Abstract
The water quality of various resources is changing everywhere, including the high-altitude region, which may have adverse health effects on animals and the human population. So far, not much study has been conducted on high-mountain region water resources. Therefore, this study was conducted at Leh-Ladakh, a high-altitude region, to know the water quality of different sources. For this, water samples were collected from irrigation, stagnant (pond), and Indus river water resources and analyzed different physicochemical parameters as per standard methods and heavy metals using inductively coupled plasma-optical emission spectrometry (Optima 7000 DV, Perkin Elmer) at the laboratory. The results revealed that the total mean values of pH (7.58 ± 0.04), electrical conductivity (EC-243.78 ± 18.05 µS/cm), salinity (0.12 ± 0.01%), total dissolved solids (TDS-121.519.75 mg/L), turbidity (1.17 ± 0.22 NTU) and chemical oxygen demand (COD-31.45 ± 0.73 mg/L) of Indus river water, pH (7.43 ± 0.05), EC (231.86 ± 11.00 µS/cm), salinity (0.11 ± 0.01%), TDS (113.31 ± 5.48 mg/L), turbidity (0.85 ± 0.11 NTU) and COD (29.74 ± 0.49 mg/L) of irrigation water, and pH (7.46 ± 0.03), EC (233.14 ± 11.41 µS/cm), salinity (0.12 ± 0.01%), TDS (115.03 ± 5.78 mg/L), turbidity (0.67 ± 0.13 NTU) and COD (29.65 ± 0.57 mg/L) of pond water were within the prescribed limit by World Health Organization (WHO) for drinking water. However, variances in the range of heavy metals were observed in Indus river water, irrigation water, and in stagnant water at different sites. Our results revealed that the As, Cd, and Pb were above the permissible limit of WHO for drinking water. At the same time, the Ni and Cr levels were observed below the maximum permissible limit. Therefore, these water resources, if used for more extended periods, may pose health-related issues to humans and animals from these elements. So, this study finding will help develop specific mitigation strategies for water management for drinking and other purposes.
Similar content being viewed by others
1 Introduction
In nature, the definitive minerals nutrient cycle occurs between soil and plants, plants and animals, and animals and soil [1]. Homeostasis of these minerals is maintained through tightly regulated mechanisms of uptake, storage, and secretion. The breakdown of minerals homeostasis can lead to the deficiency or toxicity of a particular mineral and may affect the health and productivity of animals [2, 3]. Heavy metal pollution is a major global problem, posing serious risks to humans, animals, and the environment [4]. Similarly, the presence of certain heavy metals in different water sources from plain areas is one of the most important concerns that have received the attention of their toxicological importance in ecosystems and impact on public health [5, 6]. The levels of heavy metals are varied in the soils, water, river sediments, and plants, even in the Arctic region, due to natural and anthropogenic causes [7, 8]. However, in most developing countries, including India, water pollution is often attributed to industrialization with improper waste disposal and leaching of some heavy metals from the parent materials, causing toxicity to animal and human populations [9]. Further, irrigation using this water to crops and forage plants grown in polluted environments may absorb toxic metals from the soil, irrigation water, and thereafter deposition in crop produce and may become the metal source to animals and humans [10]. So, a high amount of heavy metals was reported in serum samples of animals consuming the forages grown in contaminated land due to irrigation using polluted water [11, 12].
Heavy metals, at a higher level, are toxic to the body and are responsible for various cellular and tissue damages due to free radical generation, oxidative stress, and suppression of immunity [4, 11,12,13,14]. Pb, Cd, As, etc., have recently been implicated in gallbladder cancer, colon cancer, and neurodegenerative diseases [4, 10,11,12,13]. So, environments like soil, plant, and food derived from animals and plants exposed to high heavy metal exposure may also pose biomagnifications in the food chain. Therefore, there is a need to monitor all environmental sources, including water, to control heavy metal-induced diseases in the higher food chain, viz., human and animal [4, 14, 15].
