Abstract
Groundwater and surface water are major sources of water supply to the inhabitants of Bertoua. Hydrogeochemical study conducted in the study area aimed at identifying the processes that control the chemistry of groundwater sources and to examine the quality of the water sources for domestic and agricultural purposes. Fifty water samples were collected from boreholes, open wells, springs, and rivers within the study area in January 2022 (the dry season). The samples were analyzed for physicochemical characteristics including pH, electrical conductivity (EC), total dissolved solids (TDS) and major ions. The water samples were acidic with 94% of pH values less than 6.5. The EC varied from 21 to 776 µS/cm and TDS (8.5–388 mg/l). The low EC and TDS indicate low mineralization and fresh water. The relative abundance of major ions (meg/l) was Ca2+> Mg2+> K+>Na+ for cations and \({\text{H}\text{C}\text{O}}_{3}^{-}\)>Cl−>NO3−>SO42− for anions. These major ions concentrations were low and within the WHO guideline values for drinking water. From Piper diagram three water facies were observed; Ca-SO4, Ca-HCO3 and mixed Ca-Mg-Cl. Rock-water interaction, ion exchange, silicate weathering and anthropogenic activities were the processes responsible for the groundwater chemistry with some minor evaporative effects. Based on Sodium Adsorption Ratio and Residual Sodium Bicarbonate all samples fall in the excellent category for agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The utilization of ground and surface water for drinking and agricultural purposes has increased drastically over the years worldwide. Groundwater is under severe threat in developing countries, particularly because of huge population, increasing urbanization, and lack of sustainable practices [1]. According to [2], Sub-Saharan Africa has the lowest water supply coverage, with only 61% of its population having access to improved water supplies. It is estimated that 50% of Africa’s population will be affected by water stress by 2025 [3]. Water quality issues such as nitrate, chloride, and sewage contaminations have been noticed to pose a lot of health problems to humans [4,5,6] and a concern in many cities in Cameroon as most of the population depends on ground and surface water resources with questionable qualities [7,8,9].
Water quality greatly depends on the composition of the recharge water, the interaction between water and soil, the rocks which it comes in contact with in the unsaturated zone, the resident time and reactions that occur within the aquifer [10]. Undesirable groundwater quality affects the economy and restrains the improvement in the living conditions of the population. Consequently, it is essential for an orderly evaluation and observation of groundwater quality to look at its suitability for drinking, and agricultural uses and adopt proper measures for it management [11,12,13,14,15].
Recently groundwater resources are under severe stress because they are a major source of fresh water available to humans and this issue is of worldwide concern. The ever-increasing rate of population growth and the inherent water supply demand have led to intensive water exploitation of fresh water resources. Groundwater is of great importance for domestic, drinking, irrigation, and industry purposes especially where the water resource is availability scarce [11]; especially in Africa where economic development and poverty reduction programmes drive the improvement of groundwater assets [12].
Geochemical studies of surface and groundwater can provide a better understanding of the potential water quality variations due to land use and the geology [10, 16].
Recent studies in Cameroon show that many semi-urban communities are water stressed, confronted with the issue of lack of access and continuous availability of water supply for their day to day domestic and drinking water needs [17]. Sustainable groundwater management requires an adequate understanding of the complexity of natural and anthropogenic processes and how they affect groundwater quality [18].
Studies on groundwater in most major cities in Cameroon include Nsimi, Yaoundé, Mundemba, Adamawa, Douala, Bamenda [17,18,19,20,21,22,23] Works on the effects of mining activities on the environment in the East Region of Cameroon, indicates that the soils and waters are polluted by heavy metals [14, 24, 25]. However, there is still paucity of research works on groundwater geochemistry and anthropogenic effects on the water in Bertoua. The absence of such valuable information that are needed for harnessing and management of water resources led to this research aimed at assessing the physico-chemical characteristics and the processes that controls the water chemistry and quality for domestic and agricultural purposes. The results from this research are useful towards the better development and management of the water sources.
1.1 Location, climate and drainage
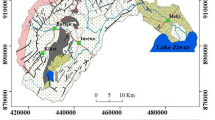
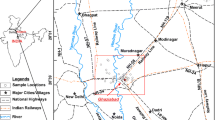
Bertoua is the capital of the Eastern Region of Cameroon and of the Lom and Djerem Division. It is located between latitude 4.579195 and longitude 13.676796 (Fig. 1). It has a population of 100,462 inhabitants and is the conventional home of the Gbaya people [26]. Hot humid equatorial climate with two rainy seasons (mid-March to mid –June and mid-August to mid-November) and two dry seasons (mid-November to mid-March and mid-June to mid-August). High temperatures (average, 24.7 °C), rainfall (mean annual range, 1500–2000 mm) and humidity support the luxuriant vegetation cover and enhance deep weathering of rocks and the formation of iron-rich, red ferallitic soils [27]. The area is drained by the Lom River and its tributaries. Some of the lower order streams have no water flow during the dry season. During the wet period, the stream discharge increases transporting most of the stream sediments within the catchment [14]. The study area is covered mostly by shrubs and herbaceous savanna in the north and an evergreen forest in the south.
