Abstract
Efficient and economical wastewater treatment has presented itself as a global challenge. In this context, adsorption is one of the most effective methods to remove contaminants from wastewater. The present study evaluated the feasibility of chemically modified pine bark biochar’s nitrate adsorption ability. Pine bark biochar was modified with urea and sulfuric acid to remove nitrate from an aqueous solution. The physicochemical properties of the biochar samples, such as pH, pH at point of zero charges, surface atomic composition, surface morphology, and surface area, were evaluated. The equilibrium adsorption data were fitted to the Langmuir and Freundlich isotherm models. The kinetic data were fitted to different kinetic models (pseudo-first order, pseudo-second order, intraparticle diffusion, and Elovich). The adsorption data fitted well with the Langmuir and pseudo-first order models. The maximum nitrate adsorption capacity was found to be 1.548 mg g−1. Mass transfer studies were conducted to identify the rate-limiting step, values of the external mass transfer coefficient, and diffusion coefficient in the nitrate adsorption process by the modified biochar. The external mass transfer coefficients were in the range of 2.2 × 10–11–2.86 × 10–10 m s−1. The intraparticle diffusion coefficient ranged from 6.53 × 10–10 to 1.78 × 10–9 m2 s−1. The Biot number value less than 100 indicated that the adsorption was controlled by film diffusion. Interaction energies between nitrate ions and model biochar structures were calculated DFT-based quantum chemical software (Gaussian). The positive interaction energy values (2.3485–2.485 eV) suggested nitrate adsorption on model biochar structures was thermodynamically not feasible.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, nitrate contamination, which also causes eutrophication in water bodies, has become one of the main concerns for water pollution [1, 2]. One of the primary sources of nitrate pollution is the runoff fertilizers from agricultural fields. In addition, the discharge of nutrient-rich waste from aquacultural facilities can also be a source of nitrate contamination. Other sources of nitrate contamination include thermal power plant water discharge, municipal wastewater, detergent manufacturing, leakage from septic systems, and others [3]. Nitrate, being highly soluble in water, can easily enter the surface and ground waters, posing a severe environmental and public health threat [3]. Apart from eutrophication, nitrate contamination can result in—(1) methemoglobinemia or commonly called ‘Blue baby syndrome’ in babies, (2) reduced intelligence due to a drop in oxygen supply in body cells, (3) formation of carcinogenic compounds such as nitrosamines and nitrosamides [4]. Thus, developing efficient, economical, and environmentally friendly technology to remove nitrate from wastewater streams has attracted the attention of researchers worldwide.
Adsorption is considered one of the effective methods in wastewater treatment due to its ease of operation, lower operational cost, and design simplicity [5, 6]. Several other physicochemical processes, such as osmosis, electrodialysis, ion exchange, biological and chemical denitrification, have been applied for nitrate removal from wastewater [3]. However, most of these methods suffer a few drawbacks which are outlined by Bhatnagar et al. and Hu et al. [3, 7]. In addition, adsorption can be effective against different organic and inorganic contaminants [8, 9]. Literature shows that adsorption has been applied to remove various anions such as fluoride, nitrate, phosphate, bromate, and perchlorate [10,11,12,13,14]. However, adsorption can still be more fruitful if agro-industrial wastes could be utilized to make effective, low-cost adsorbents benefiting the environment.
Recently, biochar has gained popularity due to its applicability in various environmental applications, including, but not limited to, electrochemical storage, gas scrubbing, soil amendment, wastewater treatment, and others [15,16,17,18,19,20,21]. Biochar is a porous carbon material generally prepared by pyrolyzing agricultural waste biomass under a limited or total absence of oxygen conditions [22]. Utilizing agricultural waste as feedstock in biochar preparation aids waste management and locks carbon from biomass in biochar’s structure and prevents it from returning to the atmosphere to make carbon dioxide, thus protecting the environment [23, 24]. In addition, preparing biochar is simpler, faster, and cheaper than preparing activated carbon [25]. In several instances, it has been reported that the adsorption performance of biochar surpassed that of activated carbon toward toxic pollutants [25]. Moreover, the nutrient-laden biochar after adsorption could be used as a soil amendment to enhance soil fertility [26].
The adsorption performance of unmodified biochar towards aqueous contaminants is limited due to less developed pore structures and poor surface functionality [27]. Thus, various modification techniques have been developed to improve the adsorption performance of biochar [3, 28]. Although modified biochar has been reported to have excellent adsorption capacity toward many contaminants, nitrate adsorption by biochar can be particularly challenging [29, 30]. For example, Fidel et al. reported 1.71–2.72 mg N g−1 by CaCl2-modified biochars produced at three temperatures ranging from 400–600 °C [31]. Similarly, Yang et al. reported a nitrate adsorption capacity between 0.08 and 0.12 mg N g−1 by tannic acid-modified biochars produced from different feedstocks and temperatures [32]. Wang et al. prepared HCl-modified hydrophyte biochars and reported a nitrate adsorption capacity of 3.31 mg N g−1 [33]. Although the reason behind such behavior is unclear, the poor adsorption ability toward nitrate by biochar might be due to the repulsion between negative charges as the biochar’s surface is known to be negative. Thus, a systematic analysis of nitrate adsorption by modified biochar is needed.