Further, cold desert high altitude (CDHA) water systems are also susceptible to climate change because numerous hydro-ecological processes react to even a small change in the climate [9, 16]. The river, pond, and lakes water ecosystem hydrology are strongly linked with climate change, which further impacts flora and fauna. However, there is not much information available on water quality and heavy metal statuses of various water resources in the high-altitude mountain region. Therefore, the present work is designed to know the current status of physico-chemical and heavy metals levels in different water resources at high altitude regions and the variation of water quality in different locations of this high altitude region. This study may give deep insight into high-altitude water quality and their management for providing safe drinking water in mountain regions and further helpful for local habitants for their healthy life.
2 Materials and methods
2.1 Survey of the study site
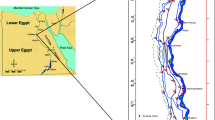
The present study was conducted in Leh district of UT Ladakh, which lies in the trans-Himalayan region of India. Leh district is located between latitude 32° to 36°N and longitude 75° to 80°E and at an altitude ranging from 2946 to 5994 m above mean sea level. The study area lies between latitude 33°59.362 and 34°17.722 N and longitudes 77°12.023 and 77°45.669 E. Further details of study area and sites are given in Additional file 1: Tables S1, S2, and S3 (shown in file ‘Supplementary material’).
2.2 Water sample collection
Water samples were collected from the Leh-Ladakh area scattered over 70 km of ranges where most of the population resides. Irrigation water, stagnant water (pond and lakes), and Indus River water were collected from eleven, eleven, and seven sites, respectively, and from each site, six water samples were collected. So, a total of 66, 42, 66 irrigation, Indus River, and stagnant water samples were collected, respectively. These water samples were collected in nitric acid (10% v/v) pretreated plastic bottles, about 1.0 L of water sample was collected, and immediately 2–3 drops of pure toluene were added as a preservative. Samples were directly taken into the laboratory and stored at 4 °C for further analysis.
2.3 Chemical and reagents
For the laboratory analysis of various water quality parameters, chemicals and reagents were GR grade purchased from Merck Germany, and some acquired from Sigma Aldrich United States of America were AR grade. Standards used in estimating heavy metals (As, Cd, Pb, Cr, and Ni) that were single and multi-element were purchased from Merck, Germany.
2.4 Laboratory methods for physico-chemical analysis
For the pH measurement, Orion 420 a (Thermo Orion, USA) pH meter was used with a glass electrode. In order to minimize the change in pH due to interaction with the atmospheric CO2 measurement was done at the time of sample collection. The TDS measurement was done by dipping the TDS electrode in standard solution and calibrating at 500 mg/L, after the calibration samples were tested. The COD was estimated by using the Closed Reflux using a Titration method. For the estimation of heavy metals, 50 mL water samples were taken from the frost chamber and brought in a 500 mL conical flask, and added 10 mL of 70% HNO3 and kept at 80 °C for 30 min to evaporate up to 10 mL of sample, then cooled and made volume 50 mL with Milli Q water as per APHA method [16].
2.5 Laboratory methods for heavy metals analysis
Thereafter, As, Cd, Pb, Cr, and Ni were estimated using ICP-OES (Optima 7000 DV, Perkin Elmer, USA) as the method provided by ICP-OES operating manual. Instrument validation was performed regularly for mineral detection limit through running blank, and calibration standards, of different minerals. So when we got a satisfactory reading as the conc. of particular minerals in the standards and their calibration curves, then only test samples were run. The limit of detection was investigated using Kicinska’s [17] formula. After subtracting a signal of any contamination from the blank, the solution was examined, and a correction was performed [18,19,20].
2.6 Statistical analysis
Data generated through the study were analyzed for mean and standard error (SE). Significant level (p < 0.05) was determined among the different water resources (Indus river water, stagnant water (pond), and irrigation water by one-way ANOVA using the SPSS statistical software (SPSS Inc. USA).