1.2 Geology
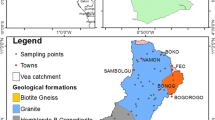
This area is located in the Adamawa–Yadé domain (AYD) or the Central Domain of the Pan African fold belt, which extends east of the Tcholliré–Banyo shear area. The geology is mostly meta-volcanic rocks, meta-sedimentary rocks and intrusive granites of the Lom series [28]. These formations are characterized by schist, micaschist, orthogneis, quartzites that are croos-cut by Pan-African granitoids, conglomerates and post tectonic granites [25, 29]. The foliations have a NE-SW steeply dipping orientations indicating a senestral and dextral shearing direction that is related to the central Cameroon shear zone [21]. This basin is well known to host gold mineralization and other economically important mineral deposits of granitic intrusion origin such as Mo, Pb and Bi. [26].
The AYD represents a Paleoproterozoic basement that changed into failed arm during the Pan-African orogeny. These deformation phases have greatly influence the topography, which is of great influence on the chemistry of the aquifers systems.
The area is covered with thick ferralitic soils with brown, reddish brown and brown colour. The soils are hydromorphic in marshy areas, the upper part of the soil profile is dark in colour indicating the abundance of organic materials [22].
2 Methodology
2.1 Field measurements
Water samples were collected from 50 water sources in January 2022 (dry season). The sources comprised of 36 open wells, 5 boreholes, 5 springs and 4 rivers. Garmin GPS was used to collect coordinates and altitude of the sampling points. The measurements of physicochemical parameters; temperature EC, pH, and TDS was measured in situ using a multipurpose H1991300 hand held HANNA meter due to their unstable nature to avoid unpredictable changes in characteristics as per the standard procedures (APHA/AWWA, 1998). This meter was calibrated before usage with buffer solutions as prescribed by the manufacturer.
At each sampling site, the water sample to be analyzed was collected using a plastic beaker after proper rinsing (3 times with water to be sampled). At borehole sites the water was purged for about 5 min before sampling. Open wells were sampled using buckets with a rope tied to its handle. Rivers were sampled as deep as possible along a flow path. Springs were sampled at the point of release by the natural pressure.
All samples were placed in plastic bottles (50ml and 1500ml in two sets). These bottles were thoroughly rinsed with the water samples to ensure that they were free from contaminants. The 50ml samples were sent to SHREE RAM testing laboratory services India within two weeks for major ions analysis. The 1500ml samples were used to determine the total alkalinity as bicarbonate (HCO3−) measured within 8 h of sampling through titration using a volume of 0.02 M H2SO4. Alkalinity kit6 was used to measure the bicarbonate (HCO3−). The measurement was done by adding drops of the acid to the samples. The drops of acid required to bring the pH of the sample to 4.5 and 4.3 is noted. The counts from the kit6 cartridge also noted. The alkalinity is then processed following methodology recommend by the manufacturer.
2.2 Chemical analyses
Major cation: Na+, K+, Mg2+, and Ca2+ were determined by flamed Technique in High resolution Continuum source AAS (Contr AA 700) as prescribed in [15]. Samples with EC values > 100µS/cm were diluted 5 to 10 times to acquire an absorbance within the range of the used standards Na+, K+, and Ca2+. Measurements of anaion: phosphate (PO42−), sulphate (SO42−), flouride (F−) and bromine (Br−) were determined by using an Ion Chromatography (Dionex ICS-900) while dissolved silica (Si(OH)4), hereafter considered as SiO2, was determined by determined by ICP-AES. Both cation and anion were analyzed following IS:3025P-45 standards. These results were tested for reliability using an ionic balance error (IBE) Eq. [6]. The values were within ± 5%, hence suitable for geochemical interpretations.
2.3 Water quality index
Brown [30] Groundwater Quality Index was used in this work. This method of evaluating the quality of groundwater is used worldwide because of its ability to express information on the quality of groundwater. It is one of the most effective tools for the evaluation and management of groundwater quality [31].