Our present work stems from our recently published works on biochar surface modification with nitrogen and oxygen-rich chemicals where we modify the pine bark biochar with different oxygen and nitrogen-rich chemicals to understand the speciation and surface chemistry of biochar after using different modifying precursors [34, 35]. The research question for this study was: Is nitrogen and oxygen modified biochar feasible for nitrate adsorption from aqueous solution? It is hypothesized that the increased electronic charges due to nitrogen and oxygen doping would hamper the nitrate adsorption due to negative charge repulsion. To test the hypothesis, this study evaluated the potential of nitrogen and oxygen-enriched pine bark biochars for removing aqueous nitrate. The study involved assessing the parameters influencing nitrate removal using pine bark biochars modified with urea and sulfuric acid. The physicochemical properties of the modified biochars were evaluated using scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and wet chemistry characterization. Batch adsorption of nitrate was carried out with the modified adsorbents, and the equilibrium and kinetic data were evaluated using different isotherm and kinetic models. Mass transfer kinetics of nitrate adsorption have also been studied to understand thoroughly the limiting factors of adsorption. Most importantly, density functional theory (DFT) based quantum chemical calculations were performed on model biochar structures to quantify the structure–activity relationship. Thus, this research connects the theoretical and experimental observations through mass transfer and DFT based calculations involving nitrate adsorption on biochar, providing a better understanding for modification strategies of biochar for enhancing the nitrate adsorption.
2 Materials and methods
Pine bark nuggets (6.65 \(\pm\) 2.51 cm in length) were secured locally from Oldcastle Lawn and Garden Inc., GA. After washing with deionized (DI) water, the nuggets were dried in a mechanical oven (Lindberg/Blue M MO1490SA-1) at 105 °C for three days and stored in air-tight containers. All the analytical grade chemicals were used to prepare and characterize the samples. Sulfuric acid (97.3% w/w, CAS: 7664–93-9), Nitric acid (69.0%, CAS: 7697-37-2), Potassium nitrate (CAS: 7757-79-1), Urea (BP169-500) and Hydrochloric acid (25% v/v, CAS: 7732–18-5) were purchased from Fisher Chemical.
2.1 Sample preparation
Figure 1 shows the steps involved in pristine and modified biochar sample preparation. Pristine biochar was prepared by pyrolyzing the washed and dried pine bark chips at 800 °C for 3 h with a heating rate of 10 °C min−1 under nitrogen flow. The prepared biochar was then ground and sieved to a particle size below a mesh size of 200. The pristine biochar sample thus obtained was labeled BC and was stored in an air-tight container. The modified biochar samples were prepared by steeping the pine bark nuggets in urea solution for 48 h. After this period, the steeped biomass, along with the urea solution, was pyrolyzed at 800 °C for 3 h with a heating rate of 10 °C min−1 under nitrogen flow. The samples were put in sulfuric acid (18.25 M) for 30 min after cooling them. After the sulfuric acid treatment, the samples were washed under flowing water for 30 min. The washed samples were dried in an oven (Lindberg/Blue M MO1490SA-1) at 105 °C for 48 h to obtain modified biochars. Finally, the biochar samples were ground and sieved to a particle size below a mesh size of 200. The samples prepared with urea solution were labeled UBC.
2.2 Sample characterization
The total nitrogen of the biochar samples was determined using the K2SO4-CuSO4 method and salicylate-nitroprusside-hypochlorite colorimetry with Lachat QuickChem 8500 (Loveland, CO) [36]. To determine the pH values, 0.4
g of biochar samples were equilibrated in 20 mL of DI water at 150 rpm for 24 h. The pH of the samples was obtained by filtering the biochar and then measuring the filtrate's pH (Model: AB150; Manufactured by Fisher Scientific, Waltham, MA, USA) [37]. Biochar samples’ pH at the point of zero charges (pHpzc) was determined by mixing 0.2 g of samples with 40 mL 0.1 N potassium nitrate solution at 150 rpm for 24 h in a pH range of 2–14. The pH was adjusted by adding either 0.1 N nitric acid or 0.1 N potassium hydroxide solution. After filtering the samples, the pH of the filtrate was recorded. The final pH of the filtrates was plotted against their initial pH. The intersection point of the curve with the 45° straight line (pHinitial = pHfinal) was recorded as the sample's pHpzc [38]. XPS was used to gain information on biochar samples’ surface elemental composition. A SPECS XPS system with a PHOIBOS 150 Analyzer using Mg Kα radiation under a pressure of about 3 × 10–10 mbar was used for this purpose. The XPS data were analyzed in XPSPeak software (version 4.1). The spectra were fitted with Gaussian–Lorentzian peaks with a Shirley-type background.
Surface morphology was examined with a Variable Pressure SEM—Hitachi SU3900c with high vacuum mode coupled with energy-dispersive X-ray spectroscopy (EDX). The biochar samples were dispersed on carbon tape and then fixed on an aluminum stub for analysis. The biochar samples' surface area (SBET) was determined via the Brunauer–Emmett–Teller method using Micromeritics HPVA II (Norcross, GA). All the samples were degassed before analysis at 110 °C to eliminate any weakly bound moisture in the samples. Nitrogen adsorption isotherms within the relative pressure (P/Po) range of 0.05–0.35 was used to determine SBET. Liquid nitrogen was used to maintain 77 K temperature for all the experiments [39].
2.3 Adsorption experiments
All the batch adsorption experiments were conducted in duplicates with BC, and UBC. 4 g of adsorbents were mixed with various concentrations of nitrate solutions (10–35 ppm) on a magnetic stirrer plate at 150 rpm, similar to the process described by Fitzgerald et al. [40]. While the adsorption was progressing, aliquots (2 mL) were withdrawn periodically using a hypodermic syringe. The nitrate samples were analyzed using the cadmium reduction-sulfanilamide colorimetry method with Lachat QuickChem 8500 (Loveland, CO) [41]. The adsorption capacities of the adsorbents were calculated via mass balance (Eq. 1).
where, \({C}_{o}\) and \({C}_{t}\) are the concentrations (mg L−1 or ppm) of nitrate initially and at time \(t\) (min), \(V\) is the solution volume (L), \(m\) is the mass of the adsorbent (g), and \({q}_{t}\) is the adsorption capacity (mg/g) of the adsorbents.