3 Results
3.1 Physico-chemical parameters of irrigation water, stagnant (ponds) water, and Indus river water
All the findings were compared among the sample collection sites, and the WHO standard was taken for further comparison and drawing a conclusion on water quality of the pond and Indus River. The physicochemical parameters of the different water resources of cold desert high altitude microclimate are presented in Tables 1, 2 and 3. The results of our investigation revealed pH ranged from 7.55–7.71, EC ranged from 195.20–418.20 µS/cm, salinity ranged from 0.10–0.20, TDS ranged from 96.38–204.66 mg/L, turbidity ranged from 0.00–1.80 NTU and COD ranged from 25.79–36.06 mg/L in irrigation water from all study locations (Table 1). However, the lowest pH, EC, salinity, TDS, turbidity, and COD level were observed at sites 11, 7, 6–8, 3, 1, and 5, respectively (Table 1). The highest pH, EC, salinity, TDS, turbidity, and COD level were observed at sites 7, 2, 2, 2, 8, and 4, respectively (Table 1).
The value of pH, EC (µS/cm), Salinity (%), TDS (mg/L), Turbidity (NTU), and COD (mg/L) of stagnant (ponds) water is presented in Table 2 and in Fig. 1A–E, which indicates these parameters are within the normal range and WHO standards. The results of our investigation revealed that the pH ranged from 7.19–7.77, EC ranged from 119.4–324.6 µS/cm, salinity ranged from 0.06–0.16%, TDS ranged from 56.58–168.44 mg/L, turbidity ranged from 0.00–1.80 NTU, and COD ranged from 25.30–33.78 mg/L in stagnant (ponds) water from all study locations (Table 2). However, the lowest pH, EC, salinity, TDS, turbidity, and COD level were observed at sites 9–10, 9–11, 11, 11, and 4, respectively. The highest pH, EC, salinity, TDS, turbidity, and COD level were observed at sites 2, 5, 5, 5, 7, and 2, respectively (Table 2).
The value of pH, EC (µS/cm), Salinity (%), TDS (mg/L), Turbidity (NTU), and COD (mg/L) of Indus river water is presented in Table 3 and in Fig. 1A–E, which indicates these parameters are within the normal range and WHO standards. The results of our investigation revealed pH ranged from 7.26–7.87, EC ranged from 195.20–410.20 µS/cm, salinity ranged from 0.06–0.20%, TDS ranged from 61.32–210.4 mg/L, turbidity ranged from 0.00 to 2.0 NTU, and COD ranged from 28.21–34.70 mg/L in Indus river water from all study locations (Table 3). However, the lowest pH, EC, salinity, TDS, turbidity, and COD level were observed at sites 2, 1, 1, 1, 2, and 1, respectively. The highest pH, EC, salinity, TDS, turbidity, and COD level were observed at sites 4, 3, 3, 3, 5, and 5, respectively (Table 3).
3.2 Heavy metals level in irrigation water, stagnant (ponds) water, and Indus river water
Again, all the findings on heavy metal were compared among the sample collection sites and the standard of WHO was taken for further evaluation and concluding the water quality (Tables 4, 5, 6). These findings revealed that As, Cd, Pb, Cr, and Ni levels in irrigation water ranged from 0.016–0.102 ppm, 0.012–0.031 ppm, 0.058–0.129 ppm, 0.021–0.03 ppm, 0.001–0.03 ppm, respectively (Table 4). These heavy metal concentrations were compared with WHO standards, which were higher than the permissible limit for As, Cd, and Pb. However, their levels varied significantly (p < 0.05) between the different collection sites (Table 4).
