Abstract
Recovered metallic waste can be used in additive manufacturing as a feedstock if the subsequent steps of the waste-to-product process are sufficiently mastered. In this study, impact of recycling of Z100 duplex steel mixed with 316L steel on the resulting powders microstructure and chemical composition was investigated. The utility of the original method of recycling stainless steels into a high-grade powder suitable for additive techniques has been demonstrated. By examining three gradations of powders, namely 20–50 μm, 50–100 μm and 125–250 μm, differences in selected properties in relation to the average particle size are shown. The results suggest that with increasing the particle diameter, fine-crystalline γ-austenite is favoured to precipitate at the boundaries and within the volume of the originally formed large δ-ferrite grains. It is reflected by a decrease of δ/γ fraction ratio from 0.64 in the 20–50 μm powders to 0.20 in the 125–250 μm, respectively. Obtained results indicate non-diffusional, shear or semi-shear character of δ → γ + δ phase transformation. The resulting fine-crystalline austenite is characterised by a significant dislocation density, which induces dislocation strengthening effect, responsible for an increase in Vickers hardness from 145 HV and Young's modulus from 29 GPa in the 20–50 μm group to 310 HV and 146 GPa in the 125–250 μm fraction, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Manufacturing and other materials processing technologies of steel components are associated with the production of the significant quantities of metallic waste. These scraps, in a variety of forms, shapes and often coated with various organic lubricants, require special handling and additional processing before they can be successfully reused. On the other hand, they still contain valuable elements and their recycling is increasingly profitable. Metallic waste is becoming an important raw material for the production of new alloys and cast products, with a positive impact on raising the profile of sustainability. The use of scrap as a raw material in the steel industry saves several tonnes of iron ore, coking coal and limestone for each tonne of steel recycled [1]. The issue is even more relevant when stainless steels are being considered, due to their chemical composition. Popular austenitic 304 and 316 grade steels or family of duplex steels can withstands corrosive high-temperature gaseous environments mostly due to the presence of chromium, nickel and molybdenum, which are distinguished as Critical Raw Materials by a recent European Commission report [2].
One of the greatest challenges regarding sustainable steels recycling technologies is scraps contamination [3]. The introduction of undesired elements may occur unintentionally due to the incomplete parts dismantling or during sorting. The type and scale of tramp elements incorporation depends on many factors. An example is the finding of increased copper and tin content in scrap processed in Western European countries, compared to metallic waste from China, which appeared to correlate strongly with the predominance of mechanical or manual sorting methods [4].
The presence of steel impurities are reflected in the altered mechanical properties and corrosion resistance of the materials as well as the components formed from it. Daigo et al. [5] have identified the influence of impurities on the major mechanical properties of carbon steel. Tramp elements show detrimental effect on properties such as maximum elongation, fracture toughness or soundness in the welding area, but at the same time, they improve a tensile strength. Liou et al. [6] have shown that Ni and N surpluses cause decrease in strength and an increase in the material toughness, while additional presence of Cr and Mo improves strength but becomes detrimental to the toughness of the 2205 duplex stainless steel.
Regarding a hot corrosion resistance, the effect varies depending on the chosen contaminant. Incorporation of Cu impurities causes one of the most serious issues in the recycling stainless steel industry. It may induce a hot shortness, which is surface cracking during high temperature deformation and oxidation. The phenomenon occurrence is associated with appearance of the liquid Cu-enriched phase and its penetration into the austenite grain boundaries [7]. Yeşiltepe and Şeşen [8] have studied the high-temperature oxidation behaviours of Cu bearing steel and have reached similar results. They have corelated the higher corrosion rates with the formation of liquid copper layer at the steel/scale interface. The copper impurities may promote corrosion degradation in the salt-chloride containing solutions. Wang et al. [9] have studied copper-containing (3% wt.) 304 steel through neutral salt spray and FeCl3 pitting corrosion tests. They have shown that Cu presence decreases a long-term corrosion resistance in both NaCl and FeCl3 solutions, by increasing the number of non-penetrating and the size of penetrating pits on the steel’s surface. Additional tin inclusions foster hot shortness by promoting significant grain-boundary liquid penetration and their broadening, after a relatively short-time exposure to the oxidizing atmosphere [10]. A microstructure of duplex stainless steel can be affected by incorporating elements such as Ni, C, N and Cu, known for being γ-austenite phase stabilizers, or Cr, Mo and Nb, which stabilize δ-ferrite [11]. Contamination with Cr, Mo, W and Si may promote intermetallic phases (σ and χ) precipitation on the γ—δ grain boundary [12], with detrimental effect on the steel mechanical performance [13].
