Abstract
This study was dedicated to the detailed characterization of the microstructural changes and the phase analysis of the interfaces formed in explosive welding of multilayered Ti Gr.1/A1050 clads. The significant effect of the detonation source localization on the microstructure of the welded materials interfaces after collision, and consequently on the diffusion processes induced by the elevated temperature was showed. Annealing process at 550 °C for series of time intervals of the Ti/Al clad allowed to determine the growth mechanism of the Al3Ti phase formed along particular interfaces. Moreover, the microstructure observations and calculations evidenced different growth mechanisms related to the localization of the Ti/Al interface in respect to the detonation source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Intermetallic phases of the Al–Ti system have received considerable attention due to their moderate price, high strength at low density, and chemical resistance at elevated temperatures, making them attractive for aerospace, missile, chemical, and mechanical engineering [1]. Among several phases, the Al3Ti phase draws attention as the one with the lowest density, highest specific stiffness, and oxidation resistance [2]. However, an obvious disadvantage of Al3Ti that limits its use is the brittleness at room temperature.
Explosive welding (EXW) is the joining technique that enables permanent bonding between aluminum and titanium sheets. The quality, durability, morphology, and microstructure of the weld largely depend on the welding process parameters (stand-off distance, collision angle, type, and amount of the explosive). Due to the extreme process conditions (high pressure and temperature), other phases may also be formed at the interface areas, such as: Al2Ti, AlTi, and the metastable AlTi2. However, the degree of complexity increases if a multilayered Ti/Al composite is considered. This study is dedicated to reveal whether and how the localization of the interfaces in respect to the detonation source influences their morphology, microstructure, and most importantly changes aroused due to the annealing. According to the literature, Al3Ti phase is one that grows at the interface of the weld during further annealing. This is associated with the lowest energy of the interface; Al3Ti nucleation at the Ti/Al interface is of 450 mJ/m2 (compared to 480 mJ/m2 for Al2Ti, 534 mJ/m2 for AlTi and 598 mJ/m2 for AlTi3) [3]. This stays in agreement when considering the work of van Loo and Rieck [4], where the Ti/Al3Ti/AlTi/Al2Ti/Al3Ti/Al diffusion couple was annealed at 625 °C for 15 h, resulting in the diffusion and formation of the Ti/Al3Ti/Al phase system, while the remaining intermetallic phases completely disappeared.
The reference [5], showing the SEM/EDS microstructure observations of the 1st, 7th, and 14th interfaces created in explosively welded fifteen-layer Ti Gr.1/A1050 set, may serve as the starting point of this study. However, in [5], the multi-layered Ti/Al clads were annealed at a temperature of 600 °C, and using additional pressure of 44 MPa, for the set of intervals, to describe the Al3Ti phase growth kinetics. However, authors focused only at the increase of the intermetallic Al3Ti layer formed at one selected interface (7th), omitting other interfaces. By lowering the annealing temperature from 600 to 550 °C, significant practical and economical advantages for the production line can be achieved. In this way, our study compared to the previously reported one [5] fulfills a literature gap by revealing possible advantage of lowering of the annealing temperature. Moreover, we intend to present how the growth kinetics varies with respect to the localization of the interface (top, middle and bottom side of the multi-layered set). The annealing time in this study is extended up to 100 h, to predict the possible microstructure transformations, as the diffusion phenomena will continuously take place during working of the final elements, especially when subjected to the elevated temperatures. In our study, we have also increased the accuracy of calculations, by taking into account set of eight annealing times, same as in [6], where only bilayered Ti/Al welds were subjected to the same annealing regimes. This allowed to determine the growth mechanism of the Al3Ti phase at each of the studied interface zone.
2 Materials and methods
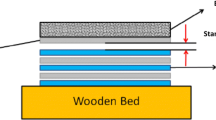
Multilayered Ti/Al weld consisted of eight sheets of Ti Gr. 1 (hereafter Ti) and seven sheets of A1050 alloy (hereafter Al) was obtained in explosive welding process using detonation velocity of 2320 m/s and 1 mm stand-off distance in Explomet company. The dimensions of the sheets to be joined were 210 × 300 mm2 and their thickness was of 1 mm, while their chemical compositions are given in Table 1. The sheets were welded in one shot to form a fifteen-layer plate composed of alternating Ti and Al sheets, with Ti as the top and bottom layer.