The level of heavy metals concentration (ppm) in stagnant water (pond) of cold desert high altitude region revealed As, Cd, Pb, Cr, and Ni levels in the range of 0.021–0.1 ppm, 0.02–0.034 ppm, 0.05–0.171 ppm, 0.021–0.034 ppm, 0.002–0.026 ppm, respectively (Table 5). These heavy metal concentrations were compared with WHO standards, which were higher than the permissible limits for As, Cd, and Pb. Furthermore, their levels varied significantly (p < 0.05) between the different collection sites (Table 5). In stagnant water, the lowest As, Cd, Pb, Cr, and Ni levels were observed at sites 8, 1–2, 9, 3, and 5, respectively. The highest As, Cd, Pb, Cr, and Ni levels were observed at sites 4, 11, 8, 8, and 11, respectively (Table 5).
The level of heavy metals concentration (ppm) in Indis river water of cold desert high altitude region revealed As, Cd, Pb, Cr, and Ni levels in the range of 0.005–0.094 ppm, 0.02–0.028 ppm, 0.05–228 ppm, 0.020–0.035 ppm, 0.001–0.051 ppm, respectively (Table 6). These heavy metal concentrations were compared with WHO standards, which were higher than the permissible limit for As, Cd, and Pb. Furthermore, their levels varied significantly (p < 0.05) between the different collection sites (Table 6). In Indus river water, the lowest As, Cd, Pb, Cr, and Ni levels were observed at sites 1, 1, 1, 7, and 3, respectively. The highest As, Cd, Pb, Cr, and Ni levels were observed at sites 4, 3, 3, 1, and 2, respectively (Table 6).
4 Discussion
4.1 Physico-chemical characteristics in irrigation water, stagnant (ponds) water, and Indus river water
Results revealed that the pH values of stagnant, Indus River and irrigation waters (7.46 ± 0.03) were similar as compared to permissible range (Tables 1, 2, 3) of 6.5 to 9.5 for the drinking water. This pH range of water is indicative of slightly alkaline, which may be due to the presence of carbonate and bi-carbonates ions [23]. A water pH between 6.0 and 7.0 is normally considered to be the most desirable for irrigation. As it was reported that pH affects the dissolved oxygen level in the water, photosynthesis of aquatic plants, metabolic rates of aquatic organisms, and the sensitivity of these organisms to pollution, parasites, and disease [15, 24]. A change in water pH can also affect aquatic life, indirectly altering water chemistry [25]. Furthermore, the present study showed normal ranges of turbidity for the Indus River, irrigation, and stagnant waters (Tables 1, 2, 3) as compared to FAO and WHO [22]. Furthermore, the desirable limit of 5 NTU is for drinking water for humans and animals.
The primary water quality guideline for crop productivity is the salinity hazard, as measured by EC [20]. In the present study, the EC of Indus river water, irrigation water, and stagnant water level estimated as 243.78 ± 18.05, 86.16 ± 1.72, and 418.20 ± 11.98 μS/cm (Tables 1, 2, 3); these values are under the permissible limit (< 1500 μmohs/cm) for domestic use. The BIS [26] has also recommended a drinking water standard of EC 750 µS/cm for humans and animals. Results from our present study revealed the low salinity of the Indus River, irrigation, and stagnant waters (Tables 1, 2, 3), which are safe for all kinds of animal's drinking purposes rearing in this region. Most applications of COD determine the number of organic pollutants found in surface water, so making COD a useful measure of water quality. In this study, COD for irrigation, stagnant (pond), and Indus river water are near the permissible limit as per the standard guideline [18]. According to BIS [27] and WHO [22] recommendations, water having a TDS of more than 1000 mg/L is not suitable for drinking purposes, and the maximum desirable limit of TDS is 500 mg/L [24, 28]. In this study, the TDS level in all the sources is within the higher limit (Tables 1, 2, 3). This high TDS level may cause calculi in various organs and disrupt the secretary ability of the endocrine system in humans and animals. So, it is concluded that water needs to be filtrated before drinking.