2.4 Adsorption equilibrium studies
Equilibrium adsorption data are vital to study any given adsorption system. Different theoretical and empirical relationships are available for modeling the equilibrium adsorption process. In this study, the equilibrium adsorption data were modeled using the non-linear forms of Langmuir and Freundlich isotherm models (Eqs. 2 and 3, respectively) by reducing the sum of squares error in Origin (2021b) software.
where, \({C}_{e}\) and \({q}_{e}\) are the nitrate concentration (mg L−1) and adsorption capacities of the adsorbents (mg g−1) at equilibrium. \({q}_{max}\)(mg g−1) and \({k}_{L}\) (L mg−1) are the Langmuir model constants relating to the theoretical maximum adsorption capacity and rate of adsorption, \({k}_{F}\) (mg/g. (L mg−1)1/n) and \(n\) (g L−1) are the Freundlich model constants signifying distribution and favorability of adsorption, respectively.
2.5 Adsorption reaction kinetics
The kinetic data from the adsorption experiments were analyzed using kinetic models. The non-linear forms of pseudo-first order, pseudo-second order, and Weber-Morris intraparticle diffusion model (Eqs. 4, 5, 6, respectively). In addition, the linear model form of the Elovich model (Eq. 7) was also employed [40, 42,43,44].
where, \({k}_{1}\) (min−1), \({k}_{2}\) (mg g−1 min−1), and \({k}_{P}\) (mg g−1 min−0.5) are the first-order, second-order, and intraparticle diffusion rate constants, respectively. \(\alpha\) (mg g−1 min−1) is the initial adsorption rate, and \(\beta\) is a desorption constant that relates chemisorption energy to the extent of surface coverage from the Elovich model.
2.6 Adsorption mass transfer kinetics
In addition to the equilibrium and reaction kinetics, it is also vital to study the extent of adsorbate transfer from bulk to the surface of the solid adsorbent and at the interface of liquid and solid particles. The slowest of these steps will be rate controlling. Hence, various diffusion-controlled kinetic models were studied with dimensionless numbers to determine the rate-limiting step.
The external diffusion model assumes that the pollutant concentration tends to zero at the adsorbent surface and that the intraparticle diffusion is neglected at the early contact times. Mathews and Weber's model (Eq. 8) describes external diffusion [45].
where \(a\) is the total surface area of the adsorbent in the system (m2), and \({k}_{mwext}\) (m min−1) is the external film transfer coefficient from Mathews and Weber's model. The linear portion of the plot of \(\mathrm{ln}\frac{{C}_{t}}{{C}_{o}}\) vs. \(t\) can be used to estimate the external mass transfer coefficient through this method. Furusawa and Smith have developed an alternative approach to determine the external mass transfer coefficient as provided in Eq. 9 [45].
where \({k}_{fsext}\) (m min−1) is the external mass transfer coefficient from Furusawa and Smith model and \({k}_{L}\) is the Langmuir isotherm constant. The linear plot of Eq. 9 can be used to estimate the external mass transfer coefficient through this method.
The diffusion process on the exterior and interior surfaces of the adsorbents was studied using Fick's law of diffusion. The equation at the initial period is represented in Eq. 10 [45].
where, r is the adsorbent particle radius (m), and \({D}_{f}\) (m2 min−1) is the film diffusion coefficient.
Because the adsorbents are porous particles, intraparticle diffusion can be the rate-limiting step. Thus, the sum of pore and surface diffusion was also studied using Eq. 11 [45].
where \(r\) is the radius of the particle, and \({D}_{p}\) (m2 min−1) is the coefficient of intraparticle diffusion. A linear plot of Eq. 11 can be used to estimate the intraparticle diffusion coefficient.
After determining the external and internal mass transfer coefficient, Biot number (\(Bi\)), a dimensionless constant defined by the ratio of the transport rate across the liquid layer to the rate of internal mass transfer, was calculated to determine the rate-limiting step. \(Bi\) is calculated per Eq. 12 [45].
If \(Bi\) is less than 100, the adsorption is controlled by the film diffusion, and when \(Bi\) is more than 100, the adsorption is governed by the internal diffusion.
2.7 DFT-based quantum chemical computation
The molecular structure of the model biochars and nitrate was designed using Avogadro 1.2.0 software, and the three-dimensional structures were adjusted and optimized using the UFF force field. The structures were then submitted to DFT calculations using the Gaussian software. Beck’s three-parameter hybrid and the functional correlation of Lee–Yang–Parr (B3LYP) were used to perform the calculations. 6-31G (d, p) was used as the basis set to optimize the geometry to reduce the energy and obtain a stable conformation.
Frequency calculations were performed using the same theoretical level as the optimization process. All calculations were performed at a temperature of 298 K and a pressure of 1.0 atm. The presence of no negative frequencies confirmed the local minimum structures. The interaction energies (\({E}_{int}\)) was calculated using Eq. 13. The HOMO (\({E}_{H}\)) and LUMO (\({E}_{L}\)) orbital energies were extracted from the Gaussian software. The HOMO–LUMO gap (\(H\)) was calculated using Eq. 14. Molecular orbitals are important to understand chemical reactivity. The molecules with a low value of \(H\) have high reactivity, whereas the molecules with a high \(H\) value show greater stability. In addition, quantum chemical descriptors, such as chemical potential (\(\mu\)), signify the electron force when attached to an atom, and chemical hardness (\(\eta\)) explains the electron redistribution resistance in a molecule. The electrophilicity index (\(\omega\)) measures the electron accepting ability of chemical species [46]. Chemical potential (\(\mu\)), chemical hardness (\(\eta\)), and electrophilicity index values (\(\omega\)), were approximated from HOMO–LUMO orbital energies (Eqs. 15–17) [46, 47].