WQI values are classified into five classes (i.e.) excellent, good, fair, poor, and unacceptable for human use [32].
2.4 Irrigational qualities of water
In order to determine the suitability of waters for irrigational purposes, the following parameters were evaluated.
The sodium absorption ratio (SAR) by [33]
Residual Sodium carbonate(RSC) by [34]
In order to determine the anthropogenic input in the groundwater, the Cl−/Br− ratio was used. This ratio is use to identify the sources of groundwater like Urban waste water, agricultural pollutant, leaching and septic tank effluent [3, 35, 36].
3 Results and discussions
3.1 Physico-chemical characteristics of water sources
The temperature of water ranged from 23.6 to 31.90 C giving a mean temperature of 26.30 C. The pH ranged from 4.71 to 7.12 with an average of 5.49 indicating an acidic aquifer system. 94% of the samples had pH < 6.5. As pointed by [10], the consumption of carbonic acid during the dissolution of silicate minerals results in increased alkalinity and lower pH values. This result is similar to those obtained in crystalline environments in humid tropical climates (pH between 3.5 and 5.6) where surface material (laterite) is depleted in basic cations and recharged by rainwater [3, 35, 36]. This indicates that the oxidation of certain minerals (pyrite) and production of CO2 in the surface soil cover under the action of biological activities can be a source of water acidity in the environment [3, 10, 37].
The TDS ranged from 8.5 to 388 mg/l with a mean value of 76.47 mg/l. The samples are classified as fresh water (TDS < 1000 mg/l). The Electrical conductivity values varied from 21 to 776 µS/sm with a mean value of 157.7µS/cm, indicating low mineralization of water sources. Similar results were obtained by [18] in hard rock aquifers of Yaounde but differ from the works of [14] and other researchers that had worked in most water systems in woody areas (without impact of mining ) in southern Cameroon where water flow in plutono-metamorphic basement [37, 38] and other residual aquifers in Africa (37.3–75.1µS/cm) [39, 40].
The concentration of HCO3− vary from 0 to 147 mg/l with an average of 16.98 mg/l. Two possible mechanisms can be suggested for the concentration of HCO3− in water; high partial pressure of CO2 (pCO2) relating to the anthropogenic organic matter [37] or alteration of silicate minerals. The correlation of HCO3− with Mg2+ and no correlation between HCO3 with Ca2+, (Fig. 1) and the absence of carbonate lithologies further suggest the predominance of weathering by organic acids to the detriment of carbonic acid [39].
The nitrate concentrations ranged from 0 to 12.9 mg/l (Table 1). All the samples have values below the WHO 2017 limit of nitrate in groundwater [40]. Anthropogenic activities were noted to be the main source of nitrate in areas of high population densities. High Nitrate concentration in drinking water is noted to be the cause of some diseases for children [17].
The abundance (mg/l) of cation in the waters is in the following order: Mg2+>Ca2+>Na+>K+ and for anions bicarbonate is the dominant anion, followed by chloride and sulfate. Their concentrations were distributed as follows HCO3−> NO3 > Cl- >SO2 − 4.
Major ions concentrations were greater in groundwater samples than surface waters. Such observations depicts that groundwater has a longer residence time than surface water [41].
3.2 Hydrogeochemical facies
The hydrogeochemical processes that are often responsible for the composition of water were analyzed using the relationship that exit between dissolve species and their content. For the analysis of geochemical evolution of groundwater, the concentrations of the cations and anions where plotted on a Piper diagram [42]. The differences and similarities in composition of waters and their classifications have been widely studied and classified them into water types using the Piper diagram. A typical classification of hydrogeochemical facies for groundwater is presented in (Fig. 2). Three facies types were identified: Ca-SO4, Ca-HCO3 and Ca-Mg-Cl Bon et al. [18] reported similar results in hardrock aquifers of Yaounde.
3.3 Sources of solute
Ion exchange reactions were assessed by calculating the Chloro-Alkaline Indices (CAI). Two Chloro-Alkaline Indices CAI 1 and CAI 2 were used for the interpretation of ion exchange between groundwater and host environment. Equation 3 and 4 express the CAI with concentration in meq/L.
A positive Chloro-Alkaline Indices indicate exchange of Na+ and K+ from the water with Mg2+. The CAI is negative when there is an exchange of Mg2+ and Ca2+ of the water with Na+ and K+ of the rocks. A majority of the samples have negative indices indicating exchange of Mg2+ and Ca2+ with Na+ and K+ in the groundwater [43, 44]. Some also had positive CAI which indicates the reverse process that is Na+ and K+ in the waters substituting Mg2+ and Ca2+ in the rocks [45].