The traditional approach for scrap recycling is either based on a direct introduction to liquid steel during smelting and casting or based on downcycling them into lesser value cast materials. The latter approach assumes that processed materials are more tolerant of chemical composition variations [14]. In the present work, the possibility of recycling through casting and gas atomisation of mixed scraps of austenitic 316L and ZERON®100 (Z100) duplex stainless steels (DSS) into high-quality powder feedstock exclusively from metal waste, for use in obtaining anti-corrosion coatings and high temperature corrosion resistant additively manufactured parts, was investigated. The main advantage of the proposed process is the efficient management of steel waste in various forms, allowing the implementation of the European Commission's recommendations on the realization of circular economy policies in industries within the European Union. For companies involved in producing the feedstock for additive techniques, the use of scrap as a raw material can be economically attractive through, for example, a reduction in the energy consumption. Set proportions of mixed materials were 30–35%wt. of 316L and 65–70%wt. of Z100, and the main goal was to maintain the extraordinary corrosion protection offered by the DSS family in diluted state. By addition of cheaper 316L steel, the possibility of increasing recycling yield without making concessions in the valued characteristics of DSS-type materials was demonstrated. From an economic point of view, 316L and Z100 steels were chosen because of their wide usage in various industries for forming corrosion-resistant components, consequently having the potential to generate significant amounts of post-processing scraps. Furthermore, the addition of austenitic steel made it possible to study the effect of changing the chemical composition of duplex steel on the austenite and ferrite phases formation trends. The effect of the manufacturing route on the chemistry of the recycled materials and their microstructure was assessed using current state-of-the-art characterization techniques, while microindentation technique was applied to correlate microstructural features with mechanical response of the powders.
2 Materials and methods
2.1 Batch materials (scrap processing)
The post-processing, metallic chips of the 316L and Z100 alloys were collected, crushed and cold pressed (press pressure up to 160KN) into cylindrical compacts of 10 cm in diameter and 15 cm in height in 30–35/70–65 ratio by weight by GESCRAP (Basque country, Spain). Then, scraps were further treated at Łukasiewicz-Krakow Institute of Technology (Kraków, Poland) At first, they were degreased using an semi-industrial ultrasonic bath with capacity of 100 litters at 40 °C for at least 30 min. The process was carried out in isopropanol and up to three times due to high degree of organic contamination of the delivered, pressed scrap material. The alcohol was replaced after each cleaning round. The degreased pressed coupons were pre-heated at 150–200 °C in air for at least 24 h using a chamber furnace, to remove any residual moisture and to carbonize remaining organic compounds.
Subsequently, scraps were melt in an induction furnace by heating to 100–150 °C above melting point, using foundry crucibles with a capacity of 50 kg each. Compacts were added gradually, as soon as the metal bath appeared on the surface to avoid boiling and contamination. Slag was collected and around 20 g of aluminium was added to deoxidize the metal bath prior to casting. Melted steels were cast into bentonite moulds, giving several 30 kg truncated cone-shaped ingots. These were sand-blasted in order to remove oxide-layer presented on the surface.
2.2 Fabrication of powders
The atomization process of stainless steel ingots was performed by an in-house manufactured gas atomization setup with a melting capacity of approximately 4 litters. The system is schematically presented in Fig. 1. The main parts of an experimental setup included: tilt-able induction alloy melting system (1), six-meter long atomization tower (2), tundish and atomization nozzle system (3), high-pressure gas system for atomization and atomization tower pouring, cyclone separator (4), filtration system (5) and water cooling system.
The atomization process was carried out following few steps. Firstly, the feedstock ingots were melted under nitrogen atmosphere. After obtaining proper melt homogeneity, the furnace was tilted and the melt was poured into a tundish. The high-pressure flow of argon, as an atomization gas, was activated afterwards. The gas was supplied under approximately 20 bar pressure and was purging the tower until the fine stream of melt flew through the 8 mm tundish orifice. Then, the stream was atomized by several micro jets formed by the axisymmetric atomization nozzle system. The micro-droplets solidified in-flight in the atomization tower and were finally collected in the cyclone separator.
The overall manufacturing route is described in more details under two pending patents relating to recycling of stainless steel [15] and nickel-based steel [16] chips.
The powders were sifted into three different fractions, using sieves with a mesh size corresponding to the aimed extremes of powder size in the group, namely 20–50 μm, 50–100 μm and 125–250 μm. Powders size distribution (volume weighted) was established by using Anton Paar PSA 1190 particle size analyser, by dispersion of the powders in water. Table 1 and Fig. 2 show the averaged results of five measurements for each group. In Table 1, D10, D50 and D90 are percentile values corresponding to the size below which 10%, 50% and 90% of all particles can be found.
In the 125–250 μm fraction, there is a noticeable discrepancy between the mesh of the sieve used and the upper limit of the powder size (Table 1, D90 = 341.34 μm). This is due to the irregularity of the shape of the material and the defects present on the surface, the volume of which increases as the average grain size increases, effect addressed in the “Results” section. The used granulometer operates based on the phenomenon of laser diffraction. When the laser beam is diffracted during measurement, a perfectly spherical shape of the particle is assumed in order to calculate the diameter of the powder under test. When defects are presented on a particle’s surface or the shape of the powder deviates from spherical (it has a finite number of axes of symmetry), the measurement result begins to depend on the spatial orientation of the particle in question, i.e. on its position relative to the laser light source. For this reason, inflation of the average powder grain size in this group is observed. In this study, the mesh of the sieves used is included in the name to distinguish the powder fractions tested.