3 Results and discussions
3.1 Sample characterization
The pH values of the pristine and modified biochars are shown in Table 1. The pH values are an essential property of biochar to understand its applicability in different agricultural and environmental scenarios [48]. The pH value of BC was 6.2, whereas the pH value of UBC was 1.38. The decrease in pH values after sulfuric acid treatment is consistent with the previous studies [35]. Table 1 also reports the pHpzc values of the studied biochar samples. The pHpzc is the pH value at which the surface charges on the biochar cancel out, making the surface neutralized. This property is critical for wastewater treatment applications because if the pH of the contaminant solution is higher than pHpzc, the biochar surface becomes negatively charged, favoring the removal of cationic species. In contrast, if the pH of the surrounding solution is lower than pHpzc, the biochar surface becomes positively charged, favoring the adsorption of cationic pollutants [49]. BC had a pHpzc value of 5.2, whereas UBC had a pHpzc value of 1.5, suggesting that at neutral pH, the modified samples will not favor the adsorption of anionic pollutants such as nitrate. The surface area is another important parameter for studying adsorption. The values of SBET for all the biochar samples are shown in Table 1. It was observed that the SBET value of the pristine biochar BC was 266.57 m2 g−1. However, there was a significant reduction in the surface area, 3.765 m2 g−1, after the modification, as evident from Table 1. This may be due to the treatment of biochar with concentrated sulfuric acid, which resulted in the destruction of the pore structure in biochar. Similar observations were reported by Yakout et al., where the specific surface area of rice husk biochar was decreased after sulfuric acid treatment [50].
The surface morphology and the micro compositions of BC and UBC were studied using SEM–EDX. Figure 2 shows the biochar samples' SEM images and EDX spectra. A rough surface on UBC was observed compared to BC, indicating etching action by sulfuric acid. The EDX spectra obtained for the biochar samples (Table 1 and Fig. 2) indicated that the oxygen content increased from 4.7% in BC to 30.1% in UBC. This indicates that the sulfuric acid treatment increased the oxygen content of the biochar. However, no nitrogen peak was observed in the EDX spectra.
High-resolution XPS spectra of the biochar samples were obtained to study the surface composition. The atomic percentages of carbon and oxygen on the biochar samples are shown in Table 1. It is observed that the oxygen percentages increased from 13.4% in BC to 47.1% in UBC. These results are consistent with the EDX results. No nitrogen peak was observed in the XPS spectra as well, like EDX. However, the bulk nitrogen analysis showed an overall increment in nitrogen content from 2437.14 mg kg−1 in BC to 12418.5 in UBC (Table 1). The absence of nitrogen peak from the EDX and XPS spectra is because the EDX can probe sample depths from 0.2 to 8 µm. At the same time, XPS can probe sample depths at 5–10 nm from the surface [51]. This signifies that although nitrogen was successfully incorporated in the biochar samples due to modification with urea, the surface nitrogen was probably washed away due to the sulfuric acid treatment.
The C1s spectra obtained from XPS were further deconvoluted into three peaks (C1, C2, and C3), as presented in Fig. 3. C1 (284.7–284.8 eV) represents sp2 hybridized carbon, C2 (285.5–286.2 eV) represents C-O bonds, and C3 (287–288.2 eV) represents C=O bonds [49, 52]. The relative contents of these peaks are shown in Table 2.
The deconvolution of O1s peaks is depicted in Fig. 4. Four component peaks (O1, O2, O3, and O4) were fitted to the O1s spectra. O-1 (531.2–531.3 eV) depicts carbonyl and quinone type oxygen bonds, O-2 (532.4–532.5 eV) signifies oxygen in esters, and anhydrides, O-3 (533.6–533.8 eV) represents ether-type oxygen in esters and anhydrides, and O-4 (534.3–534.8 eV) shows carboxylic groups [53]. The relative contents of various O1s components are shown in Table 2. As shown in Table 2, the relative content of the O2 peak increased after sulfuric acid modification. This trend is in agreement with our previous study [35]. However, the relative content of the individual peaks is different. This may be due to the reaction between sulfuric acid and urea, which resulted in the different O1s component concentrations.
3.2 Adsorption analysis
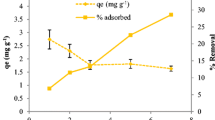
Nitrate batch adsorption data for BC and UBC are shown in Fig. 5. It is observed from the figure that BC demonstrated no removal of nitrate. Compared to pristine biochar, UBC has demonstrated higher removal of aqueous nitrate. This suggests that the nitrogen and oxygen modification improved the adsorption characteristics of the biochar. Further adsorption data analysis is presented for UBC only.
Adsorption isotherms relate to the amount of contaminant adsorbed by a unit weight of adsorbent at various equilibrium concentrations and a constant temperature. Langmuir model is one of the most commonly used isotherm models to describe adsorbent performances [54]. It assumes uniform monolayer adsorption where the adsorbent surface is homogeneous and has several specific adsorption sites [55]. The adsorbed ions or molecules do not interact with each other. Freundlich is another commonly used model for indicating adsorbent performance [54]. However, unlike the Langmuir model, the Freundlich model assumes multilayer adsorption on a heterogenous surface of the adsorbents [55].
The results of Langmuir and Freundlich isotherm analysis are presented in Table 3 and Fig. 6. In this study, the Langmuir model for the adsorption of nitrate by UBC demonstrated a better fit, evidenced by higher R2 values (0.960 and 0.942) and lower values of χ2 (0.018 and 0.02). Other researchers also reported the applicability of the Langmuir model for nitrate adsorption [56, 57]. This would indicate a monolayer nitrate adsorption on the surfaces of UBC. For UBC, the maximum adsorption capacity was found to be 1.548 mg g−1. The fitting of the Langmuir model on experimental data for UBC without any negative constant values indicates equal adsorption energy for the surface-active sites on UBC. A dimensionless separation factor, \({R}_{L}\), can be defined (Eq. 13) from the Langmuir equation, which determines the favorability of the adsorption.
