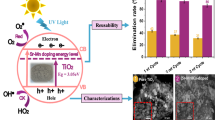
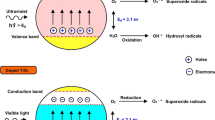
Abstract
The photocatalytic and mechanical performance of TiO2 nanotubular coatings obtained by anodic oxidation of commercial titanium, using an NH4F and 3.5% v/v water in ethylene glycol solution as electrolyte was investigated. After the anodization, the coatings were thermally treated at 450 °C for 2 h. The effects of the anodizing voltage (40–80 V) and NH4F concentration (0.06, 0.15, 0.27 M) on the formation of the nanotube arrays were evaluated. Nanotube diameters (57 to 114 nm), wall thicknesses (4 to 13 nm), and lengths (5 to 17 µm) increased with the anodizing voltage and the NH4F concentration. The photocatalysts were characterized by scanning electron microscopy, glancing incidence X-ray diffraction, and UV-Vis diffuse reflectance spectroscopy. The mechanical properties of the photocatalysts were determined: adhesion using the tape test (ASTM D3359) and erosion resistance through a 3 h accelerated test. The photocatalytic activity of the nanotubes under UV irradiation was evaluated using hexavalent chromium (Cr(VI)) in the presence of ethylenediaminetetraacetic acid (EDTA), using a 1.25 EDTA/Cr(VI) molar ratio solution at pH 2. A complete Cr(VI) transformation after 3 h of irradiation was obtained for all samples, with a better performance than that of an immobilized P25 sample. The photocatalyst obtained with 0.27 M NH4F at 40 V presented a good behavior in adherence and erosion resistance, together with a very good photocatalytic activity. This novel analysis, combining photocatalytic and mechanical tests, proved that the new TiO2 nanotubular coatings could be successfully used as immobilized photocatalysts in photoreactors for water treatment.
Graphical abstract












Similar content being viewed by others
References
Fujishima, A., & Zhang, X. (2006). Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chimie, 9, 750–760. https://doi.org/10.1016/j.crci.2005.02.055
Al-Mamun, M. R., Kader, S., Islam, M. S., & Khan, M. Z. H. (2019). Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: a review. Journal of Environmental Chemical Engineering, 7, 103248. https://doi.org/10.1016/j.jece.2019.103248
Haider, A., Al-Anbari, R., Kadhim, G., & Jameel, Z. (2018). Synthesis and photocatalytic activity for TiO2 nanoparticles as air purification. In MATEC web of conferences (Vol. 162, p. 05006). EDP Sciences. https://doi.org/10.1051/matecconf/201816205006
Tayeb, A. M., & Hussein, D. S. (2015). Synthesis of TiO2 nanoparticles and their photocatalytic activity for methylene blue. American Journal of Nanomaterials, 3, 57–63. https://doi.org/10.12691/ajn-3-2-2
Litter, M. I., Vera, M. L., & Traid, H. D. (2020). TiO2 coatings prepared by sol-gel and electrochemical methodologies. In Sol-gel derived optical and photonic materials (pp. 39–74). Woodhead Publishing. https://doi.org/10.1016/B978-0-12-818019-8.00003-X
Robert, D., Keller, V., & Keller, N. (2013). Immobilization of a semiconductor photocatalyst on solid supports: methods, materials, and applications. Photocatalysis and water purification (1st ed., pp.145–178).