2.3 Characterization methods
Chemical analysis was conducted with a Bruker S8 TIGER WDXRF spectrometer, equipped with an X-ray tube with Rh anode, using 50 kV accelerating voltage. The data were analysed by Quant-Express program of Spectra Pluss software. For the determination of carbon and sulphur, LECO CS-200 apparatus with two separated infrared (IR) cells, was used. The measuring uncertainties are: C: 20%, S: 25%, P: 25%, Cu: 20%, Mo: 8%, Si: 20%, Mn: 20%, Ni: 3%, Cr: 3% (in weight percentages). Results are presented in Table 2. Specimens for metallographic investigations were hot mounted using conductive AKASEL phenolic resin. The metallographic mounts were polished using 220–1200 grade SiC papers, followed by a mechanical polishing in diamond suspensions. Microstructural features of the powders were revealed by chemical etching with Aqua Regia. Microstructural and surface morphology investigations were conducted by using Carl Zeiss Axio Observer ZM10 light microscope (LM) and FEI Scios™ field emission gun scanning electron microscope (FEG SEM) coupled with energy-dispersive X-Ray spectroscopy module (EDS) and electron backscatter diffraction system (EBSD). Through the possible limitation of the adopted strategy for the analysis of the surface of the powders, resulting, among other things, from their rough topography, the results of the EDS analysis presented in Table 4 are given to the nearest unit for oxygen and to one decimal place for the other elements. Prior to the EBSD investigations, mounts were polished using fine colloidal silica suspensions. For each powders fraction sieved, a single representative particle was selected and X-ray scans were carried out on its cross-section. For a particle 49 μm in diameter, from 20 to 50 μm fraction, analysis step 0,067 was chosen, while for both particles, 89 μm in diameter (from 50 to 100 μm fraction) and 248 μm in diameter (from 125 to 250 μm fraction) analysis step 0.2 was applied.
The XRD study was carried out on a Empyrean Series 2 diffractometer (PANalytical instrument) equipped with a copper target (Cu Kα radiation with a wavelength of 0,15,418 nm). The analysis was conducted within the 2θ range of 20–100°, with a step size of 0.02°. The following empirical formula was used for the phase volume content determination [17]:
where \({\varvec{i}}\) is a designed symbol for either austenite or ferrite, \({{\varvec{V}}}_{{\varvec{i}}}\) is the volume fraction of the \({\varvec{i}}\) phase, \({{\varvec{I}}}_{{\varvec{i}}}^{{\varvec{j}}}\) is the integrated intensity for the next (hkl) planes considered, distinguished by \({\varvec{j}}\). Three highest peaks for each phase were taken into account: γ(111), γ(200), γ(311) and δ(110), δ(200), δ(211). The number of peaks considered for the each phase is represented by \({\varvec{n}}\), which means \(n=3\). \({{\varvec{R}}}_{{\varvec{i}}}^{{\varvec{j}}}\) is a theoretical integrated intensity for the next dhkl planes, calculated following the ASTM E975-13 standard [18]. Using the Bragg’s relation, interplanar spacing dhkl were calculated, from which lattice parameters for cubic systems presented (bcc and fcc) were determined:
The primary crystallization route was assessed using the φ-value model [19]:
where
are namely nickel—equivalent (\({\mathrm{Ni}}_{\mathrm{eq}}\)) and chromium—equivalent (\({\mathrm{Cr}}_{\mathrm{eq}}\)) factors, basing on elements known for having γ-austenite and δ-ferrite phases stabilisation effect, calculated on the basis of the weighting percentages of the consecutive components. Finally:
All these results are presented in Table 3.
The selected mechanical properties, namely Vickers hardness and Young’s modulus, were investigated using Anton Paar Micro Combi Module for ultra-nanoindentation tests (UNHT). The diamond Vickers type intender in quadrilateral pyramid shape was used. The measuring program applied: the maximum load of 0.2 N and loading/unloading rate of 0.4 N/min, with pause at contact of 10 s. At least ten measurements were done on the several particles cross-sections, for each powder fraction. Ten percent of the high and low results end were discarded, and the trimmed means were used for the further discussion. The standard deviation was used to mark errors bars on Fig. 9.
3 Results
3.1 Morphology of atomized powders
Table 2 outlines chemical composition of initially used scraps and resulting atomised powders, without significant variations with different powder fractions. The wt.% for each alloying element diverge between the extreme values in the following matter: S: 0.001%, P: 0.001%, C: 0.01%, Cu: 0.07%, Mn: 0.15%, Si: 0.19%, Mo: 0.4%, Ni: 0.4%, Cr: 1.6%, which show that most significant disparities regard Cr, Ni and Mo content. Comparing these to the constitution of standard 316L steel, one can notice higher Cr, C, Si, Cu and Mo content, at the same time with lower Ni, Mn, P and S concentrations. Regarding standard Z100 steel, C, Ni, P, Si and Mn concentrations are higher, while Cr and Cu contents are lower. This is reflected in the calculated (Table 3) chemical composition characteristics, with the highest Creq/Nieq ratio for 125–250 μm fraction and lowest for 50–100 μm fraction. No significant correlation was found between powders chemistry and powder size distribution.
Figure 3 presents the results of SEM observations carried out on powders surface, while the corresponding EDS results are collected in Table 4. Images shown in Fig. 2a, c, e were acquired in the secondary electrons (SE) mode, while these in Fig. 2b, d, f in the backscattered electrons (BSE) mode, respectively. The SE mode reveals powders morphology. The powders had a spherical shape, but with a variety of defects observed, such as metallization, bonded together and elongated particles, “clip-cap” and “satellites”. The number of observed defects increases with the powder size. The weight content of the elements decreases in the order of Fe, Cr, Ni, O, Si and Mo. Some copper impurity is revealed in Fig. 3a, b; spot 2. Generally, the EDS results concerning contents of particular elements (Table 4) are comparable to those given in Table 2, with only slightly higher contents of Cr and Si (Creq), and lower contents of Mo and Ni and with no traces of Mn (Nieq). Surface defects vary in composition, depending on the type. Satellites (Fig. 3a, b; spot 3) and elongated particles (Fig. 3c, d, spot 3) had similar constitution to the spherical particles. The “clip-cap” flaws (Fig. 3e, f; spot 2) contains mostly chromium, oxygen and silicon. Atomic content suggests the formation of chromium and silicon oxides. The BSE micrographs show the differences (contrast) in surface composition of powders. Dark areas indicate a decrease in the atomic weight of the quantified elements, which may suggest a local increase in the concentration of oxygen and silicon. Point SEM analysis (Fig. 3c, d; spot 2) confirms it.
