Abstract
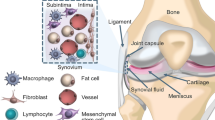
Synovium-derived mesenchymal stromal cell (Sy-MSC) is a newer member of the mesenchymal stromal cell families. The first successful demonstration of the mesenchymal stromal cell from the human synovial membrane was done in 2001 and since then its potential role for musculoskeletal regeneration has been keenly documented. The regenerative effects of Sy-MSCs are through paracrine signaling, direct cell–cell interactions, and extracellular vehicles. Sy-MSCs possess superior chondrogenicity than other sources of mesenchymal stromal cells. This article aims to outline the advancement of synovium-derived mesenchymal stromal cells along with a specific insight into the application for managing osteoarthritis knee.




Similar content being viewed by others
References
Chen, D., Shen, J., Zhao, W., Wang, T., Han, L., Hamilton, J. L., & Im, H. J. (2017). Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Research, 5, 16044. https://doi.org/10.1038/boneres.2016.44
Sophia Fox, A. J., Bedi, A., & Rodeo, S. A. (2009). The basic science of articular cartilage: Structure, composition, and function. Sports Health, 1(6), 461–468. https://doi.org/10.1177/1941738109350438
Dieppe, P. (2002). Epidemiology of the rheumatic diseases (2nd ed., p. 377). In: A. J. Silman & M. C. Hochberg (Eds.), Oxford: Oxford University Press, 2001, £95.00. ISBN: 0192631497. International Journal of Epidemiology, 31(5):1079–1080. https://doi.org/10.1093/ije/31.5.1079-a
Karuppal, R. (2017). Current concepts in the articular cartilage repair and regeneration. Journal of Orthopaedics, 14(2), A1–A3. https://doi.org/10.1016/j.jor.2017.05.001
Zhang, R., Ma, J., Han, J., Zhang, W., & Ma, J. (2019). Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. American Journal of Translational Research, 11(10), 6275–6289.
Han, Y., Li, X., Zhang, Y., Han, Y., Chang, F., & Ding, J. (2019). Mesenchymal stem cells for regenerative medicine. Cells, 8(8), 886. https://doi.org/10.3390/cells8080886
Berebichez-Fridman, R., & Montero-Olvera, P. R. (2018). Sources and clinical applications of mesenchymal stem cells: State-of-the-art review. Sultan Qaboos University Medical Journal, 18(3), e264–e277. https://doi.org/10.18295/squmj.2018.18.03.002
Ullah, I., Subbarao, R. B., & Rho, G. J. (2015). Human mesenchymal stem cells—Current trends and future prospective. Bioscience Report, 35(2), e00191. https://doi.org/10.1042/BSR20150025
Gale, A. L., Linardi, R. L., McClung, G., Mammone, R. M., & Ortved, K. F. (2019). Comparison of the chondrogenic differentiation potential of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells. Frontiers in Veterinary Science, 6, 178. https://doi.org/10.3389/fvets.2019.00178
Li, N., Gao, J., Mi, L., Zhang, G., Zhang, L., Zhang, N., Huo, R., Hu, J., & Xu, K. (2020). Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Research & Therapy, 11(1), 381. https://doi.org/10.1186/s13287-020-01885-3
Keating, A. (2006). Mesenchymal stromal cells. Current Opinion in Hematology, 13(6), 419–425. https://doi.org/10.1097/01.moh.0000245697.54887.6f
Jeyaraman, M., Muthu, S., & Ganie, P. A. (2020). Does the source of mesenchymal stem cell have an effect in the management of osteoarthritis of the knee? Meta-analysis of randomized controlled trials. Cartilage, 1947603520951623. https://doi.org/10.1177/1947603520951623.