When the ratio is negative, it indicates that groundwater has been strongly in contact with minerals which are able to yield interchangeable cation. This phenomenon of base exchanges is generally known with the alumino-silicate clays formed by layers or sheets whose cohesion is ensured by the existence of interlayer cations and water. The surfaces of the layers are negatively charged, thus promoting the possibility of cation exchange with those of the groundwater [46].
The plot of Na+ versus Cl− shows an excess of Na+ (Fig. 3a) can only be explained by different origin of both ions pointing to ion exchange as the process responsible for Na+. The molar ratio Na/Cl of approximately 1 is usually associated to halite dissolution and > 1 is typically interpreted as reflecting Na+ release from silicate weathering [47, 48]. There is no correlation between Na + and \({\text{S}\text{O}}_{4}^{2-}\) (Table 2) which may suggest another source for the addition of Na+ such as the dissolution of sodicsulphated minerals like thernadite (Na2SO4) [49]. This signifies that chloride might have originated from anthropogenic sources and precipitation/evaporation.
The mechanism of silicate weathering can be understood by estimating the ratio between (Na++ k+) and total cations. Majority of the samples plots above the trend line (Na+ + K+) = 0.5TC (Fig. 3d) which signifies the weathering of silicate minerals rich in Na+ such as albite [49, 50]. The plot of (Ca2+ + Mg2+) versus HCO3+ + SO42− was used to determine ion exchange processes (Fig. 3b). All the samples are above the line (Fig. 3b) indicating reverse cation exchange.
The Spearsman correlation coefficient was used to study the relationship among the various hydrogeochemical parameters. The correlation coefficient of r > 0.5 and − 0.5 (Table 1) were considered as strongly positive correlation and weakly negative correlation respectively. EC and TDS showed a very high to almost perfect correlation (0.85). This shows the absence of less soluble charged elements in groundwater that contributes to the total dissolve solids [7]. The correlation of Na+/Mg2+ (0.50), K+/Ca2+ (0.85), Na+/HCO3− (0.51), Mg2+/HCO3−(0.69), Ca2+/NO3− 0.54),K+/Cl−(0.76), and Ca2+/Cl−(0.87) indicates the varied processes are responsible for the groundwater chemistry in the study area.
Cl− and NO3− show a strong positive correlation (0.58) suggesting anthropogenic sources (domestic waste water, septic tanks, effluent, pit latrines and animal waste) for these ions. The geogenic sources of chloride can also be attributed to the weathering of chloride bearing minerals present such as amphiboles and biotite. To further investigate the anthropogenic inputs of chloride, the Cl−/Br− ratio was used (Fig. 4). Bromide and chloride ions have been used extentively to determine sources of contaminant in ground and surface water. Both ions move conservatively in water and have different abundances [50]. The Cl−/Br− ratio was analyzed for 38 samples because the concentrations of Cl- and Br- in these samples were greater than 1 mg/l. The bromide concentration varied from 2.29 mg/l in OW50 to 1442.01 mg/l in OW19. The concentration of chloride ranged from 0 mg/ in OW48 to 1153.75 mg/l in RT26. It has observed that Cl−/Br− ratio above 400 were correlated with chemical sources associated to human activities such as sewage sludge, septic tanks and higher nitrate concentrations [7]. The study indicates that Rt 26 has highest Cl−/Br− ratio. The low velocity of the river and it usuage for domestic washing may be the main source of Cl− and NO− 3. This study shows that 12% of the samples have Cl−/Br− ratio above 400, and these samples are found in highly populated areas.
3.4 Drinking water quality
The analytical results for physicochemical parameters of groundwater were compared with the standard guidelines values recommended by the World Health Organization [51] for drinking water and public health purposes (Table 1). All springs, bore holes, and some deep open wells with metal casing had colorless waters while for shallow wells and unprotected wells, and rivers the colors were creamy and greenish respectively. This coloration may be attributed to suspended solids in the water and the green colors in rivers showed evidence of eutrophication. The pH of the samples was acidic to neutral. 6% of the samples had pH between 6.5 and 7.01, which is within the WHO guideline values (Table 1), 71% falls within 5.1–6.4 which is moderate and 11% below the pH of 5.1. Generally, the water samples are weakly mineralized, hence corrosive [52].
The Total dissolve solid was low but for a few samples which have values above 500. This low TDS in groundwater is consistent with the observation that most exploited groundwater in weathered basement rocks of Africa are low in dissolve ions and generally suitable for consumption [53]. The concentration of ions such as Na+, Ca2+, Mg2+, Cl− and SO42− are within the maximum allowable limits for drinking water Table 1.