\({R}_{L}\) specifies whether adsorption is irreversible (\({R}_{L}=0\)), favorable (\({0< R}_{L}<1\)), linear (\({R}_{L}=1\)), or unfavorable (\({R}_{L}>1\)) [58]. The \({R}_{L}\) value of 0.20 for UBC indicates favorable adsorption of nitrate.
Studying adsorption kinetics is vital to obtain information on the adsorption mechanisms and reaction pathways. In the present study, the kinetics of nitrate adsorption was studied using pseudo-first order, pseudo-second order, Elovich, and intraparticle diffusion models. The pseudo-first-order model assumes that an increase in the adsorption capacity causes a linear decrement in the adsorption rate [59]. The pseudo-second-order model assumes that the interaction between reagents is the rate-limiting step of adsorption and is frequently used to describe chemisorption [59]. The Elovich equation is also used to describe chemisorption, and it neglects desorption and is suitable for systems where desorption is negligible due to low surface coverage [60]. Finally, the intraparticle diffusion model is used to understand systems where the diffusion of adsorbate towards adsorbent controls the adsorption rate [5].
The results of the kinetic parameter studies are shown in Table 4 and Fig. 7. The pseudo-first-order kinetics is well fitted to the experimental data, as noted by the R2 values. The qe values from the model agree well with the experimental qe values. The equilibrium attained in this study was not rapid, which signified that nitrate ions could not interact with the biochar's outer layer, which might be due to the resistance in film diffusion. Pseudo-second order model was also fitted to the experimental data, but the software (Origin 2021b) could not converge the non-linear fitting algorithm. A linear version of pseudo-second order was also fitted, which generated very low R2 values; hence, those data are not reported.
Contrary to other studies involving nitrate adsorption, the pseudo-second-order model did not work well in this study [4, 56]. One reason might be attaining a rapid equilibrium in other studies, which provided better interaction between the nitrate and adsorbent particles. However, the Elovich model, which also describes chemisorption, provided a good fit for the experimental data, which suggests that the surface chemistry of UBC played a partial role in the removal of nitrate ions from the solution.
Mass transfer analysis was done on the kinetic data to understand the adsorption mechanism better. Table 5 shows the external mass transfer analysis results using Mathews–Weber and Furusawa-Smith models.
For both methods, the mass transfer coefficient decreases with an increase in initial concentration. The reason might be attributed to the increase in driving force due to an increase in the concentration gradient. However, as the total active sites on the adsorbent surface are fixed, the mass transfer decreases [61].
Moreover, the external mass transfer depends on the operating line slope on the equilibrium isotherm curve. Thus, increasing the initial concentration will result in a decrease in the slope of the operating line. Thus, the mass transfer coefficient related to the slopes also decreases [62]. The data obtained indicate that external transfer might be one of the controlling factors behind nitrate adsorption in this study.
Film diffusion was studied by plotting \({\raise0.7ex\hbox{${q_{t} }$} \!\mathord{\left/ {\vphantom {{q_{t} } {q_{e} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${q_{e} }$}}\) Vs. \(t^{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-0pt} \!\lower0.7ex\hbox{$2$}}}}\) as shown in Fig. 8. The plot represented three stages of adsorption: film diffusion, intraparticle diffusion, and adsorption. The time taken for each step can be estimated from the plots. The ratio of time taken for film diffusion to intraparticle diffusion for UBC is shown in Table 6. The ratio of value less than 1 indicates that the intraparticle diffusion step limits the adsorption [63]. However, this study's ratio was greater than 1, indicating that film diffusion is the slowest step.
Plot of \({\raise0.7ex\hbox{${q_{t} }$} \!\mathord{\left/ {\vphantom {{q_{t} } {q_{e} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${q_{e} }$}}\) Vs. \(t^{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-0pt} \!\lower0.7ex\hbox{$2$}}}}\) showing three stages of nitrate adsorption by UBC
Table 7 shows the values of diffusion coefficients from the particle diffusion model. The \({R}^{2}\) values show that the model could not be validated for the system. The values of \({D}_{p}\) obtained (10–9 to 10–16 m2 s−1) suggests a chemisorption system [45]. A similar trend has been reported by Fu et al. [64].
The calculated external mass transfer coefficients and diffusion coefficients were represented as Biot numbers in Table 8. The values of Biot number less than 100 indicate that the adsorption of nitrate on UBC is controlled by the external film mass transfer. It was observed that the Biot number decreases with increasing initial concentration. It was also observed that the mass transfer decreases with the initial concentration. These results agree with the results discussed in the film diffusion model. To further investigate the type of surface functionality responsible for the adsorption, XPS spectra of spent UBC were analyzed. Contrary to the sample before adsorption (UBC), spent UBC showed a nitrogen peak in XPS, confirming adsorption. Moreover, the O2 peak decreased to 29.63% from 56.47% in the spent UBC, signifying the interaction of C-O bonds with the nitrate ions.