3.2 Microstructure
Figure 4 presents microstructure of powders. The duplex structure consisting of dendritic austenite with interdendritic inclusions of ferrite, is clearly distinguished.
Dual-phase structures can be topologically characterized depending on the relative orientation of the phases formed. In this case, the net structure is observed. It would suggest presence of only two types of phase boundaries, namely γ/γ and δ/γ [20]. Both types of phases are indicated in the corresponding colours in Fig. 4d. A formation of columns by austenite grains (Fig. 4c) is also noticeable. Their longitudinal axis is oriented towards the particle centre, pointing out heat flow directions during solidification, since austenite columns preferably grow towards highest temperature gradients presented [21]. There are no signs of precipitations of σ-, χ-phases or carbides from the M6C23 group, reported elsewhere [12]. On the other hand, sparse pores, as well as a thin layer of oxide on the particle surface are presented, what is confirmed by the results of surface EDS analysis (Fig. 3 and Table 4).
Figure 5 shows the results of XRD investigations. These confirm dual-phase character of material, by denoting lattice planes characteristic for austenite: γ(111), γ(200), γ(220), γ(311), γ(222) and for ferrite: δ(110), δ(200), δ(211), δ(220). The δ(220) plane was not recorded for the 125–250 μm fraction. Intensities of these planes vary with powder size distribution: the smaller the particle diameter, the higher the peaks specific for ferrite and the smaller the peaks specific for austenite. This observation was used to estimate the δ/γ content ratio for powders in each particular fraction (Table 3). The results indicate that the highest δ/γ content ratio, namely 0.64, was obtained for the powders with the smallest diameter size distribution, 20–50 μm, which (among all examined powder fractions) is the closest to the ideal duplex stainless steel proportion (δ/γ = 1). The smallest δ/γ content ratio, namely 0.20, is for the 125–250 μm fraction, while for the 50–100 μm powder, the ratio is 0.28. It points towards a rise in the δ/γ proportion with the decrease of the average powder size.
Figures 6, 7, 8 present the results of EBSD analyses carried out for each powder fraction. Figure 6a–c shows distribution of phases over the investigated area, proving lack of precipitations and material’s duplex character. Two types of grain boundaries are distinguished here: low angle grain boundaries (having a misorientation of 2–15°) marked with a thin black line and high angle grain boundaries (15–180°) marked with a thick black line. It is worth noting that a significant proportion of low-angle boundaries was detected in the volume of austenite grains, with no equivalent in the ferrite phase. Figure 7 presents Inverse Pole Figure (IPF) maps for ferrite and austenite, respectively. As the same colour in the subfigure indicates the same crystal orientation (in accordance with the colour code of inserted “basic triangle”), the maps (Fig. 7) show the presence of crystals with different crystallographic orientations, except for ferrite in the 120–250 μm fraction (Fig. 7c), where numerous small crystals appear to share a common crystal structure. In Fig. 8 are shown the EBSD Geometrically Necessary Dislocations (GND) maps for ferrite (a, b, c) and austenite (d, e, f), respectively. They indicate dominance of dislocation densities up to 70·1012 m−2, with local sharp increases within grains volume and at boundaries. Such density can be considered as high compared with literature data [22].
Figure 9 presents the relationship between mechanical properties and the powders phase composition. There is an apparent increase in the hardness and Young modulus (E) of the materials as the ferrite content decreases. For the finest powders fraction, the Vickers hardness is 145HV, and Young’s modulus 29 GPa. With the decrease in the δ/γ ratio for 50–100 μm fraction, from 0.64 to 0.28, there is a hardness increase of 63%, to 237 HV, and E increases of 121% to 64 GPa. With the further δ/γ ratio decrease for 125–250 μm fraction to 0.20, hardness increases by 27% to 301 HV, while elastic modulus by 128% to 146 GPa.
4 Discussion
The target phase composition of duplex stainless steel, ferrite to austenite, is 1:1 by volume fraction, since this composition ensures a good resistance against pitting corrosion, as well as attractive mechanical properties. It can be achieved by a strictly tailored chemical composition and by selecting the appropriate cooling rate of the heat treated material. Changing one or another factors affects the final phase fractions. Therefore, differences in the γ and δ phases content between the different powder grades (Fig. 5 and Table 3) observed in the present work, can be correlated with different cooling rates of powders. During gas atomisation process, a melt stream is disrupted by a high velocity gas, which results in droplets formation having a wide size distribution. The volume of the formed particles affects the rate of heat transfer with the environment [23]. It can, therefore, be assumed that the particles from the three different fractions studied solidified at different average rates [24]. Dai et. al [25] have shown that the dependence of cooling rate on particle diameter is a decreasing homographic function, until reaching the critical diameter range about 20–40 μm below which the cooling rate increases sharply. The calculated cooling rates by authors of the mentioned work for 20 μm diameter powder is 1.53 × 106 °C/s, for 50 μm is 3.64 × 105 °C/s and for 100 μm is 1.25 × 105 °C/s. In agreement with tendency reported elsewhere, rapid solidification (cooling) rates of duplex steels promotes δ-ferrite formation [26], what is further strengthen with high ratios of Creq/Nieq (Table 3) present in the steel.