The tested specimens were cut from bonded sheets, and their surfaces were prepared by the traditional method of metallographic specimen preparation. The surfaces of the samples for microstructure studies were mechanically grinded with sandpapers and polished using silica suspension. Explosively welded Ti/Al clads were subjected to annealing for 1.5, 2, 4, 5, 11, 40, 64, and 100 h at 550 °C. Observations before and after annealing using scanning electron microscopy (SEM) were performed on FEI ESEM XL30, FEI Quanta 3D FEG Dual Beam and ThermoFisher Scios 2 FEG Dual Beam microscopes in backscattered electron mode. SEM studies allowed the analysis of chemical composition changes of the interface zone by energy-dispersive X-ray spectroscopy (EDAX EDS). FEI Tecnai G2 and ThermoFisher Themis G200 FEG microscopes were used for nanoscale observations by transmission electron microscopy (TEM). Images were recorded in bright field (BF) mode, selected areas of electron diffraction (SAED), and EDS maps, respectively. Thin foils for TEM observations were cut with a focused gallium ion beam (FIB) instrument using a ThermoFisher Scios 2 FEG Dual Beam microscope equipped with an EasyLift system.
3 Results and discussion
3.1 Microstructure of explosively welded 15-layered Ti Gr.1/A1050 alloys clads
The series of microstructural observations of the interfaces formed by explosive welding of 15-layered clads of technically pure aluminum (A1050) and technically pure titanium (Ti Gr. 1) was performed. A tendency of a wavy morphology formation of the interfaces located in the closest neighborhood to the detonation source was observed (Fig. 1a). Hokamoto et al. [7] have already revealed that the amplitude of the wave linearly depends on the kinetic energy loss resulting from the collision of the sheets (ΔKE). In the case of multilayer welding in a single shot act, ΔKE decreases rapidly with each collision, leading to flattening of the interface. In addition, it should be noted that the remelting located at the waves was characterized by varying contrast, confirming the intense mixing of the components of the joined sheets (Fig. 1b). Analysis of the chemical composition in these areas revealed the possible presence of AlTi3 and AlTi intermetallic phases. However, SEM/EDS analysis should be treated with a great skepticism due to the spatial resolution of this method, and thus the high probability of collecting signal from the adjacent areas and discovering the presence of phases that are not actually present in the welded zone. From this reason, the composition and microstructure of the area of the apparent sharp Ti/Al interface located outside the melt (marked with the rectangle in Fig. 1a) were carried out with transmission electron microscopy. They evidenced the presence of mainly AlTi3 and AlTi intermetallic phases and Al3Ti phases (Fig. 1c), which was not detected by SEM.
Microstructures of the interface formed between the first (top) titanium plate (Ti1) and the second aluminum plate (Al2): an SEM–BSE image showing the wavy bond morphology (a) along with the remelting zone (b), and a TEM microstructure of the apparently sharp interface (c) taken from the area marked in (a)
It should be emphasized here that the analysis of the interfaces in multilayer Ti/Al sheets has been the subject of several studies; however, the attention was primarily drawn to the difference in their interface morphology. The authors of [8, 9] observed that in the welds with two Ti/Al interfaces there was a noticeable tendency to create a wavy morphology near the detonation source and a flat morphology at a distance further away. The simplest multilayer system of Ti/Al bond, whose phase composition of the interfaces was studied precisely in terms of differences depending on their location from the source of detonation, was a three-layer set combined of pure technical aluminum and titanium (A1050/TiGr.1/A1050), same materials like in this case. The use of synchrotron X-rays made it possible to collect global information on the phase composition in the state immediately after explosive welding of each interface zone. Diffraction rings that corresponded to the intermetallic phases were AlTi3, AlTi, and Al3Ti identified at both interfaces, but the presence of the Al2Ti phase was confirmed only at the interface located closer to the detonation source [8]. Therefore, it is worth further tracing, using the studied example of 15-layer Ti/Al clads, whether differences in phase composition were noted depending on the location in relation to the detonation source.
