spatiotemporal variation of water quality and assessment of pollution potential in Paradip port due to port activities. Indian J Geo Marine Sci. 2017;46(7):1274–1286.
Das A, Tripathy B. Water quality monitoring and its assessment on mahanadi basin. IOSR J Mech Civil Eng. 2019;18(3):39–55.
Tripathy B, Das A. Assessment of class of water in river mahanadi, Odisha: a case study. IOSR J Mech Civil Eng. 2019;16(5):1–10.
Srichandan S, Baliarsingh SK, Lotliker AA, Sahu BK, Roy R, Nair TMB. Unravelling tidal effect on zooplankton community structure in a tropical estuary. Environ Monit Assess. 2021;193(6):1–21. https://doi.org/10.1007/s10661-021-09112-z.
Pattanayak AA, Swain S, Behera RR, Sharma SD, Panda CR, Mohanty PK. Variability in water quality of two meso-tidal estuaries of Odisha, East Coast of India. J Mar Syst. 2024;241: 103919. https://doi.org/10.1016/j.jmarsys.2023.103919.
Upadhyay S. Physico-chemical characteristics of the mahanadi estuarine ecosystem, east coast of India. Indian J Marine Sci. 1988;17:19–23.
Gupta RS, Upadhyay S. Nutrient Biogeochemistry of the Mahanadi Estuary. Contributions in Marine Sciences: A Special Collection of Papers to Felicitate Dr. S.Z. Qasim, Secretary to the Government of India, Department of Ocean Development on His Sixtieth Birthday. N.p., National Institute of Oceanography, India; 1987, pp 291–305.
Das J, Das SN, Sahoo RK. Semidiurnal variation of some physico-chemical parameters in the Mahanadi estuary, east coast of India. Indian J Marine Sci. 1997;26:323–6.
Sundaray SK, Nayak BB, Bhatta D. Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—a case study. Environ Monit Assess. 2009;155(1–4):227–43. https://doi.org/10.1007/s10661-008-0431-2.
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D. Application of factor and cluster analysis for characterization of river and estuarine water systems—A case study: mahanadi river (India). J Hydrol. 2006;331(3–4):434–45. https://doi.org/10.1016/j.jhydrol.2006.05.029.
Sundaray SK, Panda UC, Nayak BB, Bhatta D. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the mahanadi river–estuarine system (India)—a case study. Environ Geochem Health. 2006;28:317–30. https://doi.org/10.1007/s10653-005-9001-5.
Schott FA, McCreary JP. The monsoon circulation of the Indian Ocean. Prog Oceanogr. 2001;51(1):1–123. https://doi.org/10.1016/s0079-6611(01)00083-0.
Shankar D, McCreary JP, Han W, Shetye SR. Dynamics of the east India Coastal Current: 1. Analytic solutions forced by interior Ekman pumping and local alongshore winds. J Geophys Res. 1996;101(C6):13975–91.
McCreary JP, Kundu PK, Molinari RL. A numerical investigation of dynamics, thermodynamics and mixed-layer processes in the Indian Ocean. Prog Oceanogr. 1993;31(3):181–244. https://doi.org/10.1016/0079-6611(93)90002-U.
Potemra JT, Luther ME, Obrien JJ. The seasonal circulation of the upper ocean in the Bay of Bengal. J Geophys Res. 1991;96(C7):12667–83. https://doi.org/10.1029/91JC01045.
Vinayachandran PN, Kagimoto T, Masumoto Y, Chauhan P, Nayak SR, Yamagata T. Bifurcation of the east india coastal current east of Sri Lanka. Geophys Res Lett. 2005;32:15606. https://doi.org/10.1029/2005GL022864.
Chen G, Li Y, Xie Q, Wang D. Origins of Eddy kinetic energy in the Bay of Bengal. J Geophys Res Oceans. 2018;123(3):2097–115. https://doi.org/10.1002/2017jc013455.
APHA. Standard Methods for the Examination of Water and Wastewater, 23rd edition, Pub: American Public Health Association (APHA), the American Water Works Association (AWWA), and the Water Environment Federation (WEF). 2010.
Satyanarayanan M, Balaram V, Rao TG, Dasaram B, Ramesh SL, Mathur R, Drolia RK. Determination of trace metals in sea water by ICP-MS after pre-concentration and matrix separation by dithiocarbamate complexes. Indian J Marine Sci. 2007;36(1):71–5.