Wolfstadt, J. I., Cole, B. J., Ogilvie-Harris, D. J., Viswanathan, S., & Chahal, J. (2015). Current concepts: The role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health, 7(1), 38–44. https://doi.org/10.1177/1941738114529727
Hass, R., Kasper, C., Böhm, S., & Jacobs, R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling: CCS, 9, 12. https://doi.org/10.1186/1478-811X-9-12
Kim, H. J., & Im, G. I. (2009). Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: Greater doses of growth factor are necessary. Journal of Orthopaedic Research, 27(5), 612–619. https://doi.org/10.1002/jor.20766
Khalifeh Soltani, S., Forogh, B., Ahmadbeigi, N., Hadizadeh Kharazi, H., Fallahzadeh, K., Kashani, L., Karami, M., Kheyrollah, Y., & Vasei, M. (2019). Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy, 21(1), 54–63. https://doi.org/10.1016/j.jcyt.2018.11.003
Hsu, S. H., Huang, T. B., Cheng, S. J., Weng, S. Y., Tsai, C. L., Tseng, C. S., Chen, D. C., Liu, T. Y., Fu, K. Y., & Yen, B. L. (2011). Chondrogenesis from human placenta-derived mesenchymal stem cells in three-dimensional scaffolds for cartilage tissue engineering. Tissue Engineering Part A, 17(11–12), 1549–1560. https://doi.org/10.1089/ten.TEA.2010.0419
De Bari, C., Dell’Accio, F., Tylzanowski, P., & Luyten, F. P. (2001). Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism, 44(8), 1928–1942. https://doi.org/10.1002/1529-0131(200108)44:8%3c1928::AID-ART331%3e3.0.CO;2-P
Fan, J., Varshney, R. R., Ren, L., Cai, D., & Wang, D. A. (2009). Synovium-derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Engineering. Part B, Reviews, 15(1), 75–86. https://doi.org/10.1089/ten.teb.2008.0586
Glenn, J. D., & Whartenby, K. A. (2014). Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World Journal of Stem Cells., 6(5), 526–539. https://doi.org/10.4252/wjsc.v6.i5.526
Spees, J. L., Lee, R. H., & Gregory, C. A. (2016). Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Research and Therapy, 7(1), 125. https://doi.org/10.1186/s13287-016-0363-7
Caplan, A. I. (2009). Why are MSCs therapeutic? New data: New insight. Journal of Pathology, 217(2), 318–324. https://doi.org/10.1002/path.2469
Sakaguchi, Y., Sekiya, I., Yagishita, K., & Muneta, T. (2005). Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis and Rheumatism, 52(8), 2521–2529. https://doi.org/10.1002/art.21212
Koga, H., Muneta, T., Ju, Y. J., Nagase, T., Nimura, A., Mochizuki, T., Ichinose, S., von der Mark, K., & Sekiya, I. (2007). Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells, 25(3), 689–696. https://doi.org/10.1634/stemcells.2006-0281
De Bari, C., Dell’Accio, F., Karystinou, A., Guillot, P. V., Fisk, N. M., Jones, E. A., McGonagle, D., Khan, I. M., Archer, C. W., Mitsiadis, T. A., Donaldson, A. N., Luyten, F. P., & Pitzalis, C. (2008). A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis and Rheumatism, 58(1), 240–250. https://doi.org/10.1002/art.23143
Yoshimura, H., Muneta, T., Nimura, A., Yokoyama, A., Koga, H., & Sekiya, I. (2007). Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and Tissue Research, 327(3), 449–462. https://doi.org/10.1007/s00441-006-0308-z
Nimura, A., Muneta, T., Koga, H., Mochizuki, T., Suzuki, K., Makino, H., Umezawa, A., & Sekiya, I. (2008). Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis and Rheumatism, 58(2), 501–510. https://doi.org/10.1002/art.23219
Tateishi, K., Ando, W., Higuchi, C., Hart, D. A., Hashimoto, J., Nakata, K., Yoshikawa, H., & Nakamura, N. (2008). Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: Potential feasibility for clinical applications. Cell Transplantation, 17(5), 549–557. https://doi.org/10.3727/096368908785096024