The second interface, on the other hand, was characterized by a microstructure completely different from the first one (Fig. 2a). There was a sharp change in the morphology of the interface—it was flat with a relatively wide (20–30 µm) zone of the remelting in the form of a continuous layer. In addition, single cracks located perpendicular to the interface plane were present in the remelting area, which did not propagate beyond the remelting area. Analysis of the chemical composition by SEM/EDS of the remelting layer showed that its average chemical composition (taken from the area) was as follows: 86.3at.% Al and 13.7at.% Ti. Comparing the microstructure and chemical composition of the second interface to the ones in the explosively welded three-layered A1050/TiGr.1/A1050 clads and described in [8], it can be noticed that a broad remelted layer with a similar microstructure was observed in the upper interface of the weld, a situation where, as in the case shown in Fig. 2, the aluminum plate was shot onto the titanium plate. In the case described in the work of Fronczek and co-authors [6], the phase composition of the remelted layer (TEM/EDS) showed a predominant proportion of comminuted aluminum grains and trace amounts of three intermetallic phases: AlTi3, AlTi and Al2Ti. In the case described here, the phase composition of the second interface (Al2/Ti3) was also examined by transmission electron microscopy, which showed the presence of phases: AlTi and Al2Ti, while it gave an inconclusive answer as to which phase of Al3Ti or AlTi3 (or both) is present in the remelting zone (Fig. 3).
Microstructures of the interface formed between the second aluminum plate (Al2) and the third titanium plate (Ti3): STEM image highlighting the presence of fine grains along with Al and Ti distribution maps. Below, TEM microstructure in bright field with the area from which electron diffractions were recorded. The results of the measured diffraction ring distances are summarized in Table 2, along with a list of possible intermetallic phases
The following interfaces had a similar microstructure—for example, Fig. 4 shows the 3rd, 4th, 7th, and 8th interfaces, counting them from the top-located detonation source.
SEM–BSE microstructures of the interfaces formed between: the third titanium plate (Ti3) and the fourth aluminum plate (Al4) (a), the fourth aluminum plate (Al4) and the fifth titanium plate (Ti5) (b), the seventh titanium plate (Ti7) and the eighth aluminum plate (Al8) (c), and the eighth aluminum plate (Al8) and the ninth titanium plate (Ti9) (d)
Each of these interfaces were characterized by the presence of a remelted layer, the width of which varied. It should be noted that the thinnest thicknesses of the remelted layers were observed for the middle interfaces. From the seventh interface (Fig. 4c), a thin lamella was taken to identify the phases using selected electron diffraction pattern in a transmission electron microscope (Fig. 5). Most reflections could be attributed to the Al3Ti phase, however, the presence of AlTi, Al2Ti and AlTi3 phases were not excluded.
Comparing the microstructures of the lower interfaces (Fig. 6), they are similar (Fig. 6b–d), with a visible thin melting zone. Observations made with a transmission electron microscope (Fig. 7) were conducted for the last interface (Al14/Ti15) being the thinnest one, where the AlTi3 and Al2Ti phases have been identified. Existence of Al3Ti phase is also possible as its measured distances on electron diffractograms coincide with some of the distances characteristic for the AlTi3 phase.
SEM–BSE microstructures of the interfaces formed between: the eleventh titanium plate (Ti11) and the twelfth aluminum plate (Al12) (a), the twelfth aluminum plate (Al12) and the thirteenth titanium plate (Ti13) (b), the thirteenth titanium plate (Ti13) and the fourteenth aluminum plate (Al14) (c), and the fourteenth aluminum plate (Al14) and the fifteenth titanium plate (Ti15) (d)
STEM microstructures recorded for the lamellas taken from the remelting zones located at the second, seventh and fourteenth interfaces are compared in Fig. 8, where aluminum and titanium distribution maps are also included. As can be seen, the large number of fine grains was present at the second interface, while a much smaller amount was observed within the middle interface. It can be related to the fact that closer to the detonation source the more drastic pressure/temperature condition. Also, the heat dissipation from the second interface is much faster and therefore the grains remain small. On the other hand, the last interface, located farthest from the explosive charge, was characterized by a very thin melted zone, as there was a certain time when the temperature remained higher (slower heat dissipation to the free surface) and this allowed for the melted zone growth.