To further delve into the biochar-nitrate adsorption properties, a 24-ring model graphene structure (g24) was employed. To model the modified biochars, two different nitrogen functionalities, pyrrolic-N (g24n5) and pyridinic-N (g24n6), were doped onto g24 along with carboxyl and hydroxyl groups. The optimized biochar model structures are presented in Fig. 9. To investigate the reactivity, quantum chemical descriptors such as HOMO–LUMO gap (\(H\)), chemical potential (\(\mu\)), chemical hardness (\(\eta\)), and electrophilicity index values (\(\omega\)) of the biochar structures were calculated. These values are shown in Table 9. The molecular orbitals are shown in Fig. 10. The values of the HOMO–LUMO energy gap, \(H\), show that biochar structures doped with surface functionalities are slightly more reactive than the undoped g24 as evidenced by the low \(H\) values. The electrophilicity index (\(\omega\)) values show that g24n5 and g24n6 are stronger electrophiles than g24, meaning they have a higher affinity towards electrons. This might explain the slightly higher nitrate removal by UBC than by BC. The chemical potential (\(\mu\)) values follow a similar trend showing that g24n5 and g24n6 are more reactive electrophiles than g24. Chemical hardness (\(\eta\)) values, which can be related to the stability of the molecules, indicate that g24 is more stable than other model biochar structures, which is consistent with the reactivity and electrophilicity values.
The optimization energies are shown in Table 10. To model the nitrate adsorption, the biochar structures were complexed with nitrate and optimized (Fig. 11). The interaction energies and the distances between nitrate and biochar are also given in Table 10.
It can be seen that the positive values of interaction energies between model complexes of nitrate and biochar are obtained, suggesting that the adsorption of nitrate on these biochar structures is thermodynamically unfavorable. This explains the modest adsorption ability of biochar toward nitrate ions, as found in the experimental results.
4 Conclusion
The current study demonstrated that urea and sulfuric acid modification has a modest effect on the adsorption capacity of biochar for nitrate adsorption. Adsorption isotherm, reaction, and mass transfer kinetics of nitrate were studied from batch kinetic data. UBC demonstrated a maximum adsorption capacity of 1.548 mg g−1. The adsorption of nitrate followed pseudo-first-order kinetics and Elovich kinetics. The external mass transfer coefficients were in the range of 2.2 × 10–11–2.86 × 10–10 m s−1. The intraparticle diffusion coefficient ranged from 6.53 × 10–10 to 1.78 × 10–9 m2 s−1. The analysis of mass transfer models demonstrated that external mass transfer was the slowest step in the adsorption process. The Biot number value obtained less than 100 indicated that adsorption was limited by film diffusion. Quantum chemical calculations suggested that nitrate adsorption was thermodynamically not favorable on model biochar structures. Based on this work, it is inferred that to improve the remediation of aqueous negative ions, such as nitrate, the biochar should be modified to incorporate positive surface charges. The modified biochar in this research could be effective for the removal of positively charged contaminants such as heavy metals and cationic dyes.
Data availability
Not applicable.
Code availability
Not applicable.
References
Zhang L, et al. La-Ca-quaternary amine-modified straw adsorbent for simultaneous removal of nitrate and phosphate from nutrient-polluted water. Sep Purif Technol. 2022;304(August 2022):122248. https://doi.org/10.1016/j.seppur.2022.122248.
Xie Z, Zhang Y, Zhang Z, Huang J. Nitrate removal mechanism in riparian groundwater in an intensified agricultural catchment. Agric Water Manag. 2023;280(November 2022):108223. https://doi.org/10.1016/j.agwat.2023.108223.
Bhatnagar A, Sillanpää M. A review of emerging adsorbents for nitrate removal from water. Chem Eng J. 2011;168(2):493–504. https://doi.org/10.1016/j.cej.2011.01.103.
Long L, Xue Y, Hu X, Zhu Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ Sci Pollut Res. 2019;26(3):3065–74. https://doi.org/10.1007/s11356-018-3815-z.
Xu X, Gao B, Yue Q, Li Q, Wang Y. Nitrate adsorption by multiple biomaterial based resins: Application of pilot-scale and lab-scale products. Chem Eng J. 2013;234:397–405. https://doi.org/10.1016/j.cej.2013.08.117.
Khojasteh H, Salavati-Niasari M, Safajou H, Safardoust-Hojaghan H. Facile reduction of graphene using urea in solid phase and surface modification by N-doped graphene quantum dots for adsorption of organic dyes. Diam Relat Mater. 2017;79(September):133–44. https://doi.org/10.1016/j.diamond.2017.09.011.
Hu Q, Chen N, Feng C, Hu WW. Nitrate adsorption from aqueous solution using granular chitosan-Fe 3+ complex. Appl Surf Sci. 2015;347:1–9. https://doi.org/10.1016/j.apsusc.2015.04.049.
Ambaye TG, Vaccari M, van Hullebusch ED, Amrane A, Rtimi S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int J Environ Sci Technol. 2021;18(10):3273–94. https://doi.org/10.1007/s13762-020-03060-w.
Huang D, et al. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem Eng J. 2019. https://doi.org/10.1016/j.cej.2018.10.138.
Xie Y, Li S, Wang F, Liu G. Removal of perchlorate from aqueous solution using protonated cross-linked chitosan. Chem Eng J. 2010;156(1):56–63. https://doi.org/10.1016/j.cej.2009.09.033.
Bhatnagar A, Choi YH, Yoon YJ, Shin Y, Jeon BH, Kang JW. Bromate removal from water by granular ferric hydroxide (GFH). J Hazard Mater. 2009;170(1):134–40. https://doi.org/10.1016/j.jhazmat.2009.04.123.
Yoon SY, et al. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles. Chem Eng J. 2014;236:341–7. https://doi.org/10.1016/j.cej.2013.09.053.
Viswanathan N, Meenakshi S. Selective fluoride adsorption by a hydrotalcite/chitosan composite. Appl Clay Sci. 2010;48(4):607–11. https://doi.org/10.1016/j.clay.2010.03.012.
Guan H, et al. Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites. J Hazard Mater. 2010;183(1–3):616–21. https://doi.org/10.1016/j.jhazmat.2010.07.069.
Kasera N, Kolar P, Hall SG. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: a review. Biochar. 2022. https://doi.org/10.1007/s42773-022-00145-2.