Shirasawa, S., Sekiya, I., Sakaguchi, Y., Yagishita, K., Ichinose, S., & Muneta, T. (2006). In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. Journal of Cellular Biochemistry, 97(1), 84–97. https://doi.org/10.1002/jcb.20546
Jones, E. A., Crawford, A., English, A., Henshaw, K., Mundy, J., Corscadden, D., Chapman, T., Emery, P., Hatton, P., & McGonagle, D. (2008). Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis and Rheumatism, 58(6), 1731–1740. https://doi.org/10.1002/art.23485
Sekiya, I., Ojima, M., Suzuki, S., Yamaga, M., Horie, M., Koga, H., Tsuji, K., Miyaguchi, K., Ogishima, S., Tanaka, H., & Muneta, T. (2012). Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. Journal of Orthopaedic Research, 30(6), 943–949. https://doi.org/10.1002/jor.22029
Jones, E. A., English, A., Henshaw, K., Kinsey, S. E., Markham, A. F., Emery, P., & McGonagle, D. (2004). Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis and Rheumatism, 50(3), 817–827. https://doi.org/10.1002/art.20203
Revell, P. A., Al-Saffar, N., Fish, S., & Osei, D. (1995). Extracellular matrix of the synovial intimal cell layer. Annals of the Rheumatic Diseases, 54(5), 404–407. https://doi.org/10.1136/ard.54.5.404
Neybecker, P., Henrionnet, C., Pape, E., Grossin, L., Mainard, D., Galois, L., Loeuille, D., Gillet, P., & Pinzano, A. (2020). Respective stemness and chondrogenic potential of mesenchymal stem cells isolated from human bone marrow, synovial membrane, and synovial fluid. Stem Cell Research and Therapy, 11(1), 316. https://doi.org/10.1186/s13287-020-01786-5
Mizuno, M., Katano, H., Mabuchi, Y., Ogata, Y., Ichinose, S., Fujii, S., Otabe, K., Komori, K., Ozeki, N., Koga, H., Tsuji, K., Akazawa, C., Muneta, T., & Sekiya, I. (2018). Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Research and Therapy, 9(1), 123. https://doi.org/10.1186/s13287-018-0870-9
Hatsushika, D., Muneta, T., Nakamura, T., Horie, M., Koga, H., Nakagawa, Y., et al. (2014). Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis and Cartilage, 22(7), 941–950. https://doi.org/10.1016/j.joca.2014.04.028
Futami, I., Ishijima, M., Kaneko, H., Tsuji, K., Ichikawa-Tomikawa, N., Sadatsuki, R., Muneta, T., Arikawa-Hirasawa, E., Sekiya, I., & Kaneko, K. (2012). Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS ONE, 7(9), e45517. https://doi.org/10.1371/journal.pone.0045517
Kaplan, J. M., Youd, M. E., & Lodie, T. A. (2011). Immunomodulatory activity of mesenchymal stem cells. Current Stem Cell Research and Therapy, 6(4), 297–316. https://doi.org/10.2174/157488811797904353
Herrero, C., & Pérez-Simón, J. A. (2010). Immunomodulatory effect of mesenchymal stem cells. Brazilian Journal of Medical and Biological Research, 43(5), 425–430. https://doi.org/10.1590/s0100-879x2010007500033
Zhao, X., Zhao, Y., Sun, X., Xing, Y., Wang, X., & Yang, Q. (2020). Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Frontiers in Bioengineering and Biotechnology, 8, 575057. https://doi.org/10.3389/fbioe.2020.575057
Noronha, N. C., Mizukami, A., Caliári-Oliveira, C., Cominal, J. G., Rocha, J. L. M., Covas, D. T., Swiech, K., & Malmegrim, K. C. R. (2019). Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Research and Therapy, 10(1), 131. https://doi.org/10.1186/s13287-019-1224-y.Erratum.In:StemCellResTher.2019;10(1):132
Wang, M., Yuan, Q., & Xie, L. (2018). Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells International, 2018, 3057624. https://doi.org/10.1155/2018/3057624
Bochev, I., Elmadjian, G., Kyurkchiev, D., Tzvetanov, L., Altankova, I., Tivchev, P., & Kyurkchiev, S. (2008). Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biology International, 32(4), 384–393. https://doi.org/10.1016/j.cellbi.2007.12.007