By taking into account, the applied cooling rates and materials chemical compositions, it is more likely that the bcc-iron form present is δ-ferrite rather than α-ferrite. Often considered the same phase, but separated by temperature gap, δ-ferrite has higher carbon solubility due to the slightly increased crystalline lattice constant a, as a result of thermal expansion [27]. According to the Fe–C phase diagram and various Fe–Cr–Ni pseudo-binary ternary diagrams (at different Fe wt. %), initially high-temperature form of ferrite, δ, crystallizes when crossing liquidus line below 1536 °C, L → δ [28]. Usually, during further cooling down and assuming equilibrium conditions, δ would be replaced by α. However, high cooling rates prohibit this phase transformation and, thereby, stabilize δ phase. It is confirmed by higher a value calculated from the XRD spectra (Table 3) compared with that reported elsewhere [29]. Moreover, alloying elements, especially chromium and silicon, are known to stabilize δ-ferrite upon cooling down to the room temperature [30].
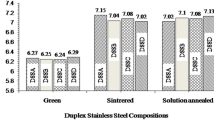
Following the Schaeffler–De Long phase diagram for the ferritic–austenitic steels, addition of 316L scraps shifted the pre-designed Creq/Nieq balance of Z100 steel towards austenitic-zone (Fig. 10). Calculated φ-values (Table 3) point towards a mechanism of ferritic primary solidification, that is typical for casting of duplex stainless steels, although resulting values are higher than for investigated Z100 scraps, namely φ = − 13.72. These discrepancies should be related to the addition of 316L steel, which decreased the Creq/Nieq ratio by increase the concentration of austenite stabilizers. It puts probable solidification sequence in ferritic–austenitic (FA) mode, which means that investigated powders solidify with the bcc (δ) as a primary phase, and with the adequate undercooling, the fcc (γ) structure starts to form. The higher the Creq/Nieq ratio, the greater the degree of undercooling required to start the δ → γ + δ transformation. The phase transformation occurs rapidly at lower temperatures by increasing the number of nucleation sites at the δ/δ boundaries, resulting in a finer microstructure [31, 32]. These observations are confirmed by the EBSD IPF maps presented in Fig. 7, which show large ferrite crystals separated by fine austenite grains having a relatively high fraction of low-angle boundaries. Involved rapid cooling rates also affects the observed phases constitution and elements segregation within a powder particle volume. The maximum solubility of alloying elements varies considering bcc (δ) or fcc (γ) structures. The maximum Creq/Nieq ratio value is greater in ferrite than in austenite phase. Thus, it is expected that the local Creq/Nieq ratio increases with the rise of ferrite content. When cooling down upon atomization, a droplet with an initially uniform temperature distribution cools most rapidly at the surface, resulting in favourable formation of ferrite in this area, as discussed before. This is confirmed by the results of EDS analyses showing an increase in chromium and silicon concentrations (Fig. 3, Table 4). Proposed solidification mechanism is visualized in Fig. 11. Lower heat treatment temperatures and slow cooling rates promote segregation by a diffusion mechanism of Creq elements into ferrite from austenite; and Nieq elements in the opposite direction [33]. However, an implementation of substantial cooling rates inhibits this mechanism [20]. These findings make it possible to narrow down the described austenite formation during solidification to non-diffusion shear/semi-shear phase transformation [34], similar in a nature to the martensitic transformation. The specific feature of newly formed phase during the shear/semi-shear transformation (in this case, γ from the δ → γ + δ) has a highly defected microstructure and similar chemical composition comparing to the parental phase [35]. It is confirmed by GND maps presented in Fig. 8, which shows relatively high dislocations density.
A scheme of proposed solidification route. I. Formation of particles with large ferrite grains directly from the melt, L → δ. II. Precipitation of fine austenite grains on δ/δ boundaries and within ferrite crystals, δ → γ + δ. III. Growth of numerous austenite crystals on further cooling. IN COLOR ON THE WEB
When comparing chemical composition of the initial scraps to the final powders, higher concentrations of carbon and silicon, are found. The raised concentration of carbon is the effect of using a graphite nozzle during the gas atomisation process. The molten steel interacts with graphite, what results in a nozzle degradation and diffusion of carbon into the melt. This may have a serious consequences regarding material’s performance in corrosive environments. Carbon concentration above 0.05% promotes precipitation of undesirable phases such as chromium carbides [37], witch exhibit negative impact on corrosion protection (behaviour), but their presence was not observed in this work. This again should be correlated with the significant cooling rate of metallic powders during solidification phase. Homogenized, precipitation-free duplex steel microstructure can be assured by a solution annealing heat treatment [20]. The chosen heat treatment temperature should be carried out at temperature above the range that thermodynamically favours precipitation of undesired phases. In the case of ferritic-austenitic stainless steels, the threshold is commonly accepted to be 1000 °C [12]. The quenching stage is achieved by free cooling of the powder in inert atmosphere, with material’s cooling rate far superior to that for standard water quenching, as described earlier. This situation can be avoided by the usage of SiC or Al2O3 ceramic nozzles. The raised content of silicon may be the effect of imprecise sandblasting of ingots prior to atomisation or the element was unintentionally introduced with the scraps. It may influence both pitting [38] and hot corrosion behaviour [39] of additively manufactured parts.