Ghosh, J. P., Achari, G., & Langford, C. H. (2016). Design and evaluation of a UV LED photocatalytic reactor using anodized TiO2 nanotubes. Water Environment Research, 88(8), 785–791. https://doi.org/10.2175/106143015X14362865226879
Bellè, U., Invernizzi, M., Polvara, E., Lucotti, A., Diamanti, M. V., Sironi, S., & Pedeferri, M. (2022). A novel nanotubular TiO2-based plug-flow reactor for gas phase photocatalytic degradation of toluene. Chemical Engineering Journal, 437, 135323. DOI: https://doi.org/10.1016/j.cej.2022.135323
Radwan, E. K., Yu, L., Achari, G., & Langford, C. H. (2016). Photocatalytic ozonation of pesticides in a fixed bed flow through UVA-LED photoreactor. Environmental Science and Pollution Research, 23(21), 21313–21318. https://doi.org/10.1007/s11356-016-7346-1
Sopha, H., & Macak, J. M. (2020). Recent advancements in the synthesis, properties, and applications of anodic self-organized TiO2 nanotube layers. In Nanostructured anodic metal oxides (pp. 173–209). Elsevier. https://doi.org/10.1016/B978-0-12-816706-9.00006-6
Stodolny, M., Zagrodnik, R., Nowaczyk, G., & Jurga, S. (2017). Size-controlled synthesis of anatase nanobrush structures with higher crystal density. Materials Research Bulletin, 94, 335–341. https://doi.org/10.1016/j.materresbull.2017.06.010
Gong, D., Grimes, C. A., Varghese, O. K., Hu, W., Singh, R. S., Chen, Z., & Dickey, E. C. (2001). Titanium oxide nanotube arrays prepared by anodic oxidation. Journal of Materials Research, 16, 3331–3334. https://doi.org/10.1557/JMR.2001.0457
Macák, J. M., Tsuchiya, H., & Schmuki, P. (2005). High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angewandte Chemie International Edition, 44, 2100–2102. https://doi.org/10.1002/anie.200462459
Macák, J. M., Tsuchiya, H., Taveira, L., Aldabergerova, S., & Schmuki, P. (2005). Smooth anodic TiO2 nanotubes. Angewandte Chemie International Edition, 44, 7463–7465. https://doi.org/10.1002/anie.200502781
Paulose, M., Shankar, K., Yoriya, S., Prakasam, H. E., Varghese, O. K., Mor, G. K., Latempa, T. A., Fitzgerald, A., & Grimes, C. A. (2006). Anodic growth of highly ordered TiO2 nanotube arrays to 134 µm in length. Journal of Physical Chemistry B, 110, 16179–16184. https://doi.org/10.1021/jp064020k
Bauer, S., Kleber, S., & Schmuki, P. (2006). TiO2 nanotubes: tailoring the geometry in H3PO4/HF electrolytes. Electrochemical Communications, 8, 1321–1325. https://doi.org/10.1016/j.elecom.2006.05.030
Zhuang, H. F., Lin, C. J., Lai, Y. K., Sun, L., & Li, J. (2007). Some critical structure factors of titanium oxide nanotube array in its photocatalytic activity. Environmental. Science and Technology, 41, 4735–4740. https://doi.org/10.1021/es0702723
Prakasam, H. E., Shankar, K., Paulose, M., Varghese, O. K., & Grimes, C. A. (2007). A new benchmark for TiO2 nanotube array growth by anodization. Journal of Physical Chemistry C, 111, 7235–7241. https://doi.org/10.1021/jp070273h
Albu, S. P., Ghicov, A., Macák, J. M., Hahn, R., & Schmuki, P. (2007). Self-organized, free-standing TiO2 nanotube membrane for flow-through photocatalytic applications. Nano Letters, 7, 1286–1289. https://doi.org/10.1021/nl070264k
Wang, J., & Lin, Z. Q. (2008). Freestanding TiO2 nanotube arrays with ultrahigh aspect ratio via electrochemical anodization. Chemistry of Materials, 20, 1257–1261. https://doi.org/10.1021/cm7028917
Liu, Z., Zhang, X., Nishimoto, S., Jin, M., Tryk, D. A., Murakami, T., & Fujishima, A. (2008). Highly ordered TiO2 nanotube arrays with controllable length for photoelectrocatalytic degradation of phenol. Journal of Physical Chemistry C, 112, 253–259. https://doi.org/10.1021/JP0772732
Wang, J., & Lin, Z. (2009). Anodic formation of ordered TiO2 nanotube arrays: Effects of electrolyte temperature and anodization potential. Journal of Physical Chemistry C, 113, 4026–4030. https://doi.org/10.1021/jp811201x
Grimes, C. A., & Mor, G. K. (2009). TiO2 nanotube arrays: synthesis, properties, and applications. Springer Science & Business Media.