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822. https://doi.org/10.1182/blood-2004-04-1559
Spaggiari, G. M., Capobianco, A., Becchetti, S., Mingari, M. C., & Moretta, L. (2006). Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood, 107(4), 1484–1490. https://doi.org/10.1182/blood-2005-07-2775
Sotiropoulou, P. A., Perez, S. A., Gritzapis, A. D., Baxevanis, C. N., & Papamichail, M. (2006). Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells, 24(1), 74–85. https://doi.org/10.1634/stemcells.2004-0359
Gao, F., Chiu, S. M., Motan, D. A., Zhang, Z., Chen, L., Ji, H. L., Tse, H. F., Fu, Q. L., & Lian, Q. (2016). Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death and Disease, 7(1), e2062. https://doi.org/10.1038/cddis.2015.327
Bifari, F., Lisi, V., Mimiola, E., Pasini, A., & Krampera, M. (2008). Immune modulation by mesenchymal stem cells. Transfusion Medicine and Hemotherapy, 35(3), 194–204. https://doi.org/10.1159/000128968
Ozeki, N., Muneta, T., Koga, H., Nakagawa, Y., Mizuno, M., Tsuji, K., Mabuchi, Y., Akazawa, C., Kobayashi, E., Matsumoto, K., Futamura, K., Saito, T., & Sekiya, I. (2016). Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage, 24(6), 1061–1070. https://doi.org/10.1016/j.joca.2015.12.018
Murata, Y., Uchida, S., Utsunomiya, H., Hatakeyama, A., Nakashima, H., Chang, A., Sekiya, I., & Sakai, A. (2018). Synovial mesenchymal stem cells derived from the cotyloid fossa synovium have higher self-renewal and differentiation potential than those from the paralabral synovium in the hip joint. American Journal of Sports Medicine, 46(12), 2942–2953. https://doi.org/10.1177/0363546518794664
Utsunomiya, H., Uchida, S., Sekiya, I., Sakai, A., Moridera, K., & Nakamura, T. (2013). Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. American Journal of Sports Medicine, 41(3), 657–668. https://doi.org/10.1177/0363546512473269
Fernandes, T. L., Kimura, H. A., Pinheiro, C. C. G., Shimomura, K., Nakamura, N., Ferreira, J. R., Gomoll, A. H., Hernandez, A. J., & Bueno, D. F. (2018). Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Engineering. Part C, Methods, 24(12), 709–716. https://doi.org/10.1089/ten.TEC.2018.0219
Li, J., Huang, Y., Song, J., Li, X., Zhang, X., Zhou, Z., Chen, D., Ma, P. X., Peng, W., Wang, W., & Zhou, G. (2018). Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomaterialia, 79, 202–215. https://doi.org/10.1016/j.actbio.2018.08.029
Suzuki, S., Mizuno, M., Sakamaki, Y., et al. (2020). Morphological changes in synovial mesenchymal stem cells during their adhesion to the meniscus. Laboratory Investigation, 100, 916–927. https://doi.org/10.1038/s41374-020-0421-8
Shimomura, K., Yasui, Y., Koizumi, K., Chijimatsu, R., Hart, D. A., Yonetani, Y., et al. (2018). First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of knee chondral lesions. American Journal of Sports Medicine, 46, 2384–2393. https://doi.org/10.1177/0363546518781825
Zeng, N., Yan, Z. P., Chen, X. Y., & Ni, G. X. (2020). Infrapatellar fat pad and knee osteoarthritis. Aging and Disease, 11(5), 1317–1328. https://doi.org/10.14336/AD.2019.1116
Favero, M., El-Hadi, H., Belluzzi, E., Granzotto, M., Porzionato, A., Sarasin, G., Rambaldo, A., Iacobellis, C., Cigolotti, A., Fontanella, C. G., Natali, A., Ramonda, R., Ruggieri, P., De Caro, R., Vettor, R., Rossato, M., & Macchi, V. (2017). Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology (Oxford), 56(10), 1784–1793. https://doi.org/10.1093/rheumatology/kex287