Table 3 compiles the values of the measured diffraction ring distances and the theoretical distances corresponding to the various intermetallic phases identified in the ring electron diffraction shown in Fig. 5.
Table 4 compiles the values of the measured diffraction ring distances and the theoretical distances corresponding to the various intermetallic phases identified in the electron diffraction shown in Fig. 7.
A set of explosively welded 10 layers of titanium and 11 layers of aluminum at a detonation velocity of 4200 m/s was described in [10]. The authors noted the difference in the morphology of the interface depending on its location relation to the detonation source—a pronounced waviness transitioned to a flat boundary on the bottom of the composite, nevertheless it should also be noted that even in the area of adjacent interfaces the mutual location of Ti and Al (interface 2 vs. 3 and 4 vs. 5) resulted in changes in morphology. Interestingly, despite the use of a similar detonation velocity as in work [11], where six alternating sheets of aluminum and titanium with initial thickness of about 0.5 mm were explosively welded together using 4500 m/s detonation velocity, in [10] the presence of no intermetallic phases was identified in the microstructure of the vortices—X-ray diffraction studies were confirmed by transmission electron microscopy, where the structure corresponded to a solid solution of titanium in aluminum. The absence of the intermetallic phases at the interface was explained by the high crystallization rate, which, according to some estimates [12], could reach 109 K/s. On the other hand, in the work of [13], 23-layer composites consisting of 12 sheets of aluminum and 11 clads of titanium were explosively welded. In this study, it was found that the most intense deformation was characterized by the material of the six–seven upper sheets, located closer to the explosive. The intense deformation of the material of the upper sheets is due to their wave shape and the presence of vortices near the crests and valleys of the waves. The geometrical parameters of the waves changed as they moved from the upper to the lower layers, with the length and amplitude of the waves becoming smaller.
3.2 Kinetics and mechanism of Al3Ti phase growth in 15-layered Ti/Al clads after annealing at different temperatures
At the Ti/Al interface formed between the first two layers after explosive welding and subjected to annealing for 1.5 h at 550 °C, a thin and continuous layer about 700 nm thick located at the apparently sharp interface was observed in addition to the remelting zones. This layer was not visible in the SEM directly after the explosive welding process, and only when observed with the aim of transmission electron microscopy. The experiment of annealing at 550 °C for different times made it possible to follow the transformation of the microstructure in the aforementioned thin layer, as shown in Fig. 9. It was characterized by a uniform contrast, and its chemical composition clearly indicated the Al3Ti phase. Measurements of the layer thickness showed that it varied from 700 nm after 1.5 h of annealing to 25 µm after 100 h of annealing (average values). The presence of aluminum-filled spaces between the growing grains of the Al3Ti phase after 5 h of annealing was also observed. After 100 h of annealing, some of the grains have merged into a continuous layer, but dark contrast spaces with high aluminum content are still visible in-between. A similar microstructure was observed in the work of [6] after annealing at 550 °C; however, in that case the thickness of the resulting layer was more than twice smaller (less than 10 µm). In contrast, the authors of the article [1] observed a much thicker remelted layer (more than 60 µm) at the Ti/Al diffusion couple interface heated at 550 °C for 36 h. However, such a large difference in thickness may be related to the initial thickness of the Al3Ti layer, which was already more than 6 μm for this system. In the case of the observed 15-layer Ti/Al weld, only TEM analyses of the first (Ti1/Al2) interface showed the presence of Al3Ti within a thin remelted interlayer (0.4 nm–1.5 µm thick).
SEM-BSE microstructures of the separation boundary formed between the first (top) titanium plate (Ti1) and the second aluminum plate (Al2), showing the growth of the Al3Ti intermetallic layer at the interface of the combined clads outside the remelting area after annealing at 550 °C for: 1.5 h (a), 2 h (b), 4 h (c), 5 h (d), 11 h (e), and 100 h (f)
On the other hand, the microstructural changes of the wide and continuous remelted layer formed at the second interface, i.e., formed between the second aluminum plate and the third titanium plate, are shown in Fig. 10. The remelted layer already after 1.5 h of annealing has a clearly different contrast, indicating the presence of Al3Ti phase grains and the surrounding aluminum. With annealing time, an increase in layer thickness was observed at each interface. At the same time, a dual morphology of the growing Al3Ti layer was noted—on the aluminum plate side, the phase grew in the form of fine, dispersed grains surrounded by aluminum; while on the titanium side, the grains were much larger. The proportion of each growing layer varied depending on its location relative to the explosion source, with the proportion of the fine-grained variety decreasing with increasing distance from the explosion source. Figure 11 shows a comparison of the morphology of the Al3Ti layer after annealing at 550 °C for 100 h depending on the location relation to the detonation source.