4.2 Heavy metals concentration in irrigation water, stagnant (ponds) water, and Indus river water
In the present study, our results revealed the high levels of arsenic in irrigation water, stagnant water, and Indus river water. However, the same trend was detected in stagnant waters at most of the study locations, whereas lower concentrations of As were determined in Indus River water samples (Fig. 2; Tables 4, 5, 6). This exposure to water could be due to anthropogenic as well as leaching from arsenic-bearing rocks [29]. The maximum desirable limit of arsenic in drinking water for human beings is 0.05 ppm [27], 0.01 ppm [30], and CCME [30] guideline (0.025 ppm) for livestock. The recommended limit of Arsenic for irrigation water for long term use is 0.10 ppm and for short-term use is 2.0 ppm [31]. High arsenic may affect renal, hepatic, brain tissues, and other tissues in humans and animals [10, 11, 13].
In the present study, results revealed that the nickel concentration in irrigation water is low in most of the studied sites as compared to the permissible limit of Ni (Fig. 2; Tables 4, 5, 6). According to WHO [22], USEPA, [31], and BIS [26], the maximum permissible limit of Ni is 0.02 ppm in drinking water for human beings [26, 31, 32], and CCME [29] recommended 1.0 ppm upper safe Ni level for livestock. However, the maximum permissible limit for Ni is 0.2 ppm for irrigation water [33]. Furthermore, it is reported that the recommended limit of Nickel (Ni) in irrigation water for plants is 0.2 ppm for long-term use and 2.0 ppm for short-term. However, it becomes toxic to several plants at 0.5 to 1.0 ppm [30]. However, irrigation water's alkaline pH decreases Ni's harmful effect on plants [30]. Water Ni level may vary due to the mobilization of groundwater and water sources from igneous rock [34, 35].
The permissible limit of Pb as per WHO [22] for human drinking water is 0.01 ppm, whereas for irrigation purposes, it is 5 ppm [22]. CCME [29] recommended 0.1 ppm safe upper levels of Pb in drinking water for livestock [29], and 0.01 ppm for human health [26]. The present study revealed that the Pb in the river, stagnant, and irrigation water are at high concentrations according to the above guidelines; in irrigation water, Pb level is higher than River and stagnant waters (Fig. 2; Tables 4, 5, 6). According to our predictions, water resources from cold deserts and high altitude are not safe for long-term drinking purposes to humans and livestock without treatment. At the same time, they may be suitable for irrigation purposes. This high concentration of heavy metal may pose cellular stress through the mediation of oxidative stress and may pose toxicity to the kidney, liver, brain, etc. [10, 11, 13]. These aspect needs to be studied further. However, no published information on high-altitude water is available to compare present findings.
In the present study, chromium level was below in all the water sources as compared to WHO and other standards. The desirable maximum limit of chromium in drinking water is 0.05 ppm for humans and livestock [22, 26, 29], for irrigation water is 0.1 ppm for the long-term [30, 31] and 0.05 ppm [16]. Hence, in conclusion, Cr exposure to humans and animals is minimal in high-altitude through water, as their concentration was low in all the water sources from the studies (Fig. 2; Tables 4, 5, 6).
The maximum desirable limit of Cd in drinking water for human beings is 0.01 ppm [22]. The irrigation standard recommended by USEPA for Cd concentration in water is 5 ppm. CCME [29] recommended a safe upper level of Cd in water is 0.08 ppm for livestock. The Cd level in studied water samples is significantly higher than the maximum permissible limit, which is 0.01 ppm [22]. So, the use of these water sources for longer periods may pose renal and hepatic damage in humans and animals [10, 11, 13]. This higher level of Cd in this cold desert high altitude region (Fig. 2; Tables 4, 5, 6) may be due to the increasing pollution level by increasing tourism, other anthropogenic activities, and leaching from Cd containing parent materials (rocks) available in this region [9, 36]. Sharma et al. [37] reported that irrigation water quality in terms of different physicochemical and mineral levels was being altered due to increased anthropogenic activities (farming, livestock breeding, tourism, small industry, etc.) near the Himalayan Rivers of Nepal. In another study on the central Himalayan rivers, Smadja et al. [38] and Abbas et al. [39] found that global warming has a big effect on the loss of water resources in high-altitude areas. Therefore, water resources of other high-altitude and mountain regions in the world may also face similar issues due to anthropogenic sources of contamination. Hence, regular water quality and metal bioaccumulation monitoring in the food chain needs to be investigated in these high-altitude areas.