There are mainly two reasons for hardness and Young’s modulus differences between powder gradations (Fig. 9). Both properties are correlated with ferrite and austenite fractional contents. The ferrite exhibits slightly lower hardness than austenite [40], due to the solid solution strengthening in the latter, provided by interstitial elements such as carbon [41]. The Young’s modulus depends on a dislocation density which is high in the austenite grains [42], which results in strain hardening effect. As a results, higher austenite content leads to higher hardness and elevated elastic modulus in the duplex stainless steels. Among the two effects described above, the second had a much greater impact on the investigated materials. The possible effect of refined microstructure and more extended grain/phase boundaries, should be also taken into account. Beside of a well-known Hall–Petch relationship, Li et al. [43] have delivered a mathematical model which proves that materials hardness increases with the grains refinement, as well as that wider grain size distribution contributes more to higher hardness than narrow grain size distribution. In other words, microstructures whose grain sizes have a bimodal distribution have greater hardness than grain sizes described by a Gaussian distribution. This may be the case here, as the coexistence of fine austenite grains and larger crystals of ferrite, was found.
5 Conclusions and future works
In this study, we investigated the possibility of recycling mixed 316L and Z100 steels into the powder for use as a batch in additive manufacturing techniques. We investigated how the addition of 316L steel affects the formation of the two-phase structure characteristic for duplex steels. The relationship between average particle size and the chemical composition, microstructure and hardness of the powder was investigated, leading to the following conclusions:
-
1.
The addition of 316L steel in amounts of 30–35 vol.% results in a disruption of the original Creq to Nieq ratio, which is reflected in the volume proportions of the austenite and ferrite phases. Despite this, the resulting microstructure of the material is still duplex type.
-
2.
The solidification mechanism of the material is defined, where the delta ferrite grains crystallise first. Subsequently, numerous austenite grains nucleate at the δ/δ grain boundaries as well as in the volume of the crystals themselves. This solidification mechanism should be referred to as non-diffusional, as indicated by the pronounced contribution of low-angle boundaries and significantly high dislocations density within the austenite grains.
-
3.
A strong correlation occurs between the fraction of austenite and ferrite phases and the particle size. It result from the change in cooling rate being a function of diameter of the particle: as the particle volume decreases, the rate of heat transfer with the environment increases. The effect of this is an increase in the relative content of ferrite as the diameter decreases. The same conclusion cannot be drawn for the chemical composition, where no significant correlation was found between powder particle size and material chemistry (chemical composition).
-
4.
A relationship was found between hardness and Young's modulus and the phase composition of the powders, where, as the ferrite content decreases, both mentioned mechanical properties increase. This is mainly related to the dislocation strengthening effect caused by fine-grained austenite. Therefore, with an increase in cooling rate, an increase in hardness and Young's modulus can be expected in the studied group of materials.
The conducted research allows to understand the impact of the recycling process and heat treatment on the key properties of the materials, paving the way for a wider application of recycling methods in the processing of stainless steels scraps and obtaining from them a high-quality substrate for additive techniques. The obtained results obtained confirm the feasibility of the applied recycling method, the products of which could be successfully used as a batch in additive manufacturing techniques. The next step would be to perform technological trials and to produce a series of samples using selected additive manufacturing technique. Subsequently, the effect of the altered chemical composition of the recycled materials on the microstructure and selected mechanical or corrosion properties of the formed components, significant for their applications, will be evaluated. This would allow to further develop stainless steels recycling technology.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tuck CC. Iron and steel scrap, U.S. geological survey, mineral commodity summaries. 2022;1–2.
Bobba S, Carrara S, Huisman J, Mathieux F, Pavel C. Critical raw materials for strategic technologies and sectors in the EU: a foresight study. 2020.
Raabe D, Ponge D, Uggowitzer PJ, Roscher M, Paolantonio M, Liu C, Antrekowitsch H, Kozeschnik E, Seidmann D, Gault B, de Geuser F, Deschamps A, Hutchinson C, Liu C, Li Z, Prangnell P, Robson J, Shanthraj P, Vakili S, Sinclair C, Bourgeois L, Pogatscher S. Making sustainable aluminum by recycling scrap: the science of “dirty” alloys. Prog Mater Sci. 2022;128:1–150. https://doi.org/10.1016/j.pmatsci.2022.100947.
Panasiuk D, Daigo I, Hoshino T, Hayashi H, Yamasue E, Tran DH, Sprecher B, Shi F, Shatokha V. International comparison of impurities mixing and accumulation in steel scrap. J Ind Ecol. 2022;26:1040–50. https://doi.org/10.1111/jiec.13246.
Daigo I, Tajima K, Hayashi H, Panasiuk D, Takeyama K, Ono H, Kobayashi Y, Nakajima K, Hoshino T. Potential influences of impurities on properties of recycled carbon steel. ISIJ Int. 2021;61:498–505. https://doi.org/10.2355/isijinternational.ISIJINT-2020-377.