Annealing of the multilayered Ti/Al clads made it possible to determine the growth rate of the Al3Ti intermetallic phase at 550 °C for various time intervals taking into account the location of the interface in relation to the detonation source. Series of the annealing times were applied to increase the accuracy of determining the growth kinetics of the Al3Ti phase (Fig. 12). To determine the growth kinetics of the Al3Ti phase formation process, the thickness of the layer (x) at 1st, 7th and 14th interface after annealing time (t) was measured, using the following relationship: x = k*tn or ln Δx = n ln t + ln k, where n is the growth factor and k is the growth rate constant. The value of n is useful, when the diffusion mechanism is to be determined: the parabolic growth with volume diffusion (n = 0.5), much faster growth due to the reaction at the interface (n = 1), and using the fast diffusion pathways, such as grain boundaries (n < 0.5) [14,15,16,17,18,19,20]. In the studied system, differences were observed in the growth mechanism of the Al3Ti phase depending on its location relatively to the detonation source. At the first (Ti1/Al2) interface, the n-factor was of 0.85 suggesting that the dominant mechanism of phase growth is reaction at the interface. For the seventh (Ti7/Al8) and fourteenth (Al14/Ti15) interfaces, the determined n values took intermediate value, i.e., n = 0.48 and n = 0.57, which corresponds to phase growth controlled mainly by volume diffusion. These results should be confronted with the morphology of the Al3Ti phase at the individual interfaces. At the first interface, a predominance of relatively large, interconnected grains can be noticed. In contrast, the layers at the middle interfaces have a dual microstructure, consisting of compact large Al3Ti grains and small Al3Ti grains distributed within the Al matrix (see Fig. 11b–d). Calculations of the kinetics of the Al3Ti intermetallic phase described in [6] for A1050/Ti Gr. 2 samples at 550 °C indicated the existence of four stages of its growth. Incubation time of up to 1.5 h of annealing, growth controlled by chemical reaction (1.5–5 h) (n = 0.9 and k = 0.21 μm/hn), mixed mechanism of both chemical reaction and volume diffusion, and growth controlled by volume diffusion (36–100 h) (n = 0.48 and k = 1.05 μm/hn). Annealing at 550 °C of the Ti/Al diffusion multilayer pair was described also in [14], where a straight line was fitted to all points in the calculations and as a result the n value reached 0.49.
The differences between the results of the present work and those of Solecka et al. [21] is mainly because of the higher temperature of annealing used in the latter work, namely 625 °C. This temperature is very close to the melting temperature of aluminum (660 °C approx.). It is well known that at such high temperatures, diffusion of atoms will happen on a large scale and the material might also experience creep deformation based on the residual stresses left behind after the explosive welding process. Thus, a higher temperature is bound to result in higher growth rate of the Al3Ti layer on account of both higher diffusion and higher heat energy available for chemical reaction at the interface. Thus, it is quite natural to expect a higher layer thickness of Al3Ti phase in Ref [21] compared to the present work, which has used a much lower annealing temperature (550 °C). The above argument is also confirmed by seeing the trend of layer thickness obtained for a given annealing time for all the studies wherein the annealing temperature was maintained greater than or equal to 625 °C (namely [9,10,11] and [21]) in Fig. 13. However, the layer thickness for a given annealing time, obtained in the work [6] is much lower than those obtained in all the above-mentioned works, but it is comparable with the present work. Thus, it can be summed up that increasing the annealing temperature and annealing time results in considerable increase in the Al3Ti layer formed in the various interfaces of the multilayered Ti/Al explosive welds.