3.5 Water quality index
The Water Quality Index (WQI) was calculated using weighted arithmetic average method. This method for calculating WQI considers maximum permissible limits for any regulation whether national or international and is adopted to the need of that study. This method has its limitations such as, it is not possible to evaluate all the risk present, and weighting is required for every boundary as per its importance which could be subjective. In the study area, the results for the WQI had values ranging from 7.94 to 366.40 having a mean value of 49.61 (Table 3). 30% of samples were excellent, 36% good, 14% fair, 4% poor and 6% unfit for drinking.
3.6 Agricultural water quality
The low EC, TDS, and major ions in groundwater makes is suitable for agriculture. The absorption of water and nutrients from the soil by plant becomes difficult with excess salinity, which reduces the osmotic activity. In view of the chloride content, 100% of water here fits for irrigation while NO3− had 50% suitable for agriculture and a 50% moderate waters.
Irrigation water rich in sodium can reduce the permeability of the soil and limit air and water flow. This is because of the exchange processes that are established between water and soil. The sodium ions are adsorbing by clay particles replacing the magnesium and calcium ions. Based on the SAR, 100% of the water samples are excellent for irrigation. To further examine water quality for irrigation, the “residual sodium carbonate” (RSC) is used to refer alkalinity hazard to soil. If the sodium in clayey soils is higher, it causes swelling and reduces the infiltration capacity of the soil. The potential of sodium vulnerability is increased as RSC rises. This index is use to identify aptness of groundwater in clayey soils. If groundwater of elevated RSC is used for irrigation, alkali soils will be form. In this region, the ground and surface waters fall in the safe for irrigation category based on this classification (Fig. 5).
4 Conclusion
This study was aimed principally to examine the geochemistry and quality of the water sources for drinking and agricultural purposes. 94% of the samples were acidic (4.71–6.4) and out of the drinking water limit. Therefore careful attention is necessary at all stages of pipe borne water treatment and supply because of corrosion caused by low acidic nature of the water. The water sources were fresh with relatively low mineralization having mean TDS of 92.47 mg/l, with the main source of ions from the weathering of schist, micaschist, orthogneiss, quartzite, granitoids, granites, conglomerates and clays.
The water types identified were: Ca-SO4, Ca-HCO3and Ca-Mg-Cl. Two main hydrogeochemical processes influenced the water chemistry; incongruent silicate dissolution and cation exchange. There is an anthropogenic finger print in the water chemistry.
The major ions of groundwater sources were within the guideline values for drinking water. The dominant cation was Ca2+ and anion HCO3. These ions vary in decreasing order of Ca2+> Mg2+> K+> Na+ for cations and HCO3−>NO3 > Cl > SO42− for anion.
The low EC, TDS and major ions in groundwater depicts low solubility of the silicates, short residence time of groundwater, and shallow nature of the aquifers with the acidic character. The water resources are suitable for agriculture but needs further purification before drinking.
Data availability
The data set generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Faizal Khan, SrinivasamoorthyKrishnaraj, Prakash Raja, GopinathSelvaraj, RabiCnaCheelil. Impact of hydrogeochemical processes and its evolution in controlling groundwater chemistry along the east coast of Tamil Nadu and Puducherry, India. Environmental Science and Pollution Research. 2020.
WHO. Guideline for drinking-water quality. 3rd ed. Geneva: World Health Organization; 2021.
Wu J, Zhou H, He S, Zhang Y. Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the chinese Loess Plateau. Environ Earth Sci. 2019;78:446.
Liu L, Wu J, He S, Wang L. Occurrence and distribution of groundwater fluoride and manganese in the Weining Plain (China) and their probabilistic health risk quantification. Expo Health. 2022;14:263–79.
Wang Y, Li P. Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environ Res. 2022;207:112210.
Kamtchueng BT, Fantong WY, Wirmvem MJ, Tiodjio RE, Takounjou AF, NdamNgoupayou JR, Kusakabe M, Zhang J, Ohba T, Tanyileke G, Hell JV, Ueda A. Hydrogeochemistry and Quality of Surface Water and Groundwater in the vicinity of Lake Monoun, West Cameroon: Approach from Multivariate Statistical Analysis and stable isotopic characterization. Volume 188. Environmental Monitoring and Assessment; 2016. p. 524.
Wirmvem MJ, Takeshi O, Fantong WY, Ayonghe SN, Justice Y, Suila Nkengmatia EA, Asaah Tanyileke G, Joseph VH. Hydrochemistry of shallow groundwater and surface water in the ndop plain, North West Cameroon. Afr J Environ Sci Technol. 2013;7(6):518–30.