Liao, A. Z., Wang, C. W., Chen, J. B., Zhang, X. Q., Li, Y., & Wang, J. (2015). Remarkably improved field emission of TiO2 nanotube arrays by annealing atmosphere engineering. Materials Research Bulletin, 70, 988–994. https://doi.org/10.1016/j.materresbull.2015.06.036
Radwan, E. K., Yu, L., Achari, G., & Langford, C. H. (2016). Photocatalytic ozonation of pesticides in a fixed bed flow through UVA-LED photoreactor. Environmental Science and Pollution Research, 23, 21313–21318. https://doi.org/10.1007/s11356-016-7346-1
Jaroenworaluck, A., Regonini, D., Bowen, C. R., Stevens, R., & Allsopp, D. (2007). Macro, micro and nanostructure of TiO2 anodised films prepared in a fluorine-containing electrolyte. Journal of materials science, 42, 6729–6734. https://doi.org/10.1007/s10853-006-1474-9
Wang, K., Liu, G., Hoivik, N., Johannessen, E., & Jakobsen, H. (2014). Electrochemical engineering of hollow nanoarchitectures: Pulse/step anodization (Si, Al, Ti) and their applications. Chemical Society Reviews, 43, 1476–1500. https://doi.org/10.1039/c3cs60150a
Dou, Q., Shrotriya, P., Li, W., & Hebert, K. R. (2019). Stress-generating electrochemical reactions during the initial growth of anodic titanium dioxide nanotube layers. Electrochimica Acta, 295, 418–426. https://doi.org/10.1016/j.electacta.2018.10.094
Lebedeva, O., Kultin, D., Kudryavtsev, I., Root, N., & Kustov, L. (2017). The role of initial hexagonal self-ordering in anodic nanotube growth in ionic liquid. Electrochemistry Communications, 75, 78–81. https://doi.org/10.1016/j.elecom.2017.01.005
Ozkan, S., Mazare, A., & Schmuki, P. (2018). Critical parameters and factors in the formation of spaced TiO2 nanotubes by self-organizing anodization. Electrochimica Acta, 268, 435–447. https://doi.org/10.1016/j.electacta.2018.02.120
Regonini, D., Bowen, C. R., Jaroenworaluck, A., & Stevens, R. (2013). A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Materials Science and Engineering: R: Reports, 74, 377–406. https://doi.org/10.1016/j.mser.2013.10.001
Anger, G., Halstenberg, J., Hochgeschwender, K., Scherhag, C., Korallus, U., Knopf, H., Schmidt, P, & Ohlinger, M. (2005). Chromium compounds. Ullmann's Encyclopedia of Industrial Chemistry, 7.