4 Conclusions
This study was dedicated to the fifteen-layered Ti Gr.1/A1050 multilayered composite formed in the explosive welding process. It comprised of the detailed characterization of the microstructure and the phase analysis of the aluminum/titanium interfaces after the welding process followed by their transformation arose due to the annealing at 550 °C. The following conclusions were formulated as a result of the series of comprehensive experiments:
-
The Ti/Al interface morphology is strongly limited to its localization in relation to the detonation source—wavy one decorated with melted zones in the closer distance and of flat shape in the far away distance. The flat shape morphology is less desirable due to possible cracking/delamination problems occurring when continuous melted layer occurs due to the diffusion at the elevated temperatures.
-
As a result of the welding process, variety of the intermetallic phases occurred: TiAl3, TiAl2, TiAl, and Ti3Al intermetallic phases, localized in the remelting areas at selected interfaces. Due to the local character of the TEM observations, the unequivocal occurrence of all phases in another area of remelting at selected boundaries cannot be ruled out.
-
Annealing process at 550 °C led to formation of a continuous Al3Ti diffusion layer along all interfaces, the width of which increased both with the annealing time and the distance from the detonation source.
-
Differences in the growth mechanism of the Al3Ti phase were observed depending on its location in relation to the detonation source. For the nearest to the detonation source interface, the dominant mechanism of phase growth was attributed to the reaction at the interface. While for the middle one (7th) and farthest (14th) interfaces, exponent coefficient values were 0.48 and 0.57, respectively, corresponding to the phase growth mainly by the volume diffusion.
-
Moreover, the prolonged time of annealing up to 100 h was implemented to completely describe the growth kinetics, and the growth mechanism of the Al3Ti phase at the individual interfaces to predict what microstructure changes can be expected with time, when the final product will be exposed to the higher temperature.
Data availability
The data analyzed during the current study will be available upon reasonable request.
References
Thiyaneshwaran N, Sivaprasad K, Ravisankar B. Nucleation and growth of TiAl3 intermetallic phase in diffusion bonded Ti/Al Metal Intermetallic Laminate. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-35247-0.
Himmler D, Randelzhofer P, Korner C. Formation kinetics and phase stability of in-situ Al3Ti particles in aluminium casting alloys with varying Si content. Results Mater. 2020. https://doi.org/10.1016/j.rinma.2020.100103.
Jiangping L, Yanqing S, Yanjin X, Liangshun L, Jingjie G, Hengzhi F. A first phase selection in solid Ti/Al diffusion couple. Rare Metal Mat Eng. 2011. https://doi.org/10.1016/S1875-5372(11)60031-3.
van Loo FJJ, Rieck GD. Diffusion in the titanium-aluminium system—I. Interdiffusion between solid Al and Ti or Ti-Al alloys Diffusion dans le systeme titane-aluminium—I. Interdiffusion a l’etat solide entre Al et Ti ou Al et les alliages Ti-AlDiffusion im system Titan-aluminium—I. Interdiffusion von festem Al mit Ti oder Ti-Al-Legierungen. Acta Metall. 1973;19:73. https://doi.org/10.1016/0001-6160(73)90220-4.
Petrzak P, Mania I, Paul H, Maj Ł, Gałka A. The kinetic of Al3Ti phase growth in explosively welded multilayered Al/Ti clads during annealing under load conditions. Arch Metall Mater. 2019. https://doi.org/10.24425/amm.2019.130125.
Fronczek DM, Wojewoda-Budka J, Chulist R, Sypien A, Korneva A, Szulc Z, Schel N, Zieba P. Structural properties of Ti/Al clads manufactured by explosive welding and annealing. Mater Des. 2016. https://doi.org/10.1016/j.matdes.2015.11.087.
Hokamoto K, Chiba A, Fujita M, Izuma T. Single-shot explosive welding technique for the fabrication of multilayered metal base composites: effect of welding parameters leading to optimum bonding condition. Compos Eng. 1995. https://doi.org/10.1016/0961-9526(95)00059-V.
Fronczek D, Chulist R, Litynska-Dobrzynska L, Lopez GA, Wierzbicka-Miernik A, Schell N, Szulc Z, Wojewoda-Budka J. Microstructural and phase composition differences across the interfaces in Al/Ti/Al explosively welded clads. Metall Mater Trans A. 2017. https://doi.org/10.1007/s11661-017-4169-8.