4.3 Variation in physico-chemical and heavy metals properties in different sites
Most of the physico-chemical properties in different water resources were higher at sites 2, 3, 4, 5, 7, 8, and 10, which are indicative of industrial and direct human factors affecting the water quality. In case of heavy metals levels in different water resources, it has been found that sites 1, 2, 3, 4, 8, and 11 had significantly higher levels of As, Cd, and Pb heavy metals. This could be due to exposure to effluents and discharge of domestic and city drains, as these sites are near to the main city and highly populated. In some sites, there are small industries are present, and the drainage system is not well developed. However, our recent studies indicated that this region has complex types of hydro-geochemical processes that control the variation of physico-chemical and heavy metals levels in different water sources [20]. So, there is a need to study these water drainage quality and further treatment before releasing to these water resources for improving the water quality of this high-altitude region. Thus human population, increasing tourism, and industrialization are becoming significant sources of metal pollution as in plain areas.
This study revealed that the water quality of high-altitude areas is also affected by heavy metals at some sites, which needs further investigation on sources of these metals and the hydrochemical cycle to know the flow of these minerals. Irrigation of land using this water should have inhibiting bioaccumulation in crops grown through crop rotation, using organic manure, microbial consortium having heavy metals chelating properties, etc., in those areas. This study will also help in the selection of water treatment technology for household and community basis and policymakers for further restoration of these water resources free from pollution.
5 Conclusions
As per the findings of physicochemical properties viz. pH, EC, TDS, turbidity, COD, and the salinity of studied water resources, the water is suitable for irrigation to various crop plants. Furthermore, this study revealed As, Pb, and Cd are above the permissible limit, whereas Ni and Cr levels were within the permissible limit. Therefore, these water resources, if used for longer periods, may pose health-related issues to humans and animals from these elements. So, this study finding will help in developing specific mitigation strategies for water if used for drinking and further planning of soil if this water is used for irrigation through crop rotation, using organic manure, microbial consortium having heavy metals chelating properties, etc. in those areas. However, a study on the minerals and heavy metals flow in different food chains should be conducted in this high-altitude region to find the existence of bioaccumulation in animals.
Availability of data and materials
Data will be available from the corresponding author upon good scientific reason and request.
References
Adelekan BA, Abegunde KD. Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. Int J Phys Sci. 2011;6:1045–58. https://doi.org/10.5897/IJPS10.495.
López-Alonso M. Trace minerals and livestock: not too much not too little. ISRN Vet Sci. 2012;2012:1–18. https://doi.org/10.5402/2012/704825.
Mehri A. Trace elements in human nutrition (II)—an update. Int J Prev Med. 2020. https://doi.org/10.4103/ijpvm.IJPVM_48_19.
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133–64. https://doi.org/10.1007/978-3-7643-8340-4_6.
Abalaka SE, Enem SI, Idoko IS, Sani NA, Tenuche OZ, Ejeh SA, et al. Heavy metals bioaccumulation and health risks with associated histopathological changes in Clarias gariepinus from the Kado fish market, Abuja, Nigeria. J Health Pollut. 2020;10:1–12. https://doi.org/10.5696/2156-9614-10.26.200602.
Eldaw E, Huang T, Elubid B, Mahamed AK, Mahama Y. A novel approach for indexing heavy metals pollution to assess groundwater quality for drinking purposes. Int J Environ Res Public Health. 2020;17:1245. https://doi.org/10.3390/ijerph17041245.