Liou H-Y, Pan Y-T, Hsieh R-I, Tsai W-T. Effects of alloying elements on the mechanical properties and corrosion behaviors of 2205 duplex stainless steels. JMEPEG. 2001;10:231–41.
Shibata K, Seo S-J, Kaga M, Uchino H, Sasanuma A, Asakura K, Nagasaki C. Suppression of surface hot shortness due to Cu in recycled steels. Mater Trans. 2002;43:292–300. https://doi.org/10.2320/matertrans.43.292.
Yeşiltepe S, Şeşen MK. High-temperature oxidation kinetics of Cu bearing carbon steel. SN Appl Sci. 2020. https://doi.org/10.1007/s42452-020-2473-1.
Wang K, Guo L, Liu T, Huang A, Zhao Y, Wang W, Peng J. Effect of Cu on the corrosion behavior of 304 stainless steel. Crystals (Basel). 2022;13:31. https://doi.org/10.3390/cryst13010031.
Yin L, Sridhar S. Effects of small additions of tin on high-temperature oxidation of Fe–Cu–Sn alloys for surface hot shortness. Metall Mater Trans B Process Metall Mater Process Sci. 2010;41:1095–107. https://doi.org/10.1007/s11663-010-9418-9.
Gunn RN. Duplex stainless steels. In: Woodhead publishing series in metals and surface engineering. New York: Woodhead Publishing, 1997.
de Paula Inácio LK, Wolf W, de Leucas BCB, Stumpf GC, Santos DB. Microtexture evolution of sigma phase in an aged fine-grained 2205 duplex stainless steel. Mater Charact. 2021;171:1–9. https://doi.org/10.1016/j.matchar.2020.110802.
Wang C, Wu Y, Guo YA, Guo J, Zhou L. Precipitation behavior of sigma phase and its influence on mechanical properties of a Ni-Fe based alloy. J Alloys Compd. 2019;784:266–75. https://doi.org/10.1016/j.jallcom.2019.01.048.
Dudziak T, Olbrycht A, Polkowska A, Boron L, Skierski P, Wypych A, Ambroziak A, Krezel A. High temperature coatings from post processing Fe-based chips and Ni-based alloys as a solution for critical raw materials. In: IOP Conf Ser Mater Sci Eng, Institute of Physics Publishing, 2018. https://doi.org/10.1088/1757-899X/329/1/012010.
Dudziak T. Sposób wytwarzania proszków w procesie recyclingu wiórów stalowych, PL 237562 B1, 2017.
Dudziak T. Sposób wytwarzania proszków w procesie recyclingu wiórów niklowych, PL 241473 B1, 2017.
De AK, Murdock DC, Mataya MC, Speer JG, Matlock DK. Quantitative measurement of deformation-induced martensite in 304 stainless steel by X-ray diffraction. Scr Mater. 2004;50:1445–9. https://doi.org/10.1016/j.scriptamat.2004.03.011.
ASTM E975-13, Standard practice for X-ray determination of retained austenite in steel with near random crystallographic orientation. ASTM International. (n.d.). https://doi.org/10.1520/E0975-13.
Hammar O, Svensson U. Influence of steel composition on segregation and microstructure during solidification of austenitic stainless steels. In: Solidification and casting of metals. London: The Metals Society; 1979. p. 401–10.
Knyazeva M, Pohl M. Duplex steels: part I: genesis formation, structure. Metallogr Microstruct Anal. 2013;2:113–21. https://doi.org/10.1007/s13632-013-0066-8.
Zhu C, Wang W, Zeng J, Lu C, Zhou L, Chang J. Interactive relationship between the superheat, interfacial heat transfer, deposited film and microstructure in strip casting of duplex stainless steel. ISIJ Int. 2019;59:880–8. https://doi.org/10.2355/isijinternational.ISIJINT-2018-747.
Muñoz JA, Chand M, Signorelli JW, Calvo J, Cabrera JM. Strengthening of duplex stainless steel processed by equal channel angular pressing (ECAP). Int J Adv Manuf Technol. 2022. https://doi.org/10.1007/s00170-022-10311-2.
Wang N, Wei B. Rapid solidification behaviour of Ag–Cu–Ge ternary eutectic alloy. Mater Sci Eng A. 2001;307:80–90. https://doi.org/10.1016/S0921-5093(00)01954-7.
Tourret D, Reinhart G, Gandin CA, Iles GN, Dahlborg U, Calvo-Dahlborg M, Bao CM. Gas atomization of Al-Ni powders: solidification modeling and neutron diffraction analysis. Acta Mater. 2011;59:6658–69. https://doi.org/10.1016/j.actamat.2011.07.023.
Dai Y, Yang M, Song C, Han Q, Zhai Q. Solidification structure of C2.08Cr25.43Si1.19Mn0.43Fe70.87 powders fabricated by high pressure gas atomization. Mater Charact. 2010;61:116–22. https://doi.org/10.1016/j.matchar.2009.11.001.
Liao L, Chumbley S. Influence of cooling rate on the ferrite prediction diagram of duplex stainless steel castings. Metall Mater Trans A Phys Metall Mater Sci. 2019;50:2435–42. https://doi.org/10.1007/s11661-019-05166-4.
Wright RN. Relevant aspects of carbon and low-alloy steel metallurgy. In: Wire technology. Amsterdam: Elsevier; 2016. p. 201–33. https://doi.org/10.1016/b978-0-12-802650-2.00014-5.