Fronczek D, Chulist R, Szulc Z, Wojewoda-Budka J. Growth kinetics of TiAl3 phase in annealed Al/Ti/Al explosively welded clads. Mater Lett. 2017. https://doi.org/10.1016/j.matlet.2017.04.025.
Bataev IA, Bataev AA, Mali VI, Pavliukova DV. Structural and mechanical properties of metallic–intermetallic laminate composites produced by explosive welding and annealing. Mater Des. 2012;15:10. https://doi.org/10.1016/j.matdes.2011.09.030.
Foadian F, Soltanieh M, Adeli M, Etminanbakhsh M. A study on the formation of intermetallics during the heat treatment of explosively welded Al-Ti multilayers. Metall Mater Trans A. 2014. https://doi.org/10.1007/s11661-013-2144-6.
Liu WD, Liu KX, Chen QY, Wang JT, Yan HH, Li XJ. Metallic glass coating on metals plate by adjusted explosive welding technique. Appl Surf Sci. 2009. https://doi.org/10.1016/j.apsusc.2009.07.033.
Bataev IA, Bataev AA, Mali VI, Pavlyukova DV, Yartsev PS, Golovin ED. Nucleation and growth of titanium aluminide in an explosion welded laminate composite. Phys Metals Metallogr. 2012. https://doi.org/10.1134/S0031918X12070022.
Xu L, Cui YY, Hao YL, Yang R. Growth of intermetallic layer in multi-laminated Ti/Al diffusion couples. Mater Sci Eng A. 2006. https://doi.org/10.1016/j.msea.2006.07.077.
Pieraggi B. Calculations of parabolic reaction rate constants. Oxid. Met. 1987. https://doi.org/10.1007/BF00667057.
Wagner G. The evaluation of data obtained with diffusion couples of binary single-phase and multiphase systems. Acta Metall. 1969;17:99–107. https://doi.org/10.1016/0001-6160(69)90131-X.
Bader S, Gust W, Hieber H. Rapid formation of intermetallic compounds by interdiffusion in the Cu-Sn and Ni-Sn systems. Acta Metall Mater. 1995;43:329–37. https://doi.org/10.1016/0956-7151(95)90289-9.
Foadian F, Soltanieh M, Adeli M, Etminanbakhsh M. The formation of TiAl3 during heat treatment in explosively welded Ti-Al multilayeres Iran. J Mater Sci Eng. 2014;11:12–9.
Garg SP, Kale GB, Patil RV, Kundu T. Thermodynamic interdiffusion coefficient in binary systems with intermediate phases. Intermetallics. 1999;7:901–8. https://doi.org/10.1016/S0966-9795(98)00139-3.
López GA, Sommadossi S, Zieba P, Gust W, Mittemeijer EJ. Kinetic behaviour of diffusion-soldered Ni/Al/Ni interconnections. Mater Chem Phys. 2002;78:459–63. https://doi.org/10.1016/S0254-0584(02)00232-8.
Solecka M, Mróz S, Petrzak P, Mania I, Szota P, Stefanik A, Garstka T, Paul H. Microstructure-related properties of explosively welded multi-layer Ti/ Al composites after rolling and annealing. Arch Civ Mech Eng. 2023. https://doi.org/10.1007/s43452-022-00577-4.
Acknowledgements
This research was financially supported by the Institute of Metallurgy and Materials Science of the Polish Academy of Sciences within the statutory work “The interface zone of the welds obtained with the use of explosion energy” Z-5/2023. The SEM and TEM research was conducted in the Accredited Testing Laboratories at the IMMS PAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare relevant to this article's content.
Ethical approval
This manuscript presents original work that has not been published previously and is not under consideration for publication elsewhere. No studies on human or animal subjects were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janusz-Skuza, M., Bigos, A., Kwiecień, I. et al. Mechanism and growth kinetics of Al3Ti phase in fifteen-layered Ti Gr.1/A1050 clads produced with the explosive welding technique. Arch. Civ. Mech. Eng. 24, 155 (2024). https://doi.org/10.1007/s43452-024-00953-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-024-00953-2