Tang D, et al. Heteroatom-doped hierarchically porous biochar for supercapacitor application and phenol pollutant remediation. Nanomaterials. 2022;12(15):2586. https://doi.org/10.3390/nano12152586.
Shafawi AN, Mohamed AR, Lahijani P, Mohammadi M. Recent advances in developing engineered biochar for CO2 capture: an insight into the biochar modification approaches. J Environ Chem Eng. 2021;9(6):106869. https://doi.org/10.1016/j.jece.2021.106869.
Choudhury A, Lansing S. Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J Environ Chem Eng. 2021;9(1):104837. https://doi.org/10.1016/j.jece.2020.104837.
Kamali M, Sweygers N, Al-Salem S, Appels L, Aminabhavi TM, Dewil R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem Eng J. 2022;428(2021):31189. https://doi.org/10.1016/j.cej.2021.131189.
Wang T, Cai J, Zheng J, Fang K, Hussain I, Husein DZ. Facile synthesis of activated biochar/BiVO4 heterojunction photocatalyst to enhance visible light efficient degradation for dye and antibiotics: applications and mechanisms. J Mater Res Technol. 2022;19:5017–36. https://doi.org/10.1016/j.jmrt.2022.06.177.
Wang T, et al. Construction of activated biochar/Bi2WO6 and /Bi2MoO6 composites to enhance adsorption and photocatalysis performance for efficient application in the removal of pollutants and disinfection. Environ Sci Pollut Res. 2023;30(11):30493–513. https://doi.org/10.1007/s11356-022-24049-7.
Liu WJ, Jiang H, Yu HQ. Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev. 2015;115(22):12251–85. https://doi.org/10.1021/acs.chemrev.5b00195.
Ding Z, Hu X, Wan Y, Wang S, Gao B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J Ind Eng Chem. 2016;33:239–45. https://doi.org/10.1016/j.jiec.2015.10.007.
Wan Z, et al. Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem Eng J. 2020. https://doi.org/10.1016/j.cej.2020.126136.
Tan X, et al. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere. 2015;125:70–85. https://doi.org/10.1016/j.chemosphere.2014.12.058.
Xiang J, Liu D, Ding W, Yuan J, Lin Y. Effects of biochar on nitrous oxide and nitric oxide emissions from paddy field during the wheat growth season. J Clean Prod. 2015;104:52–8. https://doi.org/10.1016/j.jclepro.2014.12.038.
Bhatnagar A, Hogland W, Marques M, Sillanpää M. An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J. 2013;219:499–511. https://doi.org/10.1016/j.cej.2012.12.038.
Chintala R, et al. Nitrate sorption and desorption in biochars from fast pyrolysis. Microporous Mesoporous Mater. 2013;179:250–7. https://doi.org/10.1016/j.micromeso.2013.05.023.
Zhang M, et al. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020. https://doi.org/10.1016/j.watres.2020.116303.
Cheng N, et al. Adsorption of emerging contaminants from water and wastewater by modified biochar: a review. Environ Pollut. 2021;273:116448. https://doi.org/10.1016/j.envpol.2021.116448.
Fidel RB, Laird DA, Spokas KA. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci Rep. 2018;8(1):1–10. https://doi.org/10.1038/s41598-018-35534-w.
Yang J, Li H, Zhang D, Wu M, Pan B. Limited role of biochars in nitrogen fixation through nitrate adsorption. Sci Total Environ. 2017;592:758–65. https://doi.org/10.1016/j.scitotenv.2016.10.182.
Wang B, S. yao Liu, F. yun Li, and Z. ping Fan. Removal of nitrate from constructed wetland in winter in high-latitude areas with modified hydrophyte biochars. Korean J Chem Eng. 2017;34(3):717–22. https://doi.org/10.1007/s11814-016-0308-y.
Kasera N, Hall S, Kolar P. Effect of surface modification by nitrogen-containing chemicals on morphology and surface characteristics of N-doped pine bark biochars. J Environ Chem Eng. 2021;9(2):105161. https://doi.org/10.1016/j.jece.2021.105161.
Kasera N, Augoustides V, Kolar P, Hall SG, Vicente B. Effect of surface modification by oxygen-enriched chemicals on the surface properties of pine bark biochars. Processes. 2022. https://doi.org/10.3390/pr10102136.
Baird RB, Eaton AD, Clesceri LS. Standard methods for the examination of water and wastewater. 22nd ed. Washington DC: American Public Health Association; 2012.
Augoustides V, Kasera N, Kolar P. Chemical characterization data of raw Loblolly pine bark nuggets. Chem Data Collect. 2021;33:100727. https://doi.org/10.1016/j.cdc.2021.100727.
Katuwal S, Ashworth AJ, Nur-Al-Sarah R, Kolar P. Characterization of poultry litter biochar and activated biochar as a soil amendment for valorization. Biomass. 2022;2(4):209–23. https://doi.org/10.3390/biomass2040014.
Sultana AI, Reza MT. Investigation of hydrothermal carbonization and chemical activation process conditions on hydrogen storage in loblolly pine-derived superactivated hydrochars. Int J Hydrogen Energy. 2022;47(62):26422–34. https://doi.org/10.1016/j.ijhydene.2022.04.128.
Fitzgerald S, Kolar P, Classen J, Boyette M, Das L. Swine manure char as an adsorbent for mitigation of p-cresol. Environ Prog Sustain Energy. 2015;34(1):125–31. https://doi.org/10.1002/ep.11972.
Kopp JF, McKee GD. Methods for chemical analysis of water and wastes. Washington DC: US Environmental Protection Agency; 1983.
Tan IAW, Ahmad AL, Hameed BH. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater. 2008;154(1–3):337–46. https://doi.org/10.1016/j.jhazmat.2007.10.031.