Eymard, F., Pigenet, A., Citadelle, D., Tordjman, J., Foucher, L., Rose, C., Flouzat Lachaniette, C. H., Rouault, C., Clément, K., Berenbaum, F., Chevalier, X., & Houard, X. (2017). Knee and hip intra-articular adipose tissues (IAATs) compared with autologous subcutaneous adipose tissue: A specific phenotype for a central player in osteoarthritis. Annals of the Rheumatic Diseases, 76(6), 1142–1148. https://doi.org/10.1136/annrheumdis-2016-210478
Belluzzi, E., Stocco, E., Pozzuoli, A., Granzotto, M., Porzionato, A., Vettor, R., De Caro, R., Ruggieri, P., Ramonda, R., Rossato, M., Favero, M., & Macchi, V. (2019). Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed Research International, 2019, 6390182. https://doi.org/10.1155/2019/6390182
Greif, D. N., Kouroupis, D., Murdock, C. J., Griswold, A. J., Kaplan, L. D., Best, T. M., & Correa, D. (2020). Infrapatellar fat pad/synovium complex in early-stage knee osteoarthritis: potential new target and source of therapeutic mesenchymal stem/stromal cells. Frontiers in Bioengineering and Biotechnology, 8, 860. https://doi.org/10.3389/fbioe.2020.00860
Jiang, L. F., Fang, J. H., & Wu, L. D. (2019). Role of infrapatellar fat pad in pathological process of knee osteoarthritis: Future applications in treatment. World Journal of Clinical Cases, 7(16), 2134–2142. https://doi.org/10.12998/wjcc.v7.i16.2134
Archer, C. W., Dowthwaite, G. P., & Francis-West, P. (2003). Development of synovial joints. Birth Defects Research. Part C, Embryo Today, 69(2), 144–155. https://doi.org/10.1002/bdrc.10015
Shintani, N., Kurth, T., & Hunziker, E. B. (2007). Expression of cartilage-related genes in bovine synovial tissue. Journal of Orthopaedic Research, 25(6), 813–819. https://doi.org/10.1002/jor.20345
Otero, M., & Goldring, M. B. (2007). Cells of the synovium in rheumatoid arthritis. Chondrocytes. Arthritis Research Therapy, 9(5), 220. https://doi.org/10.1186/ar2292 Erratum in Arthritis Research Therapy 2008;10(1):401.
Pei, M., Luo, J., & Chen, Q. (2008). Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthritis Cartilage, 16(9), 1110–1117. https://doi.org/10.1016/j.joca.2007.12.011
Kangari, P., Talaei-Khozani, T., Razeghian-Jahromi, I., & Razmkhah, M. (2020). Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Research and Therapy, 11(1), 492. https://doi.org/10.1186/s13287-020-02001-1
Loebel, C., & Burdick, J. A. (2018). Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell, 22(3), 325–339. https://doi.org/10.1016/j.stem.2018.01.014
Bobick, B. E., Chen, F. H., Le, A. M., & Tuan, R. S. (2009). Regulation of the chondrogenic phenotype in culture. Birth Defects Research. Part C, Embryo Today, 87(4), 351–371. https://doi.org/10.1002/bdrc.20167
Archer, C. W., & Francis-West, P. (2003). The chondrocyte. International Journal of Biochemistry and Cell Biology, 35(4), 401–404. https://doi.org/10.1016/s1357-2725(02)00301-1
Kim, Y. J., Kim, H. J., & Im, G. I. (2008). PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochemical and Biophysical Research Communications, 373(1), 104–108. https://doi.org/10.1016/j.bbrc.2008.05.183
Lefebvre, V., & Dvir-Ginzberg, M. (2017). SOX9 and the many facets of its regulation in the chondrocyte lineage. Connective Tissue Research, 58(1), 2–14. https://doi.org/10.1080/03008207.2016.1183667
Kozhemyakina, E., Lassar, A. B., & Zelzer, E. (2015). A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development, 142(5), 817–831. https://doi.org/10.1242/dev.105536
Goldring, M. B., Tsuchimochi, K., & Ijiri, K. (2006). The control of chondrogenesis. Journal of Cellular Biochemistry, 97(1), 33–44. https://doi.org/10.1002/jcb.20652
Cancedda, R., Castagnola, P., Cancedda, F. D., Dozin, B., & Quarto, R. (2000). Developmental control of chondrogenesis and osteogenesis. International Journal of Developmental Biology, 44(6), 707–714.