De Oliveira Ribeiro CA, Belger L, Pelletier É, Rouleau C. Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environ Res. 2002;90:217–25. https://doi.org/10.1016/S0013-9351(02)00025-7.
Tang W, Shan B, Zhang H, Zhang W, Zhao Y, Ding Y, et al. Heavy metal contamination in the surface sediments of representative limnetic ecosystems in eastern China. Sci Rep. 2014;4:1–7. https://doi.org/10.1038/srep07152.
Giri A, Bharti VK, Kalia S, Arora A, Balaje SS, Chaurasia OP. A review on water quality and dairy cattle health: a special emphasis on high-altitude region. Appl Water Sci. 2020;10:1–16. https://doi.org/10.1007/s13201-020-1160-0.
Singh J, Kalamdhad AS. Effects of heavy metals on soil, plants, human health and aquatic life. Int J Res Chem Environ. 2011;1:15–21.
Chang LW, Magos L, Suzuki T, editors. Toxicology of metals. CRC Press, USA; 1996.
Centeno JA, Tchounwou PB, Patlolla AK, Mullick FG, Murakat L, Meza E, Gibb H, Longfellow D, Yedjou CG. Environmental pathology and health effects of arsenic poisoning: a critical review. In: Naidu R, Smith E, Smith J, Bhattacharya P, editors. Managing arsenic in the environment: from soil to human health. Adelaide, Australia: CSIRO Publishing Corp; 2005.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60–72.
Ali H, Khan E, Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem. 2019;2019:1–14.
Srivastava V, Sarkar A, Singh S, Singh P, De Araujo AS, Singh RP. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front Environ Sci. 2017;5:64.
APHA. Standard methods for the examination of water and wastewater. (17th eds), American Public Health Association, Washington; 1989.
Kicińska A. Health risk assessment related to an effect of sample size fractions: methodological remarks. Stoch Environ Res Risk Assess. 2018;32:1867–87. https://doi.org/10.1007/s00477-017-1496-7.
Giri A, Bharti VK, Kalia S, Kumar B, Chaurasia OP. Health risk assessment of heavy metals through cow milk consumption in trans-Himalayan high-altitude region. Biol Trace Elem Res. 2021;199:4572–81. https://doi.org/10.1007/s12011-021-02593-6.
Giri A, Bharti VK, Kalia S, Acharya S, Kumar B, Chaurasia OP. Health risk assessment of heavy metals due to wheat, cabbage, and spinach consumption at cold-arid high altitude region. Biol Trace Elem Res. 2021;200:1–13. https://doi.org/10.1007/s12011-021-03006-4.
Giri A, Bharti VK, Kalia S, Kumar K, Khansu M. Hydrochemical and quality assessment of irrigation water at the trans-himalayan high-altitude regions of Leh, Ladakh, India. Appl Water Sci. 2022;12:1–20. https://doi.org/10.1007/S13201-022-01716-1.
FAO-UN. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations (FAO-UN): Rome, Italy, 1985; Volume 29. (Retrieved from https://www.fao.org/3/t0234e/t0234e.pdf)
World Health Organization. Guidelines for Drinking-water Quality First Addendum to Third Edition Volume 1 Recommendations WHO Library Cataloguing-in-Publication Data. 2006.
World Health Organization (WHO). pH in drinking water. Background document for preparation of WHO guidelines for drinking-water quality. Geneva: 2008.
Sautour M, Mary P, Chihib NE, Hornez JP. The effects of temperature, water activity and pH on the growth of Aeromonas hydrophila and on its subsequent survival in microcosm water. J Appl Microbiol. 2003;95:807–13. https://doi.org/10.1046/j.1365-2672.2003.02048.x.
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol. 2013;19:1884–96. https://doi.org/10.1111/gcb.12179.
BIS. Indian standards drinking water specification, Bureau of Indian Standard, Indian Standard 10500. New Delhi: 2005.