Alias, N., Rosli, S. A., Bashirom, N., Rozana, M., Tan, W. K., Kawamura, G., Nbelayim, P., Matsuda, A., Hussain, Z., & Lockman, Z. (2020). Oxide nanotubes formation by anodic process and their application in photochemical reactions for heavy metal removal. In Nanostructured Anodic Metal Oxides (pp. 277–303). Elsevier. https://doi.org/10.1016/B978-0-12-816706-9.00008-X
Kleiman, A., Meichtry, J. M., Vega, D., Litter, M. I., & Márquez, A. (2020). Photocatalytic activity of TiO2 films prepared by cathodic arc deposition: Dependence on thickness and reuse of the photocatalysts. Surface and Coatings Technology, 382, 125154. https://doi.org/10.1016/j.surfcoat.2019.125154
Kleiman, A., Márquez, A., Vera, M. L., Meichtry, J. M., & Litter, M. I. (2011). Photocatalytic activity of TiO2 thin films deposited by cathodic arc. Applied Catalysis B: Environmental, 101, 676–681. https://doi.org/10.1016/j.apcatb.2010.11.009
Vera, M. L., Leyva, G., & Litter, M. I. (2017). Simple TiO2 coatings by sol–gel techniques combined with commercial TiO2 particles for use in heterogeneous photocatalysis. Journal of Nanoscience and Nanotechnology, 17, 4946–4954. https://doi.org/10.1166/jnn.2017.13430
Traid, H. D., Vera, M. L., Ares, A. E., & Litter, M. I. (2017). Advances on the synthesis of porous TiO2 coatings by anodic spark oxidation. Photocatalytic reduction of Cr (VI). Materials Chemistry and Physics, 191, 106–113. https://doi.org/10.1016/j.matchemphys.2017.01.034
Dwojak, A. N., Vera, M. L., Traid, H. D., Maydana, M. F., Litter, M. I., & Schvezov, C. E. (2021). Influence of anodizing variables on Cr(VI) photocatalytic reduction using TiO2 nanotubes obtained by anodic oxidation. Environmental Nanotechnology, Monitoring & Management, 16, 100537. https://doi.org/10.1016/j.enmm.2021.100537
Vera, M. L., Traid, H. D., Henrikson, E. R., Ares, A. E., & Litter, M. I. (2018). Heterogeneous photocatalytic Cr(VI) reduction with short and long nanotubular TiO2 coatings prepared by anodic oxidation. Materials Research Bulletin, 97, 150–157. https://doi.org/10.1016/j.materresbull.2017.08.013
Candal, R. J., Rodríguez, J., Colón, G., Gelover, S., Vigil Santos, E., Jiménez González, A., Blesa, M. A. (2001). In: Blesa, M. A. (Ed.), Eliminación de Contaminantes por Fotocatálisis Heterogénea. Red CYTED VIII-G, Digital Grafic, La Plata.
Frost, M.J. (1981) Mohs scale of hardness. In: Mineralogy. Encyclopedia of Earth Science. Springer, Boston, MA. https://doi.org/10.1007/0-387-30720-6_84
Wang, Q., Yang, X., Wang, X., Huang, M., & Hou, J. (2012). Synthesis of N-doped TiO2 mesosponge by solvothermal transformation of anodic TiO2 nanotubes and enhanced photoelectrochemical performance. Electrochimica Acta, 62, 158–162. https://doi.org/10.1016/j.electacta.2011.12.009
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature methods, 9(7), 671–675. https://doi.org/10.1038/nmeth.2089
Anderson, O., Ottermann, C. R., Kuschnereit, R., Hess, P., & Bange, K. (1997). Density and Young’s modulus of thin TiO2 films. Fresenius’ journal of analytical chemistry, 358(1), 315–318.
Murphy, A. B. (2007). Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Solar Energy Materials and Solar Cells, 91, 1326–1337. https://doi.org/10.1016/j.solmat.2007.05.005
ASTM (2009). ASTM D3359 Standard Test Methods for Measuring Adhesion by Tape Test, USA, 8 pp.
Rosenberger, M. R., Guerrero, L. A., Vera, M. L.; Schvezov, C. E. (2012). Erosión de Materiales para Aplicaciones en Prótesis de Válvulas Cardíacas. Anales AFA, 24, 71–76. https://afan.df.uba.ar/journal/index.php/analesafa/article/view/1927/2300
Degremont, S.A. (1979). Manual técnico del agua. Grafo S.A., España. ISBN: 84–300–1651–1.
ASTM D1687–12. Standard Test Methods for Chromium in Water.