Wang X, Xia L, Tan K, Zheng W. Studies on adsorption of uranium (VI) from aqueous solution by wheat straw. Environ Prog Sustain Energy. 2012;31(4):566–76. https://doi.org/10.1002/ep.10582.
Wang L, Li J. Adsorption of C.I. Reactive red 228 dye from aqueous solution by modified cellulose from flax shive: kinetics, equilibrium, and thermodynamics. Ind Crops Prod. 2013;42(1):153–8. https://doi.org/10.1016/j.indcrop.2012.05.031.
Girish CR, Murty VR. Mass transfer studies on adsorption of phenol from wastewater using lantana camara, forest waste. Int J Chem Eng. 2016. https://doi.org/10.1155/2016/5809505.
de Souza TNV, de Carvalho SML, Vieira MGA, da Silva MGC, do Brasil DSB. Adsorption of basic dyes onto activated carbon: experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl Surf Sci. 2018;448:662–70. https://doi.org/10.1016/j.apsusc.2018.04.087.
Shokuhi Rad A. Adsorption of mercaptopyridine on the surface of Al- and B-doped graphenes: theoretical study. J Alloys Compd. 2016;682:345–51. https://doi.org/10.1016/j.jallcom.2016.05.004.
Igalavithana AD, et al. Advances and future directions of biochar characterization methods and applications. Crit Rev Environ Sci Technol. 2017;47(23):2275–330. https://doi.org/10.1080/10643389.2017.1421844.
Yin W, Zhang W, Zhao C, Xu J. Evaluation of removal efficiency of Ni(II) and 2,4-DCP using in situ nitrogen-doped biochar modified with aquatic animal waste. ACS Omega. 2019;4(21):19366–74. https://doi.org/10.1021/acsomega.9b02769.
Yakout SM, El A, Daifullah HM, El-Reefy SA. Pore Structure characterization of chemically modified biochar derived from rice straw. Environ Eng Manag J. 2015;14(2):473–80.
Walzak MJ, Davidson R, Biesinger M. The use of XPS, FTIR, SEM/EDX, contact angle, and AFM in the characterization of coatings. J Mater Eng Perform. 1998;7(3):317–23. https://doi.org/10.1361/105994998770347747.
Gao Y, Xu S, Yue Q, Ortaboy S, Gao B, Sun Y. Synthesis and characterization of heteroatom-enriched biochar from keratin-based and algous-based wastes. Adv Powder Technol. 2016;27(4):1280–6. https://doi.org/10.1016/j.apt.2016.04.018.
Chen W, et al. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl Energy. 2020. https://doi.org/10.1016/j.apenergy.2019.114242.
Singh NB, Nagpal G, Agrawal S, Rachna. Water purification by using adsorbents: a review. Environ Technol Innov. 2018;11:187–240. https://doi.org/10.1016/j.eti.2018.05.006.
Beni AA, Esmaeili A. Biosorption, an efficient method for removing heavy metals from industrial effluents: a review. Environ Technol Innov. 2020;17:100503. https://doi.org/10.1016/j.eti.2019.100503.
Divband Hafshejani L, Hooshmand A, Naseri AA, Mohammadi AS, Abbasi F, Bhatnagar A. Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol Eng. 2016;95:101–11. https://doi.org/10.1016/j.ecoleng.2016.06.035.
Demiral H, Gündüzoǧlu G. Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour Technol. 2010;101(6):1675–80. https://doi.org/10.1016/j.biortech.2009.09.087.
Esmaeili A, Beni AA. A novel fixed-bed reactor design incorporating an electrospun PVA/chitosan nanofiber membrane. J Hazard Mater. 2014;280:788–96. https://doi.org/10.1016/j.jhazmat.2014.08.048.
Zhen Y, et al. A pH- and temperature-responsive magnetic composite adsorbent for targeted removal of nonylphenol. ACS Appl Mater Interfaces. 2015;7(44):24446–57. https://doi.org/10.1021/acsami.5b08709.
Tan KL, Hameed BH. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng. 2017;74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024.
Karimaian KA, Amrane A, Kazemian H, Panahi R, Zarrabi M. Retention of phosphorous ions on natural and engineered waste pumice: characterization, equilibrium, competing ions, regeneration, kinetic, equilibrium and thermodynamic study. Appl Surf Sci. 2013;284:419–31. https://doi.org/10.1016/j.apsusc.2013.07.114.
Walker GM, Hansen L, Hanna JA, Allen SJ. Kinetics of a reactive dye adsorption onto dolomitic sorbents. Water Res. 2003;37(9):2081–9. https://doi.org/10.1016/S0043-1354(02)00540-7.
Singha B, Das SK. Biosorption of Cr(VI) ions from aqueous solutions: Kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf B Biointerfaces. 2011;84(1):221–32. https://doi.org/10.1016/j.colsurfb.2011.01.004.
Fu D, Zhang Y, Lv F, Chu PK, Shang J. Removal of organic materials from TNT red water by bamboo charcoal adsorption. Chem Eng J. 2012;193–194:39–49. https://doi.org/10.1016/j.cej.2012.03.039.
Funding
This research was in part funded by USDA-NIFA.
Author information
Authors and Affiliations
Contributions
NK: Conceptualization, Investigation, Experimentation, Data analysis, Data curation, Writing—original draft preparation, and revision. EG: Investigation, Experimentation, Data analysis, SH: Supervision, Review, and editing, PK: Conceptualization, Supervision, Data curation, and Review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kasera, N., Gillikin, E., Kolar, P. et al. Feasibility of nitrate adsorption from aqueous solution by nitrogen and oxygen-modified pine bark biochar: experimental and computational approach. Discov Water 3, 13 (2023). https://doi.org/10.1007/s43832-023-00037-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00037-x