Parsons TR, Maita Y, Lalli CM. A manual of chemical and biological methods for seawater analysis. Oxford: Pergamon Press; 1984.
ANZECC. Australian Water Quality Guidelines for Fresh and Marine Waters. Canberra: Australian and New Zealand Environment and Conservation Council (ANZECC). 1992.
ANZECC. Australian water quality guidelines for fresh and marine waters. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra, Australia. 2000.
CPCB. Pollution Control Acts, Rules and Notifications issued thereunder, Central Pollution Control Board, India. Law Series PCLS/02/2010. 2010; 6th edition;pp.1–1343.
CCME. Canadian water quality guidelines for the protection of aquatic life: CCME Water Quality Index 1.0, Technical Report. Technical Report. CCME, Winnipeg, Manitoba, Canada. 2001.
Brown RM, McClelland NI, Deininger RA, Tozer RG. Water quality index-do we dare? Water Sew Works. 1970;117(10):339–43.
Tyagi S, Sharma B, Singh P, Dobhal R. Water quality assessment in terms of water quality index. Am J Water Res. 2013;1(3):34–8.
Gupta N, Pandey P, Hussain J. Effect of physicochemical and biological parameters on the quality of river water of Narmada, Madhya Pradesh. India Water Sci. 2017;31:11–23. https://doi.org/10.1016/j.wsj.2017.03.002.
Miller WW, Joung HM, Mahannah CN, Garrett JR. Identification of water quality differences in nevada through index application. J Environ Qual. 1986;15(3):265–72.
Nasirian M. A new water quality index for environmental contamination contributed by mineral processing: a case study of Amang (Tin Tailing) processing activity. J Appl Sci. 2007;7:2977–87.
Simoes FS, Moreira AB, Bisinoti MC, Gimenez SMN, Yabe MJS. Water quality index as a simple indicator of aquaculture effects on aquatic bodies. Ecol Ind. 2008;8(5):476–84.
Rickwood CJ, Carr GM. Development and sensitivity analysis of a global drinking water quality index. Environ Monit Assess. 2008;156(1–4):73–90.
Hülya B. Utilization of the water quality index method as a classification tool. Environ Monit Assess. 2009;167:115–24.
Srivastava G, Kumar P. Water quality index with missing parameters. Int J Res Eng Technol. 2013;2(4):609–14.
Satpathy D, Mohapatra RK, Acharya C, Satapathy DR, Panda CR. Appraisal of temporal variation of water quality status in terms of water quality index (WQI) at Paradip port. India Res J Chem Environ. 2018;22(12):38–48.
Davies JM. Application and tests of the canadian water quality index for assessing changes in water quality in lakes and rivers of central North America. Lake Reservoir Manage. 2006;22(4):308–20. https://doi.org/10.1080/07438140609354365.
Nanda S. Chilika lake ecosystem health card. 2016. https://www.chilika.com/documents/publication1507881562.pdf. Accessed 6 Feb 2023.
Ouyang Y, Nkedi-Kizza P, Wu QT, Shinde D, Huang CH. Assessment of seasonal variations in surface water quality. Water Res. 2006;40(20):3800–10.
Shrestha S, Kazama F. Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin. Japan Environ Model Softw. 2007;22(4):464–75.
Gajbhiye S, Sharma SK, Awasthi MK. Application of principal components analysis for interpretation and grouping of water quality parameters. Int J Hybrid Inf Technol. 2015;8(4):89–96.
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A. Assessment of the surface water quality in Northern Greece. Water Res. 2003;37:4119–24.
Hamzah FM, Bahari S, Tajudin H, Abdullah SMS, Maulud KNA, Razali1 SFM, Isma ES. Statistical analysis on physical and chemical parameters and heavy metal in marine water. 5th International Conference on Mathematical Applications in Engineering, Journal of Physics: Conf Series. 2020;1489: 012036. https://doi.org/10.1088/1742-6596/1489/1/012036.
Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate data analysis with readings. 4th ed. London: Prentice-Hall; 1995.
Vega M, Pardo R, Barrato E, Deban L. Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 1998;32:3581–92.