Adelana S, Fantong W, Nedaw D, Duah A. Groundwater and health: meeting the unmet needs in Sub-Saharan Africa Sustainable groundwater resources. Int Year Planet Earth. 2011. https://doi.org/10.1007/978-90-481-3426-7_2.
Appelo CAJ, Postma D. Geochemistry, Groundwater and Pollution. 2nd ed. Rotterdam: Balkema; 2005. p. 599.
Gad M, Saleh AH, Hussein H, Farouk M, Elsayed S. Appraisal of surface water quality of nile river using water quality indices, spectral signature and multivariate modeling. Water. 2022;14:1131.
Ako AA, Shimada J, Hosono T, Ichiyanagi K, Nkeng EG, Fantong YW, Takem GEE, Ntankoua NR. Evaluation of groundwater quality and its suitability for drinking, domestic and agricultural uses in the Banana Plain (Mbanga, Njombe, Penja) of the Cameroon volcanic line. Environ Geochem Health. 2011;33:559–75.
Wirmvem MJ, Takeshi O, Anye NL, Tchakam KB, Wiylahnyuy KE, Mumbfu EM, Tasin GB, Muga Y, Eneke TG, Fantong WY, Ako AA. Effect of diffuse recharge and wastewater on groundwater contamination in Douala, Cameroon. Environ Earth Sci. 2017;76:354.
Rakotondrabe F, NdamNgoupayou JR, Mfonka Z, Rasolomanana EH, Nyangono AJ, Ako AA. Water quality assessment in the B´etar´e-Oya gold mining area (East-Cameroon): multivariate statistical analysis approach. Sci Total Environ. 2018. https://doi.org/10.1016/j.scitotenv.2017.08.080.
Paternoster M, Buccione R, Canora F, Buttitta D, Panebianco S, Rizzo G, Sinisi R, Summa V, Mongelli G. Hydrogeochemistry and groundwater quality assessment in the high Agri Valley (Southern Italy). HindawiGeofluids volume 2021. Article ID. 2020;6664164:15. https://doi.org/10.1155/2021/6664164.
Wirmvem MJ, Takeshi O, Fantong WY, Ayonghe SN, Justice Y, Suila Asobo NE, Asaah TG, Joseph VH. Hydrochemistry of shallow groundwater and surface water in the ndop plain. North West Cameroon. 2013;7(6):518–30. https://doi.org/10.5897/AJEST2013.1456.
Wotany ER, Ayonghe SN, Fantong WY, Wirmvem MJ. Physico-Chemical and Bacteriological Quality of Water Sources in the Coast of Ndian, South West Region, Cameroon: Health Implications. Int J Trend Sci Res Dev. 2019. https://doi.org/10.31142/ijtsrd26626.
Bon AF, Ngo MTA, Ewodo MG, Asone BL, Ndam NR, ., Ekodeck GE. Groundwater flow patterns, hydrogeochemistry and metals background levels of shallow hard rock aquifer in a humid tropical urban area in sub-saharan Africa- a case study from Ol´ezoa watershed (Yaound´e-Cameroon). J Hydrology: Reg Stud. 2021. https://doi.org/10.1016/j.ejrh.2021.100904.
Takem GE, Brunet F, Mortatti J, Gauthier-Lafaye F, Braun JJ, Ekodeck GE. Stable Carbon Isotopes δ13C as a Proxy for characterizing Carbon sources and processes in a small Tropical Headwater Catchment: Nsimi, Cameroon. Aquat Geochem. 2020. https://doi.org/10.1007/s10498-020-09386-8.
Kuitcha D, FouepeTakounjou A, Ndjama J. Apport de l’hydrochimie et de l’isotope de l’environnement ` a la connaissance des ressources en eauxsouterraines de Yaound´e. Cameroun J App Biosc. 2013;67:5194–208. https://doi.org/10.4314/jab.v67i0.95041.
Wotany RE, Ayonghe SN, Fantong WY, Wirmvem MJ, Takeshi O. Hydrogeochemical and anthropogenic influence on the quality of water sources in the Rio del Rey Basin, South Western, Cameroon, Gulf of Guinea. African J Environ Sci Technol. 2013;7:1053–69.
Arétouyap Z, Njandjock PN, Ekoro NH, Meli’ ILJ, Lepatio TSA. Investigation of groundwater quality control in Adamawa Cameroon Region. J Appl Sci. 2014;14(19):2309–19.