Zhang D, Liu A, Yin B, Wen P. Additive manufacturing of duplex stainless steels—a critical review. J Manuf Process. 2022;73:496–517. https://doi.org/10.1016/j.jmapro.2021.11.036.
Onink M, Brakrnan C, Tichelaar F, Mittemeijer E, van der Zwaag S, Root J, Konyer N. The lattice parameters of austenite and ferrite in Fe-C alloys as functions of carbon concentration and temperature. Scr Metall Mater. 1993;29:1011–6.
Hsieh CC, Lin DY, Chang TC. Microstructural evolution during the δ/σ/γ phase transformation of the SUS 309LSi stainless steel after aging under various nitrogen atmospheric ratios. Mater Sci Eng, A. 2008;475:128–35. https://doi.org/10.1016/j.msea.2007.04.028.
Koseki T, Flemings MC. Solidification of undercooled Fe–Cr–Ni alloys: part I thermal behavior. Metall Mater Trans A. 1995;26:2991–9. https://doi.org/10.1007/BF02663873.
Koseki T, Flemings MC. Solidification of undercooled Fe–Cr–Ni alloys: part II. microstructural evolution. Metall Mater Trans A. 1996;27:3226–40. https://doi.org/10.1007/BF02669655.
Berns H, Theisen W. Ferrous materials: steel and cast iron. Berlin: Springer; 2008. https://doi.org/10.1007/978-3-540-71848-2.
Zhu C, Zeng J, Wang W, Chang S, Lu C. Mechanism of δ → δ + γ phase transformation and hardening behavior of duplex stainless steel via sub-rapid solidification process. Mater Charact. 2020. https://doi.org/10.1016/j.matchar.2020.110679.
Haghdadi N, Cizek P, Hodgson PD, Tari V, Rohrer GS, Beladi H. Effect of ferrite-to-austenite phase transformation path on the interface crystallographic character distributions in a duplex stainless steel. Acta Mater. 2018;145:196–209. https://doi.org/10.1016/j.actamat.2017.11.057.
Schaeffler AL. Construction diagram for stainless steel weld metal. Metal Progress. 1949;56:680-680B.
Knyazeva M, Pohl M. Duplex steels. Part II: carbides and nitrides. Metallogr Microstruct Anal. 2013;2:343–51. https://doi.org/10.1007/s13632-013-0088-2.
Lee BH, Lee HJ, Kang DW, Lee HW. The effect of silicon content on the pitting corrosion of duplex stainless steel weldment, 2014. www.archivesmse.org.
AlaouiMouayd A, Koltsov A, Sutter E, Tribollet B. Effect of silicon content in steel and oxidation temperature on scale growth and morphology. Mater Chem Phys. 2014;143:996–1004. https://doi.org/10.1016/j.matchemphys.2013.10.037.
Wang XF, Yang XP, Guo ZD, Zhou YC, Song HW. Nanoindentation characterization of mechanical properties of ferrite and austenite in duplex stainless steel. Adv Mat Res. 2007;26–28:1165–70. https://doi.org/10.4028/www.scientific.net/amr.26-28.1165.
Simmons JW, Overview A. High-nitrogen alloying of stainless steels. Mater Sci Eng, A. 1996;207:159–69.
Zhang Y, Kang R, Dong Z, Bao Y, Guo D, Bai Q. Effect of dislocation density distribution in work-hardened layer on cutting characteristics in the multi-cutting of 49Fe49Co2V alloy. J Manuf Process. 2023;85:1187–96. https://doi.org/10.1016/j.jmapro.2022.12.043.
Li W, Vittorietti M, Jongbloed G, Sietsma J. The combined influence of grain size distribution and dislocation density on hardness of interstitial free steel. J Mater Sci Technol. 2020;45:35–43. https://doi.org/10.1016/j.jmst.2019.11.025.
Acknowledgements
The authors would like to acknowledge the support of GESCRAP for delivery of raw materials (scraps). The work was funded with the support of M.Era.net network with financial support of National Research and Development Centre (NCBR) in Poland and of the Basque Government (INNOBASQUE, SPRI) in Spain for the project entitled: “Additive manufacturing Parts and Coatings using Recycled Powder from waste” [Grant number: PROJECT8346]. The work was carried out as part of the Implementation Doctorate Programme, funded by Polish Ministry of Education and Science (MEiN) [Grant number: WIMiC.COP.pk-0721-191/22].
Author information
Authors and Affiliations
Contributions
FK: conceptualization, methodology, formal analysis, investigation: light microscopy, data curation, writing—original draft. AP: investigation: SEM/EDS/EBSD, light microscopy, hardness. WP: visualization, investigation: EBSD, writing—review & editing. KC: investigation: materials casting. KJ: investigation: materials casting. PS: visualization, investigation: gas atomisation. AI: supervision, funding acquisition. JL: investigation: chemical analysis. MB-S: investigation: XRD. TD: resources, writing—review and editing, supervision, funding acquisition. JJ: writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethics approval
No human or animal studies were performed in the course of the work described in the manuscript. This material is the authors' own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kateusz, F., Polkowska, A., Polkowski, W. et al. Chemistry and microstructure of duplex stainless steel powders from recycled Z100 mixed with 316L steels. Archiv.Civ.Mech.Eng 23, 246 (2023). https://doi.org/10.1007/s43452-023-00782-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-023-00782-9