BIS. Indian standards drinking water specification, Bureau of Indian Standard, Indian Standard 10500. New Delhi: 1991.
Nriagu JO, Bhattacharya P, Mukherjee AB, Bundschuh J, Zevenhoven R, Loeppert RH. Arsenic in soil and groundwater: an overview. In: Arsenic in soil and groundwater environment–biogeochemical interactions health effects and remediation. Amsterdam: Elsevier; 2007. p. 3–60. https://doi.org/10.1016/S1875-1121(06)09001-8.
Canadian Council of Ministers of the Environment (CCME). Canadian water quality guidelines for the protection of agricultural water uses 2005.
Rowe DR, Abdel-Magid IM. Handbook of wastewater reclamation and reuse. USA: CRC Press; 2020. https://doi.org/10.1201/9780138752514.
U.S. Environmental Protection Agency (USEPA). Draft Guidance for Water Quality based Decisions: The TMDL Process. Washington, D.C.: 1999.
Agency for Toxic Substances and Disease Registry. Toxicology profile for selenium. Atlanta: 2003.
Taghipour H, Mosaferi M. Heavy metals in the vegetables collected from production sites heavy metals in the vegetables collected from production sites according to chemical properties, heavy. Health Promot Perspect. 2013;3:185. https://doi.org/10.5681/hpp.2013.022.
World Health Organization (WHO). Guidelines for drinking water quality. Geneva: 2011.
Kabata-Pendias A. Soil-plant transfer of trace elements—an environmental issue. Geoderma. 2004;122:143–9. https://doi.org/10.1016/j.geoderma.2004.01.004.
Giri A, Bharti VK, Kalia S, Kumar K, Raj T, Chaurasia OP. Utility of multivariate statistical analysis to identify factors contributing river water quality in two different seasons in cold-arid high-altitude region of Leh-Ladakh, India. Appl Water Sci. 2019;9:1–15. https://doi.org/10.1007/s13201-019-0902-3.
Sharma CM, Kang S, Tripathee L, Paudyal R, Sillanpää M. Major ions and irrigation water quality assessment of the Nepalese Himalayan rivers. Environ Dev Sustain. 2021;23(2):2668–80. https://doi.org/10.1007/s10668-020-00694-1.
Abbas H, Khan MZ, Begum F, Raut N, Gurung S. Physicochemical properties of irrigation water in western Himalayas, Pakistan. Water Supply. 2020;20(8):3368–79. https://doi.org/10.2166/ws.2020.221.
Smadja J, Aubriot O, Puschiasis O, Duplan T, Grimaldi J, Hugonnet M, Buchheit P. Climate change and water resources in the Himalayas. Field study in four geographic units of the Koshi basin, Nepal. Alp Res. 2015. https://doi.org/10.4000/rga.2910.
Acknowledgements
All the authors acknowledge the financial support and facilities provided by the DRDO-Defence Institute of High Altitude Research (DIHAR), Leh, India, for conducting this study. The first author (GC) is also thankful to the DRDO-Defence Institute of High Altitude Research (DIHAR), Leh, for providing a research fellowship during this study. All the authors are also grateful to Dr. Sudhir Kumar of Jaypee University of Information Technology, Waknaghat, Solan, Himachal Pradesh, India, for his technical discussion and help during the study.
Author information
Authors and Affiliations
Contributions
GC performed sampling, physicochemical parameters, and minerals analysis, and prepared the draft. PK helped in the sampling and analysis of physicochemical parameters. AG reviewed and edited the manuscript. VKB conceived the ideas, designed the study, and interpreted the data. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no proprietary, financial, professional, nor any other personal interest of any kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional file 1
. Details of study area and sites.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Charan, G., Bharti, V.K., Giri, A. et al. Evaluation of physico-chemical and heavy metals status in irrigation, stagnant, and Indus River water at the trans-Himalayan region. Discov Water 3, 3 (2023). https://doi.org/10.1007/s43832-023-00027-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00027-z