Yoon, Y. M., Oh, C. D., Kang, S. S., & Chun, J. S. (2000). Protein kinase A regulates chondrogenesis of mesenchymal cells at the post-precartilage condensation stage via protein kinase C-alpha signaling. Journal of Bone and Mineral Research, 15(11), 2197–2205. https://doi.org/10.1359/jbmr.2000.15.11.2197
Bobick, B. E., & Kulyk, W. M. (2008). Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Research. Part C, Embryo Today, 84(2), 131–154. https://doi.org/10.1002/bdrc.20126
De Bari, C., Dell’Accio, F., Vandenabeele, F., Vermeesch, J. R., Raymackers, J. M., & Luyten, F. P. (2003). Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. Journal of Cell Biology, 160(6), 909–918. https://doi.org/10.1083/jcb.200212064
Mochizuki, T., Muneta, T., Sakaguchi, Y., Nimura, A., Yokoyama, A., Koga, H., & Sekiya, I. (2006). Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis and Rheumatism, 54(3), 843–853. https://doi.org/10.1002/art.21651
Koga, H., Muneta, T., Nagase, T., Nimura, A., Ju, Y. J., Mochizuki, T., & Sekiya, I. (2008). Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell and Tissue Research, 333(2), 207–215. https://doi.org/10.1007/s00441-008-0633-5
Shi, Y., & Massagué, J. (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell, 113(6), 685–700. https://doi.org/10.1016/s0092-8674(03)00432-x
Miyamoto, C., Matsumoto, T., Sakimura, K., & Shindo, H. (2007). Osteogenic protein-1 with transforming growth factor-beta1: Potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. Journal of Orthopaedic Science, 12(6), 555–561. https://doi.org/10.1007/s00776-007-1176-4
Nishimura, K., Solchaga, L. A., Caplan, A. I., Yoo, J. U., Goldberg, V. M., & Johnstone, B. (1999). Chondroprogenitor cells of synovial tissue. Arthritis and Rheumatism, 42(12), 2631–2637. https://doi.org/10.1002/1529-0131(199912)42:12%3c2631::AID-ANR18%3e3.0.CO;2-H
Pei, M., He, F., & Vunjak-Novakovic, G. (2008). Synovium-derived stem cell-based chondrogenesis. Differentiation, 76(10), 1044–1056. https://doi.org/10.1111/j.1432-0436.2008.00299.x
Sakimura, K., Matsumoto, T., Miyamoto, C., Osaki, M., & Shindo, H. (2006). Effects of insulin-like growth factor I on transforming growth factor beta1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells, Tissues, Organs, 183(2), 55–61. https://doi.org/10.1159/000095509
Shintani, N., & Hunziker, E. B. (2007). Chondrogenic differentiation of bovine synovium: bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis and Rheumatism, 56(6), 1869–1879. https://doi.org/10.1002/art.22701
Schmal, H., Kowal, J. M., Kassem, M., Seidenstuecker, M., Bernstein, A., Böttiger, K., Xiong, T., Südkamp, N. P., & Kubosch, E. J. (2018). Comparison of regenerative tissue quality following matrix-associated cell implantation using amplified chondrocytes compared to synovium-derived stem cells in a rabbit model for cartilage lesions. Stem Cells International, 2018, 4142031. https://doi.org/10.1155/2018/4142031
Pei, M., He, F., Boyce, B. M., & Kish, V. L. (2009). Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis Cartilage, 17(6), 714–722. https://doi.org/10.1016/j.joca.2008.11.017
Li, H., Qian, J., Chen, J., Zhong, K., & Chen, S. (2016). Osteochondral repair with synovial membrane-derived mesenchymal stem cells. Molecular Medicine Reports, 13(3), 2071–2077. https://doi.org/10.3892/mmr.2016.4795
Lee, J. C., Min, H. J., Park, H. J., Lee, S., Seong, S. C., & Lee, M. C. (2013). Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy, 29(6), 1034–1046. https://doi.org/10.1016/j.arthro.2013.02.026
Shimomura, K., Moriguchi, Y., Ando, W., Nansai, R., Fujie, H., Hart, D. A., Gobbi, A., Kita, K., Horibe, S., Shino, K., Yoshikawa, H., & Nakamura, N. (2014). Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Engineering Part A, 20(17–18), 2291–2304. https://doi.org/10.1089/ten.tea.2013.0414