Mor, G. K., Varghese, O. K., Paulose, M., Shankar, K., & Grimes, C. A. (2006). A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Solar Energy Materials and Solar Cells, 90, 2011–2075. https://doi.org/10.1016/j.solmat.2006.04.007
Oh, H. J., Kim, I. K., Jang, K. W., Lee, J. H., Lee, S., & Chi, C. S. (2012). Influence of electrolyte and anodic potentials on morphology of titania nanotubes. Metals and Materials International, 18, 673–677. https://doi.org/10.1007/s12540-012-4027-6
Naghizadeh, M., Ghannadi, S., Abdizadeh, H., & Golobostanfard, M. R. (2014). Effect of fluoride concentration and water content on morphology of titania nanotubes in ethylene glycol solution. In Advanced Materials Research (Vol. 829, pp. 907–911). Trans Tech Publications Ltd. https://doi.org/10.4028/www.scientific.net/AMR.829.907
Yasuda, K., & Schmuki, P. (2007). Formation of self-organized zirconium titanate nanotube layers by alloy anodization. Advanced materials, 19, 1757–1760. https://doi.org/10.1002/adma.200601912
So, S., Lee, K., & Schmuki, P. (2012). Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes. Journal of the American Chemical Society, 134, 11316–11318. https://doi.org/10.1021/ja301892g
Albu, S. P., & Schmuki, P. (2010). Highly defined and ordered top‐openings in TiO2 nanotube arrays. Physica status solidi (RRL)–Rapid Research Letters, 4, 151–153. https://doi.org/10.1002/pssr.201004159
Rathnayaka, V. W. S. G., Rajapakse, H. D., & Sitinamaluwa, H. S. (2020). Effect of anodization voltage and time on the structure of TiO2 nanotube arrays. In 9th YSF symposium (p. 69)
Pichat, P. (2014). Are TiO2 nanotubes worth using in photocatalytic purification of air and water? Molecules, 19, 15075–15087. https://doi.org/10.3390/molecules190915075
Yang, F., Feng, X., Ge, F., Zhang, T., Qi, J., Li, D., & Zhu, X. (2019). Rapid growth of titanium oxide nanotubes under the critical breakdown voltage: Evidence against the dissolution reaction of fluoride ions. Electrochemistry Communications, 103, 17–21. https://doi.org/10.1016/j.elecom.2019.04.010
Albu, S. P., Roy, P., Virtanen, S., & Schmuki, P. (2010). Self-organized TiO2 nanotube arrays: Critical effects on morphology and growth. Israel Journal of Chemistry, 50, 453–467. https://doi.org/10.1002/ijch.201000059
Vera, M. L., Henrikson, E. R., Traid, H. D., Ares, A. E., & Litter, M. I. (2018). Influence of thermal treatments in nanotubular anodic coatings of TiO2. Matéria (Rio de Janeiro), 23,. https://doi.org/10.1590/S1517-707620180002.0460
Varghese, O. K., Gong, D., Paulose, M., Grimes, C. A., & Dickey, E. C. (2003). Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. Journal of Materials Research, 18, 156–165. https://doi.org/10.1557/JMR.2003.0022
Velten, D., Biehl, V., Aubertin, F., Valeske, B., Possart, W., & Breme, J. (2002). Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. Journal of Biomedical Materials Research, 59, 18–28. https://doi.org/10.1002/jbm.1212
Acknowledgements
The authors would like to thank CONICET and Agencia Nacional de Promoción Científica y Tecnólogica (ANPCyT) from Argentina, PICT-2017-2133 and PICT-2017-2494 projects. The authors thank Dr. Paula Angelomé from the Nanomaterials Chemistry Group of Gerencia Química of Centro Atómico Constituyentes, Comisión Nacional de Energía Atómica, Argentina for DRS measurements, and Eng. Cristian Cegelski from the DRX Laboratory of Instituto de Materiales de Misiones (IMAM, CONICET-UNaM, Argentina) for XRD measurements.
Funding
This work has been funded by Agencia Nacional de Promoción Científica y Tecnólogica (ANPCyT) from Argentina through PICT-2017-2133 and PICT-2017-2494 projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dwojak, A.N., Vera, M.L., Traid, H.D. et al. Photocatalytic and mechanical properties of immobilized nanotubular TiO2 photocatalysts obtained by anodic oxidation: a novel combined analysis. Photochem Photobiol Sci 21, 1793–1806 (2022). https://doi.org/10.1007/s43630-022-00257-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00257-5