Sharma SK, Trinath S, Gajbhiye KS, Patil S. Application of Principal Component Analysis in Grouping Geomorphic Parameters of Uttela Watershed for Hydrological Modeling. Int J Remote Sens Geosci. 2013;2(6):63–70.
Sharma SK, Gajbhiye S, Tignath S. Application of principal component analysis in grouping geomorphic parameters of a watershed for hydrological modeling. Appl Water Sci. 2014;5(1):89–96.
Zhang S, Alford MH. Instabilities in nonlinear internal waves on the Washington continental shelf. J Geophys Res Oceans. 2015;120(7):5272–83. https://doi.org/10.1002/2014jc010638.
Ekeroth N, Kononets M, Walve J, Blomqvist S, Hall POJ. Effects of oxygen on recycling of biogenic elements from sediments of a stratified coastal Baltic Sea basin. J Mar Syst. 2016;154:206–19. https://doi.org/10.1016/j.jmarsys.2015.10.005.
Orlando L, Ortega L, Defeo O. Multi-decadal variability in sandy beach area and the role of climate forcing. Estuarine Coast Shelf Sci. 2018. https://doi.org/10.1016/j.ecss.2018.12.015.
Zheng Q, Zhao H, Shi Y, Duan M. Distribution characteristics of dissolved oxygen in spring in the Northern Coastal Beibu Gulf, a typical subtropical bay. Water. 2023;15(5):970. https://doi.org/10.3390/w15050970.
Zhang D, Zhang X, Tian L, Ye F, Huang X, Zeng Y, Fan M. Seasonal and spatial dynamics of trace elements in water and sediment from Pearl River Estuary. South China Environ Earth Sci. 2013;68(4):1053–63. https://doi.org/10.1007/s12665-012-1807-8.
Censi P, Spoto SE, Saiano F, Sprovieri M, Mazzola S, Nardone G, Di Geronimo SI, Punturo R, Ottonello D. Heavy metals in coastal water systems a case study from the northwestern Gulf of Thailand. Chemosphere. 2006;64(7):1167–76. https://doi.org/10.1016/j.chemosphere.2005.11.008.
Postacchini M, Manning AJ, Calantoni J, Smith JP, Brocchini M. A storm driven turbidity maximum in a microtidal estuary. Estuar Coast Shelf Sci. 2023;288:108350. https://doi.org/10.1016/j.ecss.2023.108350.
Senthilkumar B, Purvaja R, Ramesh R. Seasonal and tidal dynamics of nutrients and chlorophyll a in a tropical mangrove estuary, southeast coast of India. Indian J Marine Sci. 2008;37(2):132–40.
Nanjappa D, Devaganga KP, Ramkumar M, Nagarajan R, Balasubramani K. Physico-chemical Properties of the Vettar Estuarine Waters Cauvery Basin, Southern India: implications for environmental management. Thalassas Int J of Marine Sci. 2023. https://doi.org/10.1007/s41208-023-00564-3.
Krishna PV, Anil Kumar K, Panchakshari V, Prabhavathi K. Assessment of fluctuations of the physico-chemical parameters of water from Krishna estuarine region, East Coast of India. Int J Fisheries Aquatic Stud. 2017;5(1):247–52.
Vijayakumar S, Rajesh KM, Mendon MR, Hariharan V. Seasonal distribution and behaviour of nutrients with reference to tidalrhythm in the Mulki Estuary, South-West Coast of India. J mar biol Ass India. 2000;42(18):21–31.
Qasim SZ, Gupta RS. Environmental characteristics of the mandovi-zuari estuarine system in Goa. Estuarbte Coastal Shelf Sci. 1981;13:557–78.
Acharyya T, Sarma VVSS, Sridevi B, Venkataramana V, Bharathi MD, Naidu SA, Kumar BSK, Prasad VR, Bandyopadhyay D, Reddy NPC, Kumar MD. Reduced river discharge intensifies phytoplankton bloom in Godavari estuary. India Marine Chem. 2012;132–133:15–22. https://doi.org/10.1016/j.marchem.2012.01.005.
Sarma VVSS, Prasad VR, Kumar BSK, Rajeev K, Devi BMM, Reddy NPC, Sarma VV, Kumar MD. Intra-annual variability in nutrients in the Godavari estuary. India Continental Shelf Res. 2010;30(19):2005–14. https://doi.org/10.1016/j.csr.2010.10.001.