Magha A, Tita MA, Kouankap GD, Wotchoko P, Ayuk TM, Kamgang VK. Physico-chemical and bacteriological characterization of spring and well water in bamenda III (NW Region, Cameroon). Am J Environ Prot. 2015;4(3):163–73.
Kalédjé PSK, Ngoupayou JRN, Kpoumié A, Takounjou AF, Ondoua JM. Analysis of climate variability and its influence on the hydrological response of the catchment area of Kadey (East Cameroon). Int J Geosci. 2016;47:127–38.
Fodoué Y, Ismaila A, Yannah M, Wirmvem MJ, Mana CB. Heavy Metal Contamination and Ecological Risk Assessment in Soils of the Pawara Gold Mining Area, Eastern Cameroon. Earth. 2022;3:907–24. https://doi.org/10.3390/earth3030053.
Toteu SF, Van SWR, Penaye J, Michard A. New U-Pb and Sm-Nd Data from North-Central Cameroon and Its Bearing on the Pre-Pan African History of Central Africa. Precambrian Res. 2001;108:45–73. https://doi.org/10.1016/S0301-9268(00)00149-2.
Freyssinet P, Lecomte P, Edimo A. Dispersion of Gold and Base Metals in the Mborguéné Lateritic Profile, East Cameroun. J Geochem Explor. 1989;32:99–116. https://doi.org/10.1016/0375-6742(89)90050-2.
Segalen P, Sols Les. Geomorphologie du Cameroun. Cahier ORSTOM Series Pédologique. 1967;2:137–87.
Kankeu B, Greiling RO, Nzenti JP. Pan-african strike-slip tectonics in Eastern Cameroon-Magnetic fabrics (AMS) and structure in the Lom Basin and its gneissic basement. Precambrian Res. 2009;174:258–72. https://doi.org/10.1016/j.precamres.2009.08.001.
Soba D. La Serie de Lom: Etude Geologique et Geochronologique D’un Bassin Volcano-Sedimentaire de la ChainePanafricaine a L’Est de Cameroun. Ph.D. Thesis, University of Pierre and Marie Curie, Paris. 1989.
Sadat-Noori S, Ebrahimi K, Liaghat A. Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer. Iran Environ Earth Sci. 2014;71:3827–43.
Saravanan K, Srinivasamoorthy K, Prakash R, Gopinath S, Suma C. An evaluation of hydrogeochemistry of groundwater in upper elar sub-basin using mineral stability and solute transport modelling. Aquat Procedia. 2015;4:1119–25.
Richard LA. (1954). Diagnosis and Improvement of Saline and Alkali Soils. Edit. US Department of Agriculture, Agricultural Handbook n◦ 60, Washington, DC, 160. https://doi.org/10.1097/00010694-195408000-00012.
Gupta SK, Gupta IC. Management of saline soils and water. New Delhi: Oxford and IBM Publ. Co; 1987.
Alcalá FJ, Custodio E. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal J. Hydrol. 2008;359:189–207.
Alcalá FJ, Custodio E. 2004 Use of the Cl / Br ratio as tracer to identifity the origin of salinity in some Spanish coastal aquifers SWIM pp 481–97.
Boeglin J-L, Ndam J-R, Braun J-J. Composition of the different reservoir waters in a tropical humid area: example of the Nsimi catchment (Southern Cameroon). J Afr Earth Sci. 2003;37(1–2):103–10. https://doi.org/10.1016/s0899-5362(03)00041-1.
Braun J-J, NdamNgoupayou J, Viers J, Dupre B, BedimoBedimo J-P, Boeglin J-L, Robain H, Nyeck B, Freydier R, Nkamdjou LS, Rouiller J, Muller J-P. Present weathering rates in a humid tropical watershed: nsimi, South Cameroon. Geochim Cosmochim Acta. 2005;69(2):357–87. https://doi.org/10.1016/j.gca.2004.06.022.
Adeli M, Mohammadi Z, Keshavarzi B, Amjadian K, Kafi M. Heavy metal (loid) pollution of a hard-rock aquifer: evidence, distribution, and source. Environ Sci Pollut Res. 2021;28:34742–61. https://doi.org/10.1007/s11356-021-13079-2.
Yidana SM, Banoeng-Yakubo B, Akabzaa T, Asiedu D. Characterization of the groundwater flow regime and hydrochemistry of groundwater from the buem formation, Eastern Ghana. Hydrol Process. 2011;25(14):2288–301. https://doi.org/10.1002/hyp.7992.