To, K., Zhang, B., Romain, K., Mak, C., & Khan, W. (2019). Synovium-Derived mesenchymal stem cell transplantation in cartilage regeneration: A PRISMA review of in vivo studies. Frontiers in Bioengineering and Biotechnology, 7, 314. https://doi.org/10.3389/fbioe.2019.00314
Sekiya, I., Muneta, T., Horie, M., & Koga, H. (2015). Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clinical Orthopaedics and Related Research, 473(7), 2316–2326. https://doi.org/10.1007/s11999-015-4324-8
Kubosch, E. J., Lang, G., Furst, D., Kubosch, D., Izadpanah, K., Rolauffs, B., Sudkamp, N. P., & Schmal, H. (2018). The potential for synovium-derived stem cells in cartilage repair. Current Stem Cell Research and Therapy, 13(3), 174–184. https://doi.org/10.2174/1574888X12666171002111026
Kohno, Y., Mizuno, M., Ozeki, N., Katano, H., Komori, K., Fujii, S., Otabe, K., Horie, M., Koga, H., Tsuji, K., Matsumoto, M., Kaneko, H., Takazawa, Y., Muneta, T., & Sekiya, I. (2017). Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Research and Therapy, 8(1), 115. https://doi.org/10.1186/s13287-017-0572-8
Kubosch, E. J., Heidt, E., Niemeyer, P., Bernstein, A., Südkamp, N. P., & Schmal, H. (2017). In-vitro chondrogenic potential of synovial stem cells and chondrocytes allocated for autologous chondrocyte implantation—A comparison: Synovial stem cells as an alternative cell source for autologous chondrocyte implantation. International Orthopaedics, 41(5), 991–998. https://doi.org/10.1007/s00264-017-3400-y
Zhu, Y., Wang, Y., Zhao, B., Niu, X., Hu, B., Li, Q., Zhang, J., Ding, J., Chen, Y., & Wang, Y. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research and Therapy, 8(1), 64. https://doi.org/10.1186/s13287-017-0510-9
Chung, C., & Burdick, J. A. (2008). Engineering cartilage tissue. Advanced Drug Delivery Reviews, 60(2), 243–262. https://doi.org/10.1016/j.addr.2007.08.027
Pei, M., He, F., Kish, V. L., & Vunjak-Novakovic, G. (2008). Engineering of functional cartilage tissue using stem cells from synovial lining: A preliminary study. Clinical Orthopaedics and Related Research, 466(8), 1880–1889. https://doi.org/10.1007/s11999-008-0316-2
Ando, W., Tateishi, K., Hart, D. A., Katakai, D., Tanaka, Y., Nakata, K., Hashimoto, J., Fujie, H., Shino, K., Yoshikawa, H., & Nakamura, N. (2007). Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials, 28(36), 5462–5470. https://doi.org/10.1016/j.biomaterials.2007.08.030
Ando, W., Tateishi, K., Katakai, D., Hart, D. A., Higuchi, C., Nakata, K., Hashimoto, J., Fujie, H., Shino, K., Yoshikawa, H., & Nakamura, N. (2008). In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: Biological and mechanical properties and further chondrogenic potential. Tissue Engineering Part A, 14(12), 2041–2049. https://doi.org/10.1089/ten.tea.2008.0015
Koga, H., Shimaya, M., Muneta, T., Nimura, A., Morito, T., Hayashi, M., Suzuki, S., Ju, Y. J., Mochizuki, T., & Sekiya, I. (2008). Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Research and Therapy, 10(4), R84. https://doi.org/10.1186/ar2460
Sakao, K., Takahashi, K. A., Arai, Y., Inoue, A., Tonomura, H., Saito, M., Yamamoto, T., Kanamura, N., Imanishi, J., Mazda, O., & Kubo, T. (2008). Induction of chondrogenic phenotype in synovium-derived progenitor cells by intermittent hydrostatic pressure. Osteoarthritis Cartilage, 16(7), 805–814. https://doi.org/10.1016/j.joca.2007.10.021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeyaraman, M., Muthu, S., Jeyaraman, N. et al. Synovium Derived Mesenchymal Stromal Cells (Sy-MSCs): A Promising Therapeutic Paradigm in the Management of Knee Osteoarthritis . JOIO 56, 1–15 (2022). https://doi.org/10.1007/s43465-021-00439-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-021-00439-w