Hershey NR, Nandan SB, Vasu KN. Trophic status and nutrient regime of Cochin estuarine system, India. Indian J Geo Marine Sci. 2020;49(08):1395–404.
Sarma VVSS, Krishna MS, Srinivas TNR. Long-term changes in nutrient concentration and fluxes from the Godavari estuary: role of river discharge and fertilizer inputs. Estuaries Coasts. 2023;46:959–73.
Mathew J, Singh A, Gopinath A. Nutrient concentrations and distribution of phytoplankton pigments in recently deposited sediments of a positive tropical estuary. Mar Pollut Bull. 2021;168:112454. https://doi.org/10.1016/j.marpolbul.2021.112454.
Mitra S, Ghosh S, Satpathy KK, Bhattacharya BD, Sarkar SK, Mishra P, Raja P. Water quality assessment of the ecologically stressed Hooghly River Estuary, India: a multivariate approach. Mar Pollut Bull. 2018;126:592–9. https://doi.org/10.1016/j.marpolbul.2017.09.053.
Biswal D, Behera A, Pati SS, Mishra S, Nayak S, Mishra S, Panda BS, Behera R. Status and trend of coastal parameters: 2013–18, paradeep-gahirmatha-dhamra coastal environment. Odisha: ICZMP, SPCB; 2019.
Padhi RK, Biswas S, Mohanty AK, Prabhu RK, Satpathy KK, Nayak L. Temporal distribution of dissolved trace metal in the coastal waters of southwestern Bay of Bengal. India Water Environ Res. 2013;85(8):696–705. https://doi.org/10.2175/106143012X13560205144975.
Kamble SR, Vijay R. Assessment of water quality using cluster analysis in coastal region of Mumbai. India Environ Monitoring Assess Dordrecht. 2011;178(4):321–32.
Du X, Shao F, Wu S, Zhang H, Xu S. Water quality assessment with hierarchical cluster analysis based on Mahalanobis distance. Environ Monit Assess. 2017;189(7):335. https://doi.org/10.1007/s10661-017-6035-y.
Sahu G, Satpathy KK, Mohanty AK, Sarkar SK. Variations in community structure of phytoplankton in relation to physicochemical properties of coastal waters, southeast coast of India. Indian J Geo-Marine Sci. 2012;41(3):223–41.
Irvine KN, Somogye EL, Pettibone GW. Turbidity, suspended solids, and bacteria relationships in the buffalo river watershed. Middle States Geogr. 2002;35:42–51.
Seo M, Lee H, Kim Y. Relationship between Coliform bacteria and water quality factors at weir stations in the nakdong river. South Korea Water. 2019;11:1171. https://doi.org/10.3390/w11061171.
Mishra S, Kumar A, Yadav S, Singhal MK. Assessment of heavy metal contamination in water of Kali River using principal component and cluster analysis. India Sustain Water Res Manag. 2017. https://doi.org/10.1007/s40899-017-0141-4.
Satpathy KK, Mohanty AK, Sahu G, Sarkar SK, Natesan U, Venkatesan R, Prasad MVR. Variations of physicochemical properties in kalpakkam coastal waters, east coast of india, during southwest to northeast monsoon transition period. Environ Monit Assess. 2010;171:411–24.
Acknowledgements
The authors are grateful to the World Bank; SICOM (Society for Integrated Coastal Management); MOEF (Ministry of Environment, Forests and Climate Change, Government of India); Ministry of Forests and Environment, Government of Odisha; PCCF (Principal Chief Conservator of Forests (Wild Life Division), Government of Odisha), SPMU-ICZMP for their kind assistance. We would also like to thank all the officials and staff of Coastal Management Cell of the State Pollution Control Board for their on-site and laboratory assistance during the study period.
Funding
The study was conducted under the World Bank Funded Project.
Author information
Authors and Affiliations
Contributions
NM & KM came up with the idea for the article; SSP, SN, SM, & BSP conducted the literature research; SN, SM, BSP, RB, SKM & SSM conducted the analysis and authored the paper; SSP have critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All the authors declared that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pati, S.S., Nayak, S., Mishra, S. et al. A comprehensive study of the estuary sea environment in the Bay of Bengal, near the Mahanadi River confluence. Discov Water 3, 20 (2023). https://doi.org/10.1007/s43832-023-00044-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00044-y