Ouandaogo-Yameogo S, Blavoux B, Nikiema J, NindaouaSavadogo A. Caract´erisation du fonctionnement des aquif`eres de socledans la r´egion de Ouagadougou ` a partird’une ´etude de la qualit´echimique des eaux. Rev Sc Eau. 2013;26(3):173–91. https://doi.org/10.7202/1018784ar.
Wali SU, Umar KJ, Abubakar SD, Ifabiyi IP, Dankani IM, Shera IM, Yauri SG. Hydrochemical characterization of shallow and deep groundwater in Basement Complex areas of southern Kebbi State, Sokoto Basin, Nigeria. Appl Water Sci. 2019;9:169. https://doi.org/10.1007/s13201-019-1042-5.
Schoeller H. Geochemistry of groundwater. In: Brown RH, editor. Groundwater Studies – An International Guide for Research and Practice. Paris: UNESCO; 1967. pp. 1–18.
Kumar SK, Bharani R, Magesh NS, Godson PS, Chandrasekar N. Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci. 2014;4:341–50.
Jankowski J, Acworth RI, Shekarforoush S. (1998). Reverse ionexchange in deeply weathered porphyritic dacite fractured aquifers system, Yass, New South Wales, Austria, In: G.B., Arehart, J.R., Hulston, editor. Proceedings of 9 “International symposium on water rock interaction Taupo, New Zealand Rotterdam: Balkema. pp. 243–246.
Fisher RS, Mullican WF. Hydrochemical evolution of sodium sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol J. 1997;5:4–16.
Cendon DI, Larsen JR, Jones BG, Nanson GC, Rickleman D, Hankin SI, Pueyo JJ, Maroulis J. Freshwater recharge into a shallow saline groundwater system, Cooper Creek floodplain, Queensland, Australia. J Hydrol. 2010;392(2–4):150–63.
Jaladi M. Geochemistry characterization of groundwater in the agricultural area of Razan Hamadan. Iran Environ Geol. 2009;51:1479–88.
Stallard RF, Edmond JM. Geochemistry of the Amazon: the influence of geology and weathering environment on the dissolved load. J Geophys Res Oceans. 1983;88:9671–88.
Saravanan K, Srinivasamoorthy K, Prakash R, Gopinath S, Suma CS. An Evaluation of Hydrogeochemistry of Groundwater in Upper Vellar Sub-Basin using Mineral Stability and Solute Transport Modeling International Conference on Water Resources Coastal and Ocean Engineering (ICWRCOE). Aquat Procedia. 2015;4:1119–1125.
Khan F, Krishnaraj S, Raja P, Selvaraj G, Cheelil R. Impact of hydrogeochemical processes and its evolution in controlling groundwater chemistry along the east coast of Tamil Nadu and Puducherry. India. Environ SciPollut Res. 2020. https://doi.org/10.1007/s11356-020-10912-y.
WHO. Guideline for drinking-water quality. 3rd ed. World Health Organization: Geneva; 2018.
WHO. Guidelines to drinking Water Quality. Geneva: World Health Organization; 2021.
Adelana S, Fantong W, Nedaw D, Duah A. Groundwater and health: meeting the unmet needs in Sub-Saharan Africa. Sustainable groundwater resources. Int Year Planet Earth. 2011. https://doi.org/10.1007/978-90-481-3426-7_2.
Acknowledgements
We would like to thank Prince Tanyi Justin Aginga of Union Farms of Africa, for providing assistance in the handling of the samples in India, correlating with the laboratory technicians for timely analyses of Samples.
Author information
Authors and Affiliations
Contributions
MAN: Corresponding author: Wrote the main manuscript text Department of Geology, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon *Corresponding author E-mail address: mentinelson2013@gmail.com. Engome Regina Wotany: Scientific corrections and part of the field work for data collection Department of Geology, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon Email: wotanyr@gmail.com. Christopher Agyingi: Scientific corrections and prove reading of literature Department of Geology, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon Email: cmagyingi@gmail.com. Mengnjo Jude Wirmvem: Bicarbonate analyses, scientific reading and corrections and part of the fieldwork. Institute of Geological and Mining Research, P.O. Box 4110, Yaounde, Cameroon Email: judevom@gmail.com. Ngai N Jude: Data analyses and assisting in paper write up and prepared figures for the papers. Institute of Geological and Mining Research, P.O. Box 4110, Yaounde, Cameroon Email: ngaijude89@gmail.com.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Menti, A.N., Wotany, E.R., Christopher, A. et al. Hydrogeochemical characterization of ground and surface water in the eastern part of the Adamawa-Yade domain, Bertoua-Cameroon. Discov Water 3, 10 (2023). https://doi.org/10.1007/s43832-023-00